Abstract
Radiotherapy has been a longstanding treatment option for low-grade glioma. Improvements in tumor control and radiation-related toxicity may be attributed to advances in neuroimaging as well as radiotherapy planning and delivery. The discovery of various molecular prognostic factors have aided in patient selection for radiotherapy. These prognostic and predictive factors may also play a key role in determining which patients are likely to benefit most from combined systemic therapy and radiation.
KEYWORDS : astrocytoma, chemotherapy, IMRT, intensity modulated radiotherapy, low-grade glioma, OARs, oligodendroglioma, organs at risk, quality of life, radiation necrosis, radiotherapy, temozolomide
Practice points.
Several factors are considered in the decision to proceed with radiotherapy after a diagnosis of low-grade glioma: patient-specific factors (age ≥40, performance status, persistent postoperative symptoms) and tumor-specific factors (tumor volume, extent of resection, molecular and genetic features including 1p/19q status and IDH mutation).
Postoperative radiotherapy may benefit symptomatic patients, including patients with focal neurological deficits, excessive edema or raised intracranial pressure.
Current standard of care radiotherapy would include the use of MRI to define the target volume (T2 or FLAIR abnormality with a margin) and organs at risk with 3D-conformal or intensity modulated radiotherapy planning and delivery.
As recurrences are largely local and in-field following surgery and radiation, efforts have explored dose-escalation, altered fractionation and particle therapy radiation treatments but these have been associated with increased toxicity without an improvement in tumor control or survival.
Adding chemotherapy to radiotherapy for patients with low-grade glioma has resulted in improvements in survival, particularly for patients with 1p/19q codeleted tumors.
With advances in surgical techniques, improvements in neuroimaging and growing knowledge about molecular and genetic predictive and prognostic biomarkers, the management of adult patients with low-grade glioma following surgical resection is evolving. Historically, the primary postoperative treatment of low-grade gliomas has been radiotherapy. Of note, historical trials especially in grade II tumors often combined oligodendroglial and astrocytic tumors, and subset analyses distinguishing outcomes of specific tumor histologies (e.g., oligodendroglioma, astrocytoma) were completed post hoc.
Role of radiotherapy
Following biopsy or surgical resection that histologically confirms a diagnosis of grade 2 or 3 glioma, immediate postoperative radiotherapy is considered. Several factors may influence the decision to proceed with radiotherapy at initial presentation, including patient-specific and tumor-specific factors. The patient factors include the initial clinical presentation, the postoperative course and persistence of symptoms following surgical resection, as well as patient age and overall patient performance status. The tumor-specific factors include tumor grade (grade 2 vs grade 3), tumor volume, evidence of mass effect, extent of resection and more recently, consideration of the molecular and genetic features of the tumor that may predict their natural history and response to treatments.
• Radiotherapy versus observation
The role of immediate postoperative radiotherapy was challenged in a large Phase III randomized study, EORTC 22845, which randomized patients with low-grade glioma to early postoperative radiotherapy versus observation until progression. In this study, 311 adult patients with supratentorial low-grade gliomas (WHO grade 1–2 51% astrocytoma, 14% oligodendroglioma, 13% mixed oligoastrocytoma) were randomized to immediate postoperative radiotherapy (n = 154) or observation (n = 157) following surgery (42% GTR, 19% STR, 35% biopsy) [1]. Radiation aimed to cover the pre-operative, rather than postoperative, tumor volume to a total dose of 54 Gy over 30 daily weekday treatments. Patients in the observation arm were considered for treatment at progression with the majority of patients treated with radiotherapy (65%) and the remaining patients treated with chemotherapy (16%), or no treatment (12%). Progression-free survival (PFS) was significantly better with early radiotherapy compared with observation with a median PFS of 5.3 years versus 3.4 years, translating to a 5-year PFS of 55 versus 35%, respectively (p < 0.0001). However, this did not translate to an improvement in overall survival (OS) with a median survival of 7.2 years for the observation arm and 7.4 years for the radiotherapy arm. Although quality of life measurements were not specifically analyzed as they were not collected for all patients, improved tumor control was associated with better seizure control at 1 year (41% seizures with observation vs 25% seizures with radiotherapy). Therefore, this study suggested that postoperative radiotherapy may benefit symptomatic patients, including patients with focal neurological deficits, excessive edema or raised intracranial pressure. But without evidence for improvement in survival, it would be reasonable to withhold radiotherapy until progression in young patients who present with minimal symptoms.
