Abstract
Background
High sedentary time is associated with adverse metabolic health outcomes and mortality in older adults. It has been suggested that breaking up sedentary time may be beneficial for metabolic health; however population prevalence data are lacking on the patterns of sedentary behaviour which would identify opportunities for intervention.
Methods
We used data of adults aged ≥60 years (n=3705) from the population-based EPIC-Norfolk cohort to characterise the patterns of total sedentary time, breaks in sedentary time, and sedentary bouts across the day and assess their associations with participant characteristics using multi-level regression. Sedentary time was measured objectively by a hip-mounted accelerometer (Actigraph™ GT1M) worn for 7 days during awake time.
Results
More than 50% of every waking hour was spent sedentary, increasing to a peak of 83% in the evening. On average fewer breaks were accrued in the evenings compared to earlier in the day. Marginally more sedentary time was accrued on weekend days compared to weekdays (difference 7.4 minutes, 95% CI 5.0-9.7). Large proportions of this sedentary time appear to be accrued in short bouts (bouts of <10 minutes 32% of the time). Older age, being male, being retired, not being in paid employment and having a higher BMI were associated with greater sedentary time and fewer breaks.
Conclusion
Sedentary time is common throughout the day but peaks in the evenings with fewer breaks and longer bouts. We identified a number of characteristics associated with sedentary time and additionally inversely associated with sedentary breaks, which should inform the development and targeting of strategies to reduce sedentary time among older adults.
Keywords: sedentary time, older adults, epidemiology
Background
There is emerging evidence that higher levels of sedentary time are associated with adverse health outcomes (1–4). Greater sedentary time has been associated with more adverse cardio-metabolic risk profiles, higher risk of type 2 diabetes (7) and cardiovascular disease, higher risk of cardiovascular and all-cause mortality (6,7) and reduction in physical capability. Increased understanding of the patterns and impact of sedentary time has important public health implications. There may be greater opportunities for health promotion interventions to target sedentary time in comparison to moderate-to-vigorous physical activity (MVPA) which only makes up a small portion of waking hours (8), even among highly active adults. A recent meta-analysis of more than a million individuals, which examined the joint associations of self-reported MVPA and self-reported sitting time with all-cause mortality, reported that high MVPA levels, of >60 minutes/day (achieved by 25% of the population), attenuated the association between sitting time and all-cause mortality (8). Berkemeyer et al. (9) report median daily MVPA time of <15min for over 60 year olds. Despite the known health benefits of MVPA, the majority of individuals have difficulty in maintaining such high levels (10–12), so targeting sedentary time may represent a complementary strategy.
Early work on the epidemiology of bouts and breaks in sedentary time in both the general adult population, and to a lesser extent older adults, suggests that prolonged sedentary bouts (13–21) and fewer breaks in sedentary time (22–26) may be deleterious to metabolic health, independent of total sedentary time and MVPA. The recent literature on descriptive epidemiology of sedentary time among older adults predominantly focuses on mean total sedentary time as measured by accelerometers. We currently know little about how sedentary time is accrued; in particular, there are few data concerning how sedentary time is distributed across the day and the week, the length of bouts typically accrued, and how it is broken up. Consequently, there is a need for research on this topic which will contribute to greater understanding of the determinants of sedentary time and inform development of interventions to reduce sedentary time.
This study is novel in utilising the data from a large population-based cohort of older adults (the European Prospective Investigation into Cancer in Norfolk, EPIC-Norfolk), to examine the descriptive epidemiology of objectively measured total sedentary time, breaks in sedentary time and sedentary bouts at the hourly level.
Methods
Participants
The European Prospective Investigation into Cancer (EPIC) Norfolk is a large prospective cohort of over 25,000 people living in Norfolk who were recruited at baseline between 1993-1997. The participating cohort at initial recruitment was similar to the national population sample studied in the Health Survey of England in terms of anthropometry, serum lipids and blood pressure (27).
Within EPIC-Norfolk, baseline characteristics were obtained through an initial health check (28). Between 2006-2011, 8623 individuals participated in a 3rd Health-check (27). The 3rd health check included accelerometry assessment of free-living behaviour in a subsample of 4137 (based on monitor availability). The present analysis was restricted to participants aged over 60 years (n=3705). The age of 60 was used in concordance with the United Nations agreed cut-off for older adults (29).
Data Collection
Participants received questionnaires and were examined by nurses trained to adhere to the EPIC research protocol. Participants completed a Health & Lifestyle Questionnaire which addressed their personal and family history of disease, current medication, and lifestyle factors including physical activity, diet and smoking habits. The phrasing of the questions have been outlined in detail before (28). Trained nurses also took anthropometric measurements at the health assessment. Weight was measured on digital scales and height was measured using a free-standing stadiometer.
Accelerometry
Participants were invited to wear a uniaxial accelerometer (Actigraph GT1M™, Actigraph, Pensacola, USA) on the right hip for 7 days during waking hours and to remove it while showering, bathing or swimming. Monitors were initialised to record activity and step frequency at 5 second epoch intervals, which was then integrated into 60 second epochs, to maximise comparability to the existing literature (30,31). We defined sedentary behaviour as acceleration <100 counts per minute (cpm) (32,33), again to maximise comparability with the existing literature (34). This method is an established proxy for measurement of sedentary time (35–37) although there is potential for misclassification of time spent sitting or reclining from standing with the use of this method (34,38). We defined breaks in sedentary time as ≥1minute spent in acceleration of ≥100 cpm (39,40). Non-wear was identified as continuous zero count strings of ≥90 minutes. Accelerometry summary data were generated for each consecutive hour of each record. In the present analysis, we only included hours if at least 30 minutes of the hour was wear time, and we further only included records with at least 600 minutes of wear time on at least one day between the hours of 06:00 and 00:00. Sensitivity analysis utilising records with at least five days of wear time greater than 600 minutes per day was done (individual accelerometer-wear time of 5 days or more made up 96% of total accelerometer wear-data, individual accelerometer-wear time of 7 days made up 79% of total accelerometer wear-data).
Statistical Analysis
Hourly accelerometry data were indexed with hour of day and day of week, and other participant level data were merged onto this dataset. Multilevel mixed-effects linear regression models weighted for hourly wear-time were used to analyse associations, with random intercepts specified at participant level. Stratification by descriptive factors was built into models, with adjustment factors which have previously been shown to be related to sedentary time (age and sex). Descriptive factors that were examined included age (60-<65, 65-<70, 70-<75, 75-<80, 80-<85, ≥85), sex, smoking status (never, former, current), BMI (<18.5, 18.5-<25, 25-<30, 30-<35, ≥35 kg/m2), employment status (employed versus not employed), social class (I, II, IIIa, IIIb, IV, V), retirement status (retired versus not retired), education level (further education after age 16 years (yes versus no)), and marital status (single, married, other). The model was additionally tested to examine if there were differences after stratification for weekend versus weekday. Interactions were tested in the model and confidence intervals were examined for significance. All analyses were conducted using STATA 13.1 (StataCorp, TX, USA) and figures were produced in Microsoft Excel.
Results
Participant Characteristics
We included 3705 participants in this analysis (Table 1). Included individuals had similar characteristics to the excluded individuals, to those in the wider cohort of EPIC-Norfolk at the 3rd health check and as reported previously, those included in the Health Survey for England (27). Death registration data from the Office for National Statistics (41) suggests that the Norfolk area is slightly healthier than the general UK population, with a standardized mortality ratio of 0.92. Characteristics of the cohort at baseline have been described previously (27). As reported before, EPIC-Norfolk represents a healthier sample than the general UK population; in this sample only 3.4% were current smokers, and prevalence of co-morbid conditions were lower than would be expected in the general population (rates in this sample were diabetes 3.7%, stroke 2.3%, cancer 12.0%, myocardial infarction 4.0%).
Table 1. Baseline Characteristics.
Included Participants n=3705. Statistical testing for differences was done with either Student’s unpaired T-Test, Chi-squared test, or Mann-Whitney U test. Chi-squared test for trend was used for age groups and BMI groups. The ‘Other’ category of marital status includes those who were widowed, separated or divorced.
| Characteristic | Included Participants | All EPIC-Norfolk 3rd Health-check Participants Aged over 60 years | P Value For Difference between Included & all EPIC-Norfolk Participants At 3rd Health-check Aged over 60 years | ||||
|---|---|---|---|---|---|---|---|
| Frequency | Percent (%) | Frequency | Percent (%) | ||||
| Sex | Female | 1683 | 45.4 | 3463 | 45.8 | 0.15 | |
| Male | 2022 | 54.6 | 4096 | 54.2 | |||
| TOTAL | 3705 | 100 | 7559 | 100 | |||
| Age | 60-<65 | 1030 | 27.8 | 2,136 | 28.3 | 0.29 | |
| 65-<70 | 913 | 24.6 | 1,784 | 23.6 | (P for trend) | ||
| 70-<75 | 821 | 22.2 | 1,594 | 21.1 | |||
| 75-<80 | 563 | 15.2 | 1,208 | 16.0 | |||
| 80-<85 | 294 | 7.94 | 643 | 8.51 | |||
| ≥85 | 84 | 2.27 | 194 | 2.56 | |||
| TOTAL | 3705 | 100 | 7559 | 100 | |||
| Marital Status | Single | 111 | 3.16 | 251 | 3.41 | 0.25 | |
| Married | 2845 | 78.6 | 5,692 | 77.3 | |||
| Other | 663 | 18.3 | 1425 | 19.4 | |||
| TOTAL | 3619 | 100 | 7,368 | 100 | |||
| Social Class | I | 325 | 8.84 | 641 | 8.56 | 0.42(P for trend) | |
| II | 1519 | 41.3 | 3067 | 41.0 | |||
| IIIa | 588 | 16.0 | 1215 | 16.2 | |||