• Radiotherapy versus chemotherapy
There have been several studies demonstrating the efficacy of PCV (procarbazine, CCNU and vincristine) and temozolomide in the adjuvant and salvage setting for grade 2 and 3 gliomas [2–4]. Several Phase II studies have shown that temozolomide monotherapy, in lieu of radiotherapy, can result in response rates between 31 and 61%, durable PFS and 3-year OS of 57 to 66% [5–7]. This has raised the question of whether patients with low-grade glioma could be managed with postoperative chemotherapy instead of radiotherapy, thereby deferring the toxicities of radiotherapy.
In order to address this question, the efficacy of early postoperative radiotherapy was compared against temozolomide in a randomized Phase III trial, EORTC 22033–26033/CE.5. In this study, 477 patients with high-risk supratentorial low-grade gliomas were randomized to postoperative radiotherapy (50.4 Gy in 28 fractions delivered within 6 weeks) versus temozolomide (75 mg/m2 daily for 21 days every month [28 days] until progression or a maximum of 12 cycles). Patients were considered high risk if they had at least one of the following features: age ≥40 years, postoperative radiological progression, new or worsening neurological symptoms or intractable seizures that were interfering with daily activities. In comparison to the EORTC 22845 study, the radiotherapy targeted the postoperative surgical cavity and FLAIR abnormality rather than the pre-operative tumor volume. Although, this study failed to demonstrate a PFS or OS difference between the two arms for the overall study population, chromosome 1p status was identified as a promising prognostic and predictive marker. Specifically, patients with 1p retention had a trend toward inferior PFS with temozolomide and patients with 1p deletion had more favourable preliminary OS results. These findings suggest that postoperative temozolomide should be considered for patients with 1p deletion whereas radiotherapy should be offered to patients with 1p retention.
The NOA-04 trial randomized 318 patients with anaplastic glioma to one of three initial treatment arms: radiotherapy (arm A), PCV (arm B1) and temozolomide (arm B2). At the time of progression, patients who had received radiation were randomized to either PCV of temozolomide and patients who had received chemotherapy were treated with radiotherapy. The primary end point of time to treatment failure was not significantly different between radiotherapy and either chemotherapy (hazard ratio [HR]: 1.2; 95% CI: 0.8–1.8) [8].
Although there is evidence to support the use of either PCV or temozolomide following surgical resection in selected patients, current neuro-oncology practices may favor the use of temozolomide in light of the more favorable toxicity profile of temozolomide over PCV [9]. In particular, for patients with good performance status and favorable molecular profile of 1p 19q codeletion, there is interest in using chemotherapy to defer the need for radiotherapy.
Radiotherapy dose & fractionation
As recurrences are largely local and in field following surgery and radiation, there have been several studies exploring the role of dose-escalation to improve tumor control. Unfortunately, these studies have failed to demonstrate improvement in outcome with the use of higher doses of radiotherapy for low-grade gliomas. In Europe, EORTC 22844, a Phase III randomized study of 343 patients with supratentorial low-grade glioma (WHO grade 1–2), including a mixture of astrocytoma (69%), oligodendroglioma (22%) and mixed oligoastrocytoma (9%) were randomized to two doses of radiotherapy following surgery (25% gross total resection, 30% subtotal resection, 45% biopsy) [10]. The two radiation doses were 45 Gy in 25 fractions delivered within 6 weeks or 59.4 Gy in 33 fractions delivered within 8 weeks. Radiotherapy techniques were variable and included simple two-field treatment to multifield treatments encompassing the enhancing tumor and nonenhancing tumor with margins. After a median follow-up of 74 months, no differences in either OS (5-year OS of 58 vs 59%) or PFS (5-year PFS of 47 vs 50%) were demonstrated between the low-dose versus high-dose arms [10]. In this patient population treated with postoperative radiotherapy, prognostic factors associated with better OS and PFS on multivariable analysis included smaller tumor volume, gross total resection and neurological status. Age under 40 years was also associated with better survival, as shown in other studies regardless of the management approach.