| IIIb | 759 | 20.6 | 1543 | 20.6 | |||
| IV | 414 | 11.3 | 843 | 11.2 | |||
| V | 73 | 1.98 | 178 | 2.38 | |||
| TOTAL | 3678 | 100 | 7487 | 100 | |||
| Paid Employment | Yes | 719 | 19.8 | 1480 | 20.0 | 0.83 | |
| No | 2,911 | 80.2 | 5928 | 80.0 | |||
| TOTAL | 3,630 | 100 | 7408 | 100 | |||
| Retired | Yes | 3,074 | 86.2 | 6196 | 85.2 | 0.16 | |
| No | 491 | 13.8 | 1074 | 14.8 | |||
| TOTAL | 3,565 | 100 | 7270 | 100 | |||
| Further Education after Left School | Yes | 1,603 | 43.7 | 3246 | 43.4 | 0.77 | |
| No | 2,067 | 56.3 | 4235 | 56.6 | |||
| TOTAL | 3,670 | 100 | 7481 | 100 | |||
| Smoking Status | Current | 124 | 3.40 | 287 | 3.85 | 0.37 | |
| Former | 1,697 | 46.6 | 3524 | 47.3 | |||
| Never | 1,824 | 50.0 | 3634 | 48.8 | |||
| TOTAL | 3,645 | 100 | 7445 | 100 | |||
| Complying To Recommended Alcohol Limit (Males: <21units/week, Females: <14 Units/week) | Male | Yes | 1,454 | 90.3 | 2981 | 89.7 | 0.32 |
| No | 156 | 9.69 | 343 | 10.3 | |||
| TOTAL | 1,610 | 100 | 3324 | 100 | |||
| Female | Yes | 1778 | 92.0 | 3624 | 92.4 | 0.55 | |
| No | 155 | 8.02 | 297 | 7.57 | |||
| TOTAL | 1933 | 100 | 3921 | 100 | |||
| Personal History of Stroke | No | 3,619 | 97.7 | 7364 | 97.5 | 0.41 | |
| Yes | 85 | 2.29 | 193 | 2.55 | |||
| TOTAL | 3,704 | 100 | 7557 | 100 | |||
| Personal History of MI | No | 3,555 | 96.0 | 7247 | 95.9 | 0.84 | |
| Yes | 149 | 4.02 | 310 | 4.1 | |||
| TOTAL | 3,704 | 100 | 7557 | 100 | |||
| BMI (kg/m2) | <18.5 | 24 | 0.65 | 53 | 0.7 | 0.48 | |
| 18.5-<25 | 1,253 | 33.9 | 2564 | 34.0 | |||
| 25-<30 | 1,722 | 46.6 | 3477 | 46.1 | |||
| 30-<35 | 551 | 14.9 | 1124 | 14.9 | |||
| ≥35 | 149 | 3.19 | 272 | 3.27 | |||
| TOTAL | 3,699 | 100 | 7540 | 100 | |||
Among the 3705 individuals (all aged over 60 years) there was a good spread across age groups between 60-80 years old (90% of all included individuals), with a further 10% over age 80. The median age of participants was 69.5 (IQR 64.5-75.1 years). 86% of the included individuals were retired, as would be expected in a cohort of older adults.
The mean daily sedentary time was 545 minutes (SD 81.3). The mean number of daily breaks in sedentary time was 78 (SD 14.3). Average break duration was 4.6 minutes (SD 5.9). Mean daily sedentary time was predominantly accumulated in bouts of >10 minutes (mean 367 minutes (SD 123)) spent in bouts of >10 minutes, 67% of total sedentary time). Bouts of >20 minutes accounted for 47% of total sedentary time (mean 257 minutes (SD 122)), whereas only 12% of total sedentary time was accumulated through bouts of >60 minutes (mean 63 minutes (SD 82).
Distribution of Hourly Sedentary Time & Breaks across the Day
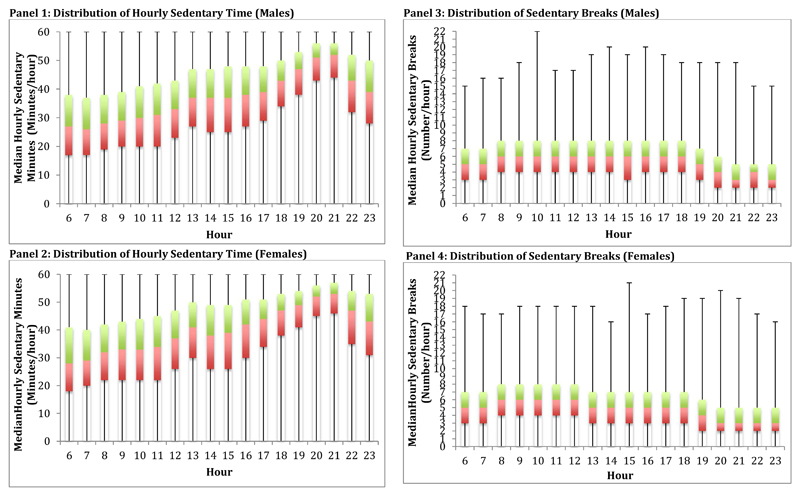
Older adults spent at least 30 minutes of each hour sedentary, with this gradually increasing across the day (Figure 1). There were two peaks in the day; a small peak around 13:00 and a much bigger peak around 20:00 to 22:00 when average sedentary time was almost 50 minutes of each hour. Conversely,, individuals spent up to 30 minutes per hour in sedentary breaks with this falling to just over 10 minutes per hour by 20:00, i.e. showing decreasing time spent in at least light intensity activity as the day progressed. On average, older adults took 5-7 breaks in sedentary activity per hour, until around 18:00 when there was a noticeable decline to a low of less than 4 breaks per hour from 20:00 until 23:00. The bottom quartile took 2 or less breaks per hour in these evening hours.
Figure 1.
Boxplots of Distributions of Hourly Sedentary Time and Breaks in Sedentary Time. Red segments are 25th to 50th centiles. Green segments are 50th to 75th centiles. Tails are minimum and maximum values.