The North American NCCTG Phase III study randomized 203 adult patients with grade 1–2 astrocytoma, oligodendroglioma and mixed oligoastrocytomas to standard-dose postoperative radiotherapy (50.4 Gy in 28 fractions) or an even higher dose of postoperative radiotherapy (64.8 Gy in 36 fractions) [11]. The radiation in the high-dose arm incorporated a lower dose to a larger volume with margin around the tumor with a conformal boost directed to the pre-operative tumor to the total dose of 64.8 Gy. Similar to the European study, there was no difference in OS or PFS. But dose-escalation up to 64.8 Gy resulted in higher rates of toxicity with 5% of patients in the high-dose arms with grade 3–5 neurotoxicity versus 2.5% in the low-dose arm at 2 years [11].
Similarly, small studies exploring the role of altered fractionation schemes have not demonstrated greater survival outcomes than conventional radiotherapy. There was a Phase II study investigating the efficacy and toxicity of hyperfractionated radiation dose-escalation in 37 adult patients with incompletely resected supratentorial low-grade gliomas. The hyperfractionated radiation regimen was composed of several phases with shrinking volumes such that the tumor plus 2 cm margin was treated with 55 Gy in 50 fractions delivered in 25 treatment days over 5 weeks, then the tumor plus 1 cm margin was treated with an additional 17.6 Gy given in 16 fractions in 8 treatment days over 1.5 weeks such that the total dose was 72.6 Gy in 66 fractions in 33 treatment days over 6.5 weeks. At the time of publication, median survival time and median time to tumor progression had not yet been reached. However, this treatment was reasonably tolerated with only grade 1 and 2 toxicities noted, and 7-year PFS and OS were similar to those resulting from standard treatment, 70 and 69%, respectively [12]. Similarly, a small series from McGill University reported the outcomes of 21 patients with well-circumscribed LGG (<4 cm) treated with 42 Gy in six fractions. These patients had an OS similar to those expected with standard therapy (10 years: 71%, 15 years: 63%) [13].
Radiotherapy treatment planning, delivery & techniques
Studies of radiotherapy for low-grade glioma have used variable target volumes with variable margins intended to treat microscopic disease in the peritumoral region. Definition of the target volume has varied from the pre-operative tumor volume to the postoperative surgical bed along with residual tumor, and it is our personal recommendation that the latter be utilized. Although low-grade gliomas are not expected to grow within weeks of surgery, the timing of image acquisition for radiotherapy planning may impact the radiotherapy treatment, as there can be substantial changes in the surgical cavity during the early postoperative period, and for this reason we recommend postoperative MRI images be obtained at the time of treatment planning.
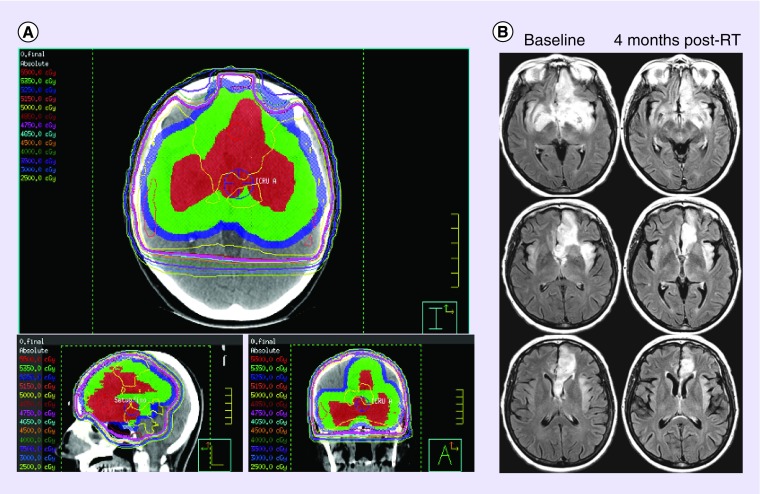
Patients are typically immobilized in a thermoplastic mask, which they wear for the CT simulation and daily treatments. Postoperative MRI images, including gadolinium-enhanced T1 and T2 or FLAIR image sets, are commonly coregistered to the CT image set to assist in target delineation. In general the target volume includes the surgical cavity and any visible residual tumor, which appears hyperintense on the FLAIR images along with a margin to encompass the region most likely to contain further microscopic tumor (Figure 1). Using the available image sets, organs at risk (OARs) are delineated and dose thresholds are respected during the planning process. These OARs include but are not limited to the lenses, orbits, optic nerves and chiasm, brainstem, cochlea, lacrimal glands and parotid glands.
Figure 1. . Radiotherapy planning and imaging response.
(A) Representative image of a radiotherapy plan delivering 50 Gy in 25 fractions to a large bifrontal oligodendroglioma. Gross tumor volume is in red, clinical target volume in green and planning target volume in blue. (B) Representative images of the tumor at the time of radiotherapy and 4 months postradiotherapy.
Most radiotherapy centers currently use 3D-conformal or intensity modulated radiotherapy (IMRT) techniques for the treatment of low-grade gliomas (Figure 1). If available, centers may use cone-beam CT to guide online correction and ensure that any displacements in patient positioning are within 2 mm, prior to treatment delivery. Although practice has evolved in most centers to exploit the readily available technology, there is no prospective data to show that advanced imaging and treatment techniques have survival impact.
Several studies have reported both the benefit and toxicity associated with the use of protons and carbon ions to treat low-grade gliomas. Fitzek et al. explored the role of using proton/photon radiotherapy with dose escalation to improve outcomes for lower grade gliomas in a prospective Phase I/II trial. In this study, 20 patients Daumas–Duport grade 2 (n = 7) and grade 3 (n = 13) gliomas were treated with postoperative proton/photon radiotherapy to a total dose of 68.2 cobalt Gy equivalent (CGE, 1 proton Gy 5 1.1 CGE) to gross tumor for grade 2 lesions and 79.7 CGE for grade 3 lesions delivered in 1.8 to 1.92 CGE daily fractions. Median age at diagnosis was 35.9 years (range 19–49), and tumor histologies included astrocytoma (n = 9), oligodendroglioma (n = 1) and mixed gliomas (n = 10). With median follow-up of 61 months for patients with grade 2 tumors, actuarial 5-year survival rate was estimated at 71% as calculated from diagnosis and median survival has not yet been reached. For grade 3 patients, with median follow-up of 55 months, the actuarial 5-year survival rate was 23% and median survival was 29 months. Tumor recurrence was the cause of death in three of seven patients with grade 2 tumors and eight of 13 patients with grade 3 tumors. The incidence of radionecrosis was remarkably high with evidence of radionecrosis in two of seven patients with grade 2 tumors and four of 13 patients with grade 3 tumors of which one patient likely died from radionecrosis [14]. Therefore the current data for dose escalation, including studies with combined proton/photon radiotherapy do report higher rates of toxicity, including radionecrosis, with no evidence of improved outcomes for patients with grade 2 and 3 gliomas.
A Phase I/II clinical trial has evaluated the outcomes of carbon ion radiotherapy for grade 2 diffuse astrocytoma. Fourteen patients with diffuse astrocytoma were treated with two doses of carbon ion radiotherapy, either a lower dose of 50.4 GyE or 55.2 GyE delivered in 24 fractions over 6 weeks. There were nine patients enrolled at the lower dose but seven patients received the entire dose of 50.4 GyE, while two patients received a reduced dose of 46.2 GyE in order to limit dose to the basal ganglia (due to deep-seated location and large size). Five patients were enrolled and received the total higher dose of 55.2 GyE. As none of the patients developed grade 3 or higher acute or late reactions, it was concluded that toxicities were acceptable with this treatment. The median PFS was 18 months for the low-dose group, but much higher at 91 months for the high-dose group (p < 0.003) and the median OS time was 28 months for the low-dose group and not reached for the high-dose group after a median follow-up of 62 months (p < 0.0208) [15]. These findings provide early provocative data to raise the question of whether high-dose carbon ion radiotherapy may lead to better PFS and OS, although this experience must be interpreted with caution in view of the small patient numbers.
Combined chemotherapy & radiation
Adding chemotherapy to radiotherapy for patients with low-grade glioma has so far resulted in modest benefits, observed in prior trials that largely evaluated nitrosurea-based chemotherapy [16,17]. However, the combination of chemotherapy with radiotherapy had failed to demonstrate significant survival benefit over radiotherapy alone for grade 2 or 3 glioma in any prospective randomized trial until recently.