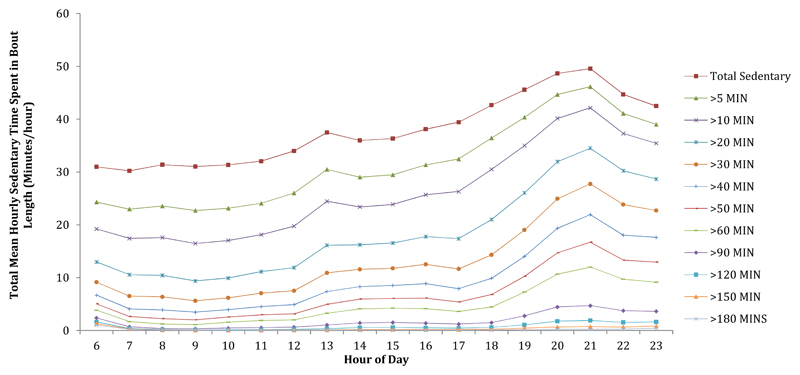
65.8% of total sedentary time was accumulated through short bouts of <30 minutes and 31.9% of total sedentary time was accumulated through bouts of <10 minutes duration. In line with results for hourly break patterns, sedentary time was accrued in varying bout durations at different times of the day. Longer bouts of sedentary time occurred more exclusively in the evening, whereas shorter bouts occurred throughout the day (Figure 2).
Figure 2. Accrual of Hourly Sedentary Time through Different Lengths of Bouts.
All models were adjusted for age and sex.
Associations with Participant Characteristics
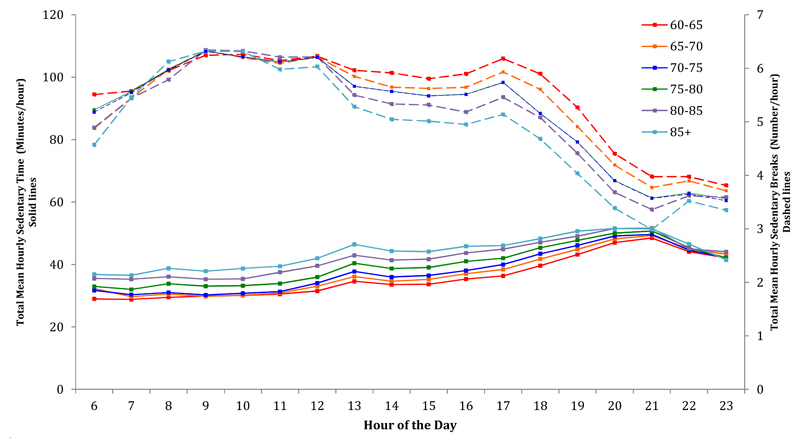
With increasing age we observed greater time spent sedentary, with this difference being greatest in the earlier hours of the day for the strata aged over 75 (Figure 3). The trend appeared to decrease gradually until the peak of sedentarinesss around 21:00, when all age groups spent similar time sedentary. Older participants broke up their sedentary time less frequently (p<0.001); this was particularly the case after midday until the end of the day. There was very little difference in break patterns between age groups in the morning. Men spent more hourly time sedentary and took more hourly breaks than women throughout the day (p<0.001) (Figure 3).
Figure 3.
Mean Total Hourly Sedentary Time & Mean Total Hourly Breaks after Stratification for Age Group. All models were adjusted for sex. P for trend<0.001 for association of age with hourly sedentary time & p value for trend<0.001 for associations of age with hourly breaks in sedentary time. Trends in hourly sedentary time are in dotted lines and trends in sedentary breaks are in solid lines.
Retired participants accrued more sedentary time across the day than those not retired (p<0.001) and had fewer breaks across every hour than those who were not retired (p<0.001). This was particularly the case across the later hours of the day (13:00-20:00) (Supplementary Figure 1).
Those who had had further education accrued marginally more sedentary time in the morning (06:00-12:00) than those without, with a crossover point at midday after which time those with further education appeared to have marginally less sedentary time per hour until 21:00 when the difference disappeared again (Supplementary figure 1). Individuals who had had further education had fewer breaks in the morning (06:00-10:00), then more breaks in the evening after 17:00 than those without further education.
From social class I to IV, there was a trend of increasing time spent sedentary in the morning, with all social classes converging to similar durations of sedentary time after lunch. However, after midday, those in social class V spent the most time sedentary (Supplementary Figure 2).
Individuals who never smoked accrued less sedentary time than current smokers between 08:00-10:00 and 14:00-18:00. Current smokers accrued marginally more sedentary time in particular in both the early hours (06:00-08:00) and late hours (21:00-23:00) (Supplementary figure 1). Current smokers had more breaks than former or never smokers, especially in the middle part of the day (12:00-16:00) (Supplementary Figure 1).
Finally, those with higher BMI spent more time sedentary per hour than their counterparts with lower BMI (p<0.001). These differences were greatest between 07:00 to 12:00, and 14:00 to 18:00, with little difference by the evening peak (Supplementary Figure 2). Participants with a higher BMI were less likely to break up their sedentary time, with the difference being greater after midday.
Sensitivity analysis utilising records with at least five valid days of wear time revealed no significant differences in the results (excluding n=141).
Weekend versus Weekday
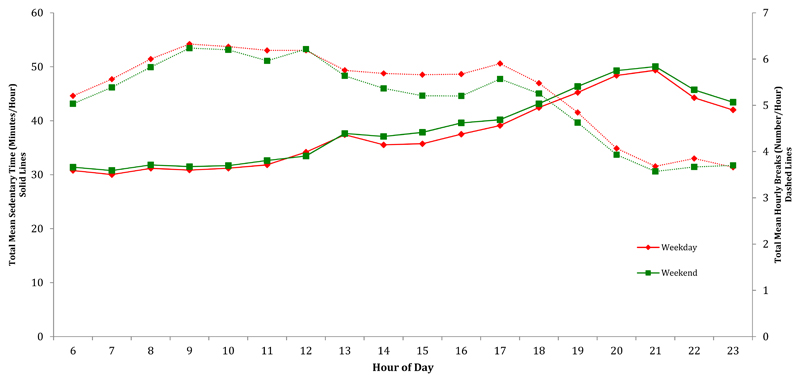
Participants were more sedentary on weekend days compared to weekdays (p<0.0001) (Figure 4). The difference was greatest in the afternoon hours between 14:00 and 18:00. On weekdays, sedentary time was broken up more frequently than on weekend days, particularly between 13:00 and 17:00.