• Nitrosurea-based chemotherapy
Based on prior studies showing greater benefit of adjuvant PCV over BCNU, there have been several studies exploring the combination of PCV with radiotherapy. The Medical Research Council compared radiotherapy to radiotherapy with PCV in patients with high-grade astrocytoma. This trial failed to show a significant survival advantage in the PCV arm, although the trend was toward improved survival with PCV (hazard ratio 0.86; 95% CI: 0.58–1.30) [18]. However, only 19% of patients in this study had anaplastic astrocytomas with the remainder having glioblastoma.
Recent updates of two large randomized trials suggest a survival benefit with the addition of PCV to radiotherapy particularly for patients with 1p/19q codeleted anaplastic gliomas containing an oligodendroglial component (either pure anaplastic oligodendroglioma or mixed anaplastic glioma). The EORTC study randomized patients to radiotherapy with or without five adjuvant five cycles of standard-dose PCV following radiotherapy and showed an increase in OS to 42.3 months with the addition of PCV versus 30.6 months with RT alone (HR: 0.75; 95% CI: 0.60–0.95) [19]. The RTOG 9402 study randomized patients to dose-intense PCV followed by radiotherapy versus radiotherapy alone and did not result in significantly better OS with median survival of 4.9 years with PCV versus 4.7 years without PCV (HR: 0.79; 95% CI: 0.60–1.04) [20]. On further subset analyses in both studies based on 1p/19q codeletion status, only the codeleted patients had improved OS with combined PCV and radiation, which was statistically significant in the RTOG 9402 study (HR: 0.67; 95% CI: 0.50–0.91) and near significant in the EORTC study (HR: 0.56; 95% CI: 0.31–1.03) [19,21].
Similar to the anaplastic glioma studies, RTOG 9802 randomized patients with grade II astrocytoma, oligodendroglioma or mixed oligoastrocytoma with high-risk features (age ≥40 or incomplete resection) to radiotherapy (54 Gy in 30 fractions directed to the tumor volume defined as FLAIR hyperintensity with a 2 cm) or radiotherapy followed by six cycles of PCV. After a median follow-up of 5.9 years, the 5-year PFS was 46% for radiation versus 63% for radiation with PCV (p = 0.005) and the median OS for the RT arm was 7.5 years but had not been reached for the combined therapy arm. Therefore, the addition of PCV to radiation again demonstrated improvement in PFS but has not shown an improvement in OS at the present time [22]. It was also noted that the separation of the survival curves for both PFS and OS only presented after 2 years of follow-up suggesting that there is a delayed benefit with PCV, and a larger OS benefit is anticipated with longer follow-up. This prediction was supported by a post hoc analysis that suggested that for patients who have survived 2 years in this study (n = 211), the rate of survival for an additional 5 years was greater with PCV (74%) than without PCV (59%) (p = 0.02).
Although many of the more recent studies have emphasized investigation with PCV in combination with RT, a retrospective study reviewing the outcomes of several prior RTOG trial regimens demonstrated no difference in survival outcome between BCNU and PCV in combination with RT in patients with anaplastic astrocytomas [23]. Therefore, both PCV and BCNU likely have a favorable impact but one chemotherapy regimen is not likely superior to the other when used in combination with radiotherapy.
• Temozolomide
Temozolomide is a well-tolerated chemotherapeutic regimen with radiosensitizing properties that has shown significant survival benefit in a Phase III randomized study of patients with glioblastoma. In this study, the addition of temozolomide, concurrently with radiation and adjuvantly for 6 months, resulted in an improvement in median survival from 12.1 months to 14.6 months (p < 0.0001) and a doubling of 2-year survival from 10 to 26%. Based on these promising results with glioblastoma and considering the mild toxicity profile even in combination with radiotherapy, the potential benefit of adding temozolomide to radiation for low-grade glioma is under investigation.
A Phase II study, RTOG 0424, evaluated a Stupp-like regimen of radiation with concurrent and adjuvant temozolomide in 136 patients with low-grade glioma with at least 3 high-risk factors. The radiation treatment consisted of conformal radiotherapy with 54 Gy delivered in 30 fractions, and Temozolomide 75 mg/m2 was administered daily concurrently with radiotherapy over 6 weeks and then continued at a dose of 150–200 mg/m2 on days 1–5, every 28 days, for up to 12 cycles. This study has reported promising results with a 3-year OS rate of 73.1%. Although not an ideal control group, the OS of this study cohort was reported to be higher than a historical control group composed of patients enrolled on a prior EORTC trial [24].
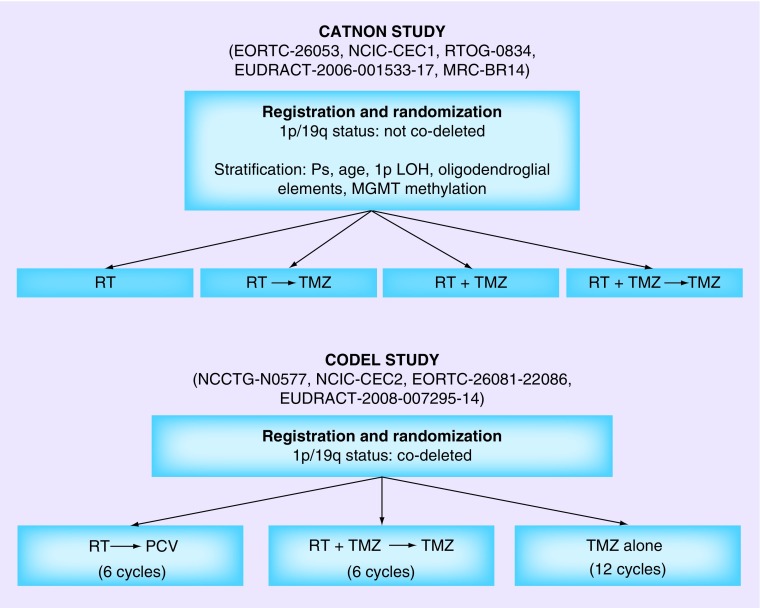
There are several ongoing Phase III studies evaluating the role of temozolomide in combination with radiation. (Table 1) The Intergroup (ECOG, RTOG, NCCTG and SWOG) Phase III study randomizes patients with grade II astrocytoma, oligodendroglioma or mixed oligoastrocytomas to radiotherapy (50.4 Gy in 28 fractions) or radiotherapy with concurrent and adjuvant temozolomide. Eligible patients need to have at least one high-risk feature: headache with mass effect, uncontrolled seizures despite two anti-epileptic medications, focal neurological or cognitive deficits, radiological tumor progression or age ≥40. For grade III gliomas, there are two tandem Phase III international studies attempting to evaluate the role of temozolomide and radiotherapy as part of initial therapy. Recognizing the prognostic and predictive value of 1p/19q status, the two trials are separated based on 1p/19q codeletion status, while both studies enroll all histologies including oligodendroglioma, astrocytoma or mixed glioma. Patients who are not codeleted tend to have a worse prognosis and require adjuvant radiotherapy, therefore the ‘CATNON’ study aims to evaluate the benefit of adding temozolomide by randomizing patients to radiotherapy with or without concurrent daily temozolomide followed by a second randomization to adjuvant temozolomide for a total of 12 cycles. Patients who are codeleted tend to have a better prognosis and are more likely to response to temozolomide therefore the initial study design randomized patients to temozolomide, radiotherapy and radiotherapy in combination with temozolomide. However, the compelling results of RTOG 9802 showing benefit of combined PCV and radiotherapy, particularly for patients with 1p/19q codeletion, have raised discussions around the appropriate control arm for this study and a major amendment has recently been made to the study design such that the control arm of radiotherapy alone is now radiotherapy with PCV (Figure 2).
Table 1. . Recruiting Phase I, II and III radiation trials of low-grade and anaplastic gliomas.