Figure 4. Mean Total Hourly Sedentary Time & Mean Total Hourly Breaks after Stratification for Weekend Versus Weekday.
All models were adjusted for age and sex. P<0.001 for difference between hourly sedentary time for weekday compared to weekend day & p<0.001 for difference between hourly breaks in sedentary time for weekday compared to weekend day. Trends in breaks are in dashed lines, trends in sedentary time are in solid lines (Sample sizes: weekday=250698 person- hours, weekend day=97193 person- hours).
Discussion
Key Findings
This is the first study to comprehensively examine sedentary time at the hourly level with simultaneous examination of the descriptive epidemiology of bouts and breaks in sedentary time in a large, population-based cohort of UK older adults. We have described patterns of sedentary time not previously reported in the literature. More sedentary time and fewer breaks occur in the evenings compared to earlier in the day. The majority of this sedentary time was accrued in reasonably short bouts. Further, marginally more sedentary time was accrued on weekend days compared to weekdays.
We also report associations with several descriptive characteristics, as well as replicating trends previously seen for age and sex. Having a higher BMI and being retired were associated with greater sedentary time and fewer breaks in sedentary time. Further, we found that sedentary time was socio-economically patterned. Those in higher social classes and with further education were more likely to accrue more sedentary time in the morning. Those with further education had fewer breaks in sedentary time in the morning. Altogether, this work meaningfully adds to the picture of the descriptive epidemiology of sedentary time, bouts and breaks, and gives new insights into the patterns of sedentary time and breaks throughout the day.
Findings in the Context of the Literature
A considerable amount of sedentary time was accumulated through short bouts of <30 minutes (65.8% of total sedentary time) and <10 minutes (31.9% of total sedentary time). This is in agreement with Jefferis et al. (42) and Shiroma et al. (43) who both reported that large proportions of sedentary time are accumulated in bouts of <30 minutes. Only 11.1% of total sedentary time was accumulated through bouts of >60 minutes. Longer bouts of sedentary time occurred predominantly in the evening, whereas shorter bouts occurred throughout the day. Longer bouts occurring in the evening may present an easier target for a planned intervention than shorter bouts occurring at other times of the day.
Older adults on average spent at least 30 minutes of each waking hour in sedentary activity, with this gradually increasing across the day peaking around 21:00 when sedentary time represented almost 50 minutes of the hour. Two other studies have reported on patterns across the day measured by accelerometry. Martin et al. (44) found similar results but suggested that the peak of hourly sedentary time was lower at 40 minutes in a population of 60-69 year olds living in the USA. The predominant prevalence of sedentary time in evenings may be because older adults have a diurnal pattern of activity that peaks in volume in the late morning related to activities that take the individual out of the house (e.g. shopping) with the evening being a time for rest (e.g while eating dinner, watching television).
Older participants accumulated more sedentary time in prolonged sedentary bouts than younger ones, particularly in the morning. Davis et al. (45) and Belletiere et al.(46) looked at patterns of hourly sedentary time across age groups, and had similar findings. We also found that older individuals took fewer hourly breaks; reinforcing findings from two earlier studies (42,43). However, this difference was predominantly seen in the afternoon and evening.
Men accumulated more sedentary time in prolonged bouts than women as Belletiere et al.(46) have also reported, and we are the first to report that older men had fewer hourly breaks in sedentary time than older women. Though Jefferis et al. (42) report a mean of 7.2 breaks per hour for men, and Shiroma et al.(43) report a mean of 9.0 breaks per hour in a study of women, there are no other studies in the literature which examine the diurnal pattern of breaks. The overall pattern of hourly breaks in sedentary time shows that there is a noticeable decline in breaks from early evening, with the fewest breaks occurring at 20:00 (5-7 breaks per hour during the day and 3-4 during the evening). This highlights that an intervention relating to increasing breaks might target the evening hours.
In a recent review of existing experimental studies examining the relationship between frequency of interruption of sedentary time and markers of adiposity and cardiometabolic health (40), the studies that were selected almost exclusively compared an intervention with 2 or 3 breaks per hour versus no breaks. Our results, and other observational studies (39,47) suggest the ‘average’ older adults achieve this but about 25% of the population do not, particularly during evening hours This is important because a dose-response relationship between breaks and health outcomes now needs to be elucidated before we know how to approach designing interventions targeting breaking up sedentary time.
In addition to number of breaks in sedentary time, it is likely that duration, posture, and intensity of breaks are important aspects of what makes them beneficial (48). A break in sedentary time might constitute simply standing or other light activity, or higher intensity activity. It has been suggested that certain types of breaks (for example, standing still) are inadequate to represent a metabolic benefit (48–51), that instead a break with at least light activity might be more beneficial (48). Though we did not examine these specific break characteristics in this work, due in part to the limitation of the employed accelerometer to not be able to distinguish postures, understanding what types of breaks are most beneficial will be useful in intervention design.
Our analysis is the first to suggest distinct associations between sedentary time and several socioeconomic factors with patterns of sedentary time and breaks in sedentary time across the day. These findings contribute to our understanding of the determinants of sedentary time and highlight potential targets for interventions such as the evening time or the ‘older’ elderly strata, the retired and those with higher BMI.
Mean hourly sedentary time on weekend days was on average longer than weekdays. Participants took fewer hourly breaks on weekdays compared to weekend days. However, the differences found here for both sedentary time and breaks were small and may not be clinically significant.
Interpretation of Study Findings
The accelerometry method used in our study is a widely implemented proxy method for measuring sedentary time (35–37). There are, however, a number of decisions in the accelerometry data collection and processing protocol which could lead to information biases. We set our analysis epoch length at 60 seconds, the commonest length used in the literature. Changes in epoch length can influence accelerometer-determined sedentary time but the most appropriate length has yet to be established (30,31). We used a cut-point of <100 cpm, however other cut-points have been suggested (37) but given that the overwhelming majority of existing studies use the <100 cpm threshold and 60 second epoch, using these settings facilitates comparisons of our data with the majority of existing literature.
Another aspect of the analysis protocol relates to the identification of non-wear. We used a time threshold of ≥90 minutes of consecutive zero counts to define non-wear, which means that shorter true episodes of non-wear would be wrongly included as sedentary time. However, the opposite is also true as too low a threshold would significantly underestimate sedentary time and evidence supports the 90-minute threshold as this yields a realistic number of 3-4 non-wear bouts per day (8,52,53). In order to account for confounding by wear-time, we only included hours with wear-time for at least 30 minutes of the hour and also weighted for wear-time. The sample sizes were large for each hour from 09:00 to 22:00 (n>21000 hours) and there were smaller sample sizes as would be expected around morning rising and going to bed (06:00 n=1635 hours, 07:00 n=5812 hours, 08:00 n=13653, 22:00 n=14692, 23:00 n=4705).
The use of three-day wear-time threshold was appropriate, given our sensitivity analysis utilising records with at least five days of wear time greater than 600 minutes/day showed no significant difference in results.
Given that age and sex have repeatedly been found to be associated with sedentary time, these factors were potential confounders and were included in regression models. Adjusted and unadjusted models were compared, and only age, sex and wear-time remained in the models as covariates. However, we cannot exclude the possibility of residual confounding.
The EPIC-Norfolk cohort has several strengths. It is a well-characterised, large population-based cohort that is similar to a nationally representative sample for anthropometric indices, blood pressure and serum lipids, albeit with a lower prevalence of cigarette smoking (54). The sample is less representative of the frailer elderly and the oldest elderly and in common with all cohorts is susceptible to healthy volunteer bias and attrition. This study and analyses were designed to minimise possible sources of bias and confounding, although there are several unavoidable sources which are recognised here.
Conclusions
High sedentary time in older adults is a large and neglected public health problem. Total sedentary time and prolonged bouts are highly prevalent and particularly common in this age group hence significant potential health gain could arise from targeting this emerging risk factor. Although some national recommendations concerning sedentary time among older adults exist (55–58), they are brief and vague, as little is known about this topic. As the health risks associated with spending too much time sedentary (5–7,59) and not breaking this time up are being recognised (22–26,60,61), the need to map the determinants of sedentary time and identify population subgroups who are most likely to benefit from interventions remains an important area for future research. We have highlighted the burden of sedentary time and described how this time is accrued through bouts and breaks in sedentary time. This, coupled with emerging evidence that excess sedentary time is associated with adverse health outcomes and sedentary breaks are inversely associated with adverse health outcomes, underlines the need for prospective studies of the determinants of sedentary time and what future interventions might look like.
Supplementary Material
Key Messages.
Older adults spent significant amounts of time sedentary throughout the day, but particularly in the evenings. They also took fewer breaks in sedentary time in the evening. Marginally more sedentary time was accrued on weekend days compared to week days.
Older adults, males, the retired, and those with a higher BMI spent more time sedentary and had fewer breaks
These findings should inform the development and targeting of strategies to reduce sedentary time among older adults
Acknowledgments
We are grateful to the participants for giving up their time to take part in this study. We thank EPIC-Norfolk study coordination, data collection, and data management teams for their assistance with the study, and we acknowledge Tom White for assistance in processing the accelerometer data. AJC, KWe, KWi, NW, SJG and SB were funded by Medical Research Council (MC_UU_12015/1, MC_UU_12015/3 and MC_UU_12015/4), and KWi was funded by the British Heart Foundation (FS/12/58/29709). EPIC Norfolk is supported by programme grants from the Medical Research Council UK G1000143 and MR/N003284/1 and Cancer Research UK 14136
References
- 1.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Ann Intern Med. 2. Vol. 162. American College of Physicians; 2015. Jan 20, Sedentary Time and Its Association With Risk for Disease Incidence, Mortality, and Hospitalization in Adults; p. 123. [DOI] [PubMed] [Google Scholar]
- 2.Koster A, Caserotti P, Patel KV, Matthews CE, Berrigan D, Van Domelen DR, et al. Association of Sedentary Time with Mortality Independent of Moderate to Vigorous Physical Activity. In: Ruiz JR, editor. PLoS One. 6. Vol. 7. Public Library of Science; 2012. Jun 13, p. e37696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocklebank LA, Falconer CL, Page AS, Perry R, Cooper AR. Accelerometer-measured sedentary time and cardiometabolic biomarkers: A systematic review. Prev Med (Baltim) 2015 Jul;76:92–102. doi: 10.1016/j.ypmed.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Schmid D, Ricci C, Leitzmann MF. PLoS One. 3. Vol. 10. Public Library of Science; 2015. Associations of objectively assessed physical activity and sedentary time with all-cause mortality in US adults: the NHANES study; p. e0119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010 Dec;35(6):725–40. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 6.Stamatakis E, Davis M, Stathi A, Hamer M. Prev Med (Baltim) 1. Vol. 54. Elsevier Inc; 2012. Jan, Associations between multiple indicators of objectively-measured and self-reported sedentary behaviour and cardiometabolic risk in older adults; pp. 82–7. [DOI] [PubMed] [Google Scholar]