| Study ID | Study title | Phase | Chemo | Year started |
|---|---|---|---|---|
| EORTC-26053, NCIC-CEC1, RTOG-0834, EUDRACT-2006–001533–17 MRC-BR14, NCT00626990 |
Radiation therapy with or without temozolomide in treating patients with anaplastic glioma (1p/19q not codeleted) | III | TMZ | 2009 |
| NCCTG-N0577, NCIC-CEC2, EORTC-26081–22086, EUDRACT-2008–007295–14,NCT00887146 |
Radiation therapy or radiation therapy and temozolomide or temozolomide alone in treating patients with newly diagnosed anaplastic glioma (1p/19q codeleted) | III | TMZ, PCV | 2009 |
| ECOG-E3F05, NCT00978458 |
Radiation therapy with or without temozolomide in treating patients with low-grade glioma | III | TMZ | 2009 |
| NCT01358058 | Proton radiation therapy for low-grade gliomas | II | N/A | 2011 |
| NCT01024907 | Proton beam radiation therapy in treating patients with low-grade gliomas | I–II | N/A | 2013 |
Figure 2. . Current study schemes for CATNON and CODEL studies of radiation and temozolomide for anaplastic gliomas.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 2.Viaccoz A, Lekoubou A, Ducray F. Chemotherapy in low-grade gliomas. Curr. Opin. Oncol. 2012;24(6):694–701. doi: 10.1097/CCO.0b013e328357f503. [DOI] [PubMed] [Google Scholar]
- 3.Brada M, Stenning S, Gabe R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J. Clin. Oncol. 2010;28(30):4601–4608. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 4.Buckner JC, Gesme D, Jr, O'Fallon JR, et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J. Clin. Oncol. 2003;21(2):251–255. doi: 10.1200/JCO.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin. Cancer Res. 2009;15(1):330–337. doi: 10.1158/1078-0432.CCR-08-0888. [DOI] [PubMed] [Google Scholar]
- 6.van den Bent MJ, Taphoorn MJ, Brandes AA, et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J. Clin. Oncol. 2003;21(13):2525–2528. doi: 10.1200/JCO.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J. Clin. Oncol. 2003;21(4):646–651. doi: 10.1200/JCO.2003.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized Phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J. Clin. Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 9.Pouratian N, Schiff D. Management of low-grade glioma. Curr. Neurol. Neurosci. Rep. 2010;10(3):224–231. doi: 10.1007/s11910-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2002;20(9):2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 12.Jeremic B, Shibamoto Y, Grujicic D, et al. Hyperfractionated radiation therapy for incompletely resected supratentorial low-grade glioma. A Phase II study. Radiother. Oncol. 1998;49(1):49–54. doi: 10.1016/s0167-8140(98)00074-7. [DOI] [PubMed] [Google Scholar]
- 13.Roberge D, Souhami L, Olivier A, Leblanc R, Podgorsak E. Hypofractionated stereotactic radiotherapy for low grade glioma at McGill University: long-term follow-up. Technol. Cancer Res. Treat. 2006;5(1):1–8. doi: 10.1177/153303460600500101. [DOI] [PubMed] [Google Scholar]
- 14.Fitzek MM, Thornton AF, Harsh GT, et al. Dose-escalation with proton/photon irradiation for Daumas-Duport lower-grade glioma: results of an institutional Phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2001;51(1):131–137. doi: 10.1016/s0360-3016(01)01589-9. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa A, Mizoe JE, Tsujii H, et al. Experience with carbon ion radiotherapy for WHO Grade 2 diffuse astrocytomas. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(1):100–106. doi: 10.1016/j.ijrobp.2011.06.1952. [DOI] [PubMed] [Google Scholar]
- 16.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71(8):2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Eyre HJ, Crowley JJ, Townsend JJ, et al. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low-grade gliomas: a Southwest Oncology Group study. J. Neurosurg. 1993;78(6):909–914. doi: 10.3171/jns.1993.78.6.0909. [DOI] [PubMed] [Google Scholar]
- 18.Medical Research Council Brain Tumor Working P. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J. Clin. Oncol. 2001;19(2):509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 19.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intergroup Radiation Therapy Oncology Group T. Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J. Clin. Oncol. 2006;24(18):2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 21.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 22.Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J. Clin. Oncol. 2012;30(25):3065–3070. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prados MD, Scott C, Curran WJ, Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: a retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J. Clin. Oncol. 1999;17(11):3389–3395. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- 24.Fisher BJ, Hu C, Macdonald DR, et al. Phase 2 study of temozolomide-based chemoradiationtherapy for high-risk low-grade gliomas: preliminary results of RadiationTherapy Oncology Group 0424. Int. J. Radiat. Biol. Phys. 2015;91(3):497–504. doi: 10.1016/j.ijrobp.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]