- 7.Wijndaele K, Brage S, Besson H, Khaw K-T, Sharp SJ, Luben R, et al. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk study. Int J Epidemiol. 2011 Feb;40(1):150–9. doi: 10.1093/ije/dyq105. [DOI] [PubMed] [Google Scholar]
- 8.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008 Apr 1;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkemeyer K, Wijndaele K, White T, Cooper AJM, Luben R, Westgate K, et al. The descriptive epidemiology of accelerometer-measured physical activity in older adults. Int J Behav Nutr Phys Act. 2016;13(1) doi: 10.1186/s12966-015-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakicic JM, Gregg E, Knowler W, Kelley DE, Lang W, Miller GD, et al. Activity patterns of obese adults with type 2 diabetes in the look AHEAD study. Med Sci Sports Exerc. 2010 Nov;42(11):1995–2005. doi: 10.1249/MSS.0b013e3181e054f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007 Feb;30(2):203–9. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 12.Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J, et al. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005 Jan;28(1):3–9. doi: 10.2337/diacare.28.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Jennings G, Nelson L, Nestel P, Esler M, Korner P, Burton D, et al. The effects of changes in physical activity on major cardiovascular risk factors, hemodynamics, sympathetic function, and glucose utilization in man: a controlled study of four levels of activity. Circulation. 1986 Jan;73(1):30–40. doi: 10.1161/01.cir.73.1.30. [DOI] [PubMed] [Google Scholar]
- 14.Lipman RL, Raskin P, Love T, Triebwasser J, Lecocq FR, Schnure JJ. Glucose intolerance during decreased physical activity in man. Diabetes. 1972 Feb;21(2):101–7. doi: 10.2337/diab.21.2.101. [DOI] [PubMed] [Google Scholar]
- 15.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003 Sep 1;551(Pt 2):673–82. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am J Physiol. 1998 Dec;275(6 Pt 1):E1016–22. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- 17.Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J Appl Physiol. 2006 Jan;100(1):249–57. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol. 2011 Oct;111(4):1201–10. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 19.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012 May;35(5):976–83. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M, Hamilton D, Zderic T. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(November):2655–67. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 21.Saunders TJ, Larouche R, Colley RC, Tremblay MS. Acute sedentary behaviour and markers of cardiometabolic risk: a systematic review of intervention studies. J Nutr Metab. 2012 Jan;2012 doi: 10.1155/2012/712435. 712435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healy G, Dunstan DW, Salmon J, Cerin E, Shaw J, Zimmet P, et al. Beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 23.Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardiometabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011 Mar 1;32(5):590–7. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson V, Wong SL, Winkler E, Healy GN, Colley RC, Tremblay MS. Prev Med (Baltim) Vol. 65. Elsevier Inc.; 2014. Patterns of sedentary time and cardiometabolic risk among Canadian adults; pp. 23–7. [DOI] [PubMed] [Google Scholar]
- 25.Cooper AR, Sebire S, Montgomery AA, Peters TJ, Sharp DJ, Jackson N, et al. Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia. 2012 Mar 14;55(3):589–99. doi: 10.1007/s00125-011-2408-x. [DOI] [PubMed] [Google Scholar]
- 26.Henson J, Yates T, Biddle SJH, Edwardson CL, Khunti K, Wilmot EG, et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013 May;56(5):1012–20. doi: 10.1007/s00125-013-2845-9. [DOI] [PubMed] [Google Scholar]
- 27.Hayat SA, Luben R, Keevil VL, Moore S, Dalzell N, Bhaniani A, et al. Int J Epidemiol. 4. Vol. 43. Oxford University Press; 2014. Cohort profile: A prospective cohort study of objective physical and cognitive capability and visual health in an ageing population of men and women in Norfolk (EPIC-Norfolk 3) pp. 1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MRC Epidemiology. EPIC-Norfolk: health check and laboratory protocol. 2015 Available from: http://www.srl.cam.ac.uk/epic/about/protocols.shtml.
- 29.World Health Organisation. WHO | Ageing and life-course. World Health Organization; 2013. Ageing and lifecourse. Available from: http://www.who.int/ageing/en/ [Google Scholar]
- 30.Ojiambo R, Cuthill R, Budd H, Konstabel K, Casajús JA, González-Agüero A, et al. Impact of methodological decisions on accelerometer outcome variables in young children. Int J Obes (Lond) 2011;35(Suppl 1):S98–103. doi: 10.1038/ijo.2011.40. [DOI] [PubMed] [Google Scholar]
- 31.Edwardson CL, Gorely T. Epoch length and its effect on physical activity intensity. Med Sci Sports Exerc. 2010;42(5):928–34. doi: 10.1249/MSS.0b013e3181c301f5. [DOI] [PubMed] [Google Scholar]
- 32.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012 Feb;95(2):437–45. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridgers ND, Salmon J, Ridley K, O’Connell E, Arundell L, Timperio A. Agreement between activPAL and ActiGraph for assessing children’s sedentary time. International Journal of Behavioral Nutrition and Physical Activity. 2012:15. doi: 10.1186/1479-5868-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilar-Farías N, Brown WJ, Peeters GMEEG. ActiGraph GT3X+ cut-points for identifying sedentary behaviour in older adults in free-living environments. J Sci Med Sport. 2014 May;17(3):293–9. doi: 10.1016/j.jsams.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17(1):17–30. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- 36.Lee I, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. 2014 Feb;48(3):197–201. doi: 10.1136/bjsports-2013-093154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011 Aug;43(8):1561–7. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 38.Lyden K, Kozey Keadle SL, Staudenmayer JW, Freedson PS. Validity of Two Wearable Monitors to Estimate Breaks from Sedentary Time. Med Sci Sport Exerc. 2012 Nov;44(11):2243–52. doi: 10.1249/MSS.0b013e318260c477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Breaks in Sedentary Time: Beneficial associations with metabolic risk. Diabetes Care. 2008 Apr 1;31(4):661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 40.Chastin SFM, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity. 2015 Sep;23(9):1800–10. doi: 10.1002/oby.21180. [DOI] [PubMed] [Google Scholar]
- 41.Office for National Statistics. Deaths by local authority of usual residence, numbers and standardised mortality ratios (SMRs) by sex, 2008 registrations: Population Trends. 2008 Available from: http://www.statistics.gov.uk/StatBase/ssdataset.asp?vlnk=9876&Pos=1&ColRank=1&Rank=272.
- 42.Jefferis BJ, Sartini C, Shiroma E, Whincup PH, Wannamethee SG, Lee I-M. Duration and breaks in sedentary behaviour: accelerometer data from 1566 community-dwelling older men (British Regional Heart Study) Br J Sports Med. 2014 Sep 17; doi: 10.1136/bjsports-2014-093514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiroma EJ, Freedson PS, Trost SG, Lee I. Patterns of accelerometer-assessed sedentary behavior in older women. Jama. 2013;310(23):2562–3. doi: 10.1001/jama.2013.278896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin KR, Koster A, Murphy RA, Van Domelen DR, Hung M, Brychta RJ, et al. Changes in Daily Activity Patterns with Age in U.S. Men and Women: National Health and Nutrition Examination Survey 2003-04 and 2005-06. J Am Geriatr Soc. 2014 Jul;62(7):1263–71. doi: 10.1111/jgs.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis MG, Fox KR, Hillsdon M, Sharp DJ, Coulson JC, Thompson JL. Objectively measured physical activity in a diverse sample of older urban UK adults. Med Sci Sports Exerc. 2011 Apr;43(4):647–54. doi: 10.1249/MSS.0b013e3181f36196. [DOI] [PubMed] [Google Scholar]
- 46.Bellettiere J, Carlson JA, Rosenberg D, Singhania A, Natarajan L, Berardi V, et al. PLoS One. 8. Vol. 10. Public Library of Science; 2015. Gender and Age Differences in Hourly and Daily Patterns of Sedentary Time in Older Adults Living in Retirement Communities; p. e0136161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith L, Hamer M, Ucci M, Marmot A, Gardner B, Sawyer A, et al. Weekday and weekend patterns of objectively measured sitting, standing, and stepping in a sample of office-based workers: the active buildings study. BMC Public Health. 2015 Jan 17;15(1):9. doi: 10.1186/s12889-014-1338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chastin SFM, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity. 2015;23(9):1800–10. doi: 10.1002/oby.21180. [DOI] [PubMed] [Google Scholar]
- 49.Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport. 2015 May;18(3):294–8. doi: 10.1016/j.jsams.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Pulsford RM, Blackwell J, Hillsdon M, Kos K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: A randomised cross-over study in inactive middle-aged men. J Sci Med Sport. 2016 Aug; doi: 10.1016/j.jsams.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Lord S, Chastin SFM, McInnes L, Little L, Briggs P, Rochester L. Exploring patterns of daily physical and sedentary behaviour in community-dwelling older adults. Age Ageing. 2011 Mar;40(2):205–10. doi: 10.1093/ageing/afq166. [DOI] [PubMed] [Google Scholar]
- 52.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mailey EL, Gothe NP, Wójcicki TR, Szabo AN, Olson EA, Mullen SP, et al. J Aging Phys Act. 2. Vol. 22. Human Kinetics Publishers Inc.; 2014. Influence of allowable interruption period on estimates of accelerometer wear time and sedentary time in older adults; pp. 255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999 Jul;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 55.National Ageing Research Institute. National Ageing Recommendations for older Australians. National Ageing Research Institute; 2006. [Google Scholar]
- 56.Chief Medical Officers Of England Scotland Wales ANI. Start Active, Stay Active. A report on physical activity for health from the four home countries’ Chief Medical Officers. Crown; 2011. Strategy. [Google Scholar]
- 57.The Australian Government. Australia’s Physical Activity and Sedentary Behaviour Guidelines. The Department of Health website. Australian Government Department of Health; 2014. Available from: http://www.health.gov.au/internet/main/publishing.nsf/content/health-pubhlth-strateg-phys-act-guidelines. [Google Scholar]
- 58.Jefferis BJ, Sartini C, Lee I-M, Choi M, Amuzu A, Gutierrez C, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health. 2014 Jan;14(1):382. doi: 10.1186/1471-2458-14-382. BMC Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Rezende LFM, Rey-López JP, Matsudo VKR, do Carmo Luiz O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. 2014 Jan;14:333. doi: 10.1186/1471-2458-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011 Feb;34(2):497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sardinha LB, Santos DA, Silva AM, Baptista F, Owen N. Breaking-up Sedentary Time Is Associated With Physical Function in Older Adults. Journals Gerontol Ser A Biol Sci Med Sci. 2015 Jan 1;70(1):119–24. doi: 10.1093/gerona/glu193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.