Abstract
Oligodendroglioma is the quintessential molecularly-defined brain tumor. The characteristic whole-arm loss of the long arm of chromosome 1 and the short arm of chromosome 19 (1p/19q-codeletion) within the genome of these tumors facilitated the reproducible molecular identification of this subcategory of gliomas. More recently, recurrent molecular genetic alterations have been identified to occur concurrently with 1p/19q-codeletion, and definitively identify these tumors, including mutations in IDH1/2, CIC, FUBP1, and the TERT promoter, as well as the absence of ATRX and TP53 alterations. These findings provide a foundation for the consistent diagnosis of this tumor type, upon which a generation of clinical investigators have assembled a strong evidence base for the effective treatment of this disease with radiation and chemotherapy.
KEYWORDS : 1p/19q loss, CIC mutation, FUBP1 mutation, IDH mutation, oligodendroglioma, TERT mutation
Oligodendrogliomas are the second most common intraparenchymal brain tumor in adults [1]. Classified under WHO neuropathologic criteria, oligodendrogliomas can be either low-grade (grade II) or anaplastic (grade III) [2]. Long-recognized as a distinct histological entity within the family of diffuse gliomas [3,4], oligodendrogliomas were established as the prototypic ‘molecularly-defined’ brain tumor by a series of discoveries at the end of the 20th century. These clinical-translational studies linked the recognition of a marked chemotherapeutic responsiveness and long survival in histologically typical anaplastic oligodendrogliomas [5–7] with the characteristic genomic alteration of whole-arm chromosomal loss of 1p and 19q in these gliomas [8,9].
With the advent of large-scale genome sequencing technology, molecular genetic alterations in IDH1/2, CIC, FUBP1 and the TERT promoter have now been identified in the majority of oligodendrogliomas. These alterations have afforded investigators with an increasing number of overlapping and mutually reinforcing criteria to diagnose these lesions. Similarly, genetic mutations identified as characteristic of other subgroups of diffuse gliomas, such as ATRX and TP53 mutations in astrocytomas, have allowed for molecular tests that can nearly exclude the diagnosis of oligodendroglioma. In aggregate, these discoveries have led to a greater appreciation of the limits of both histologic sampling [10,11] and molecular diagnostic testing [12–14]. Indeed, it seems likely that the diagnosis of oligodendroglioma will become one in which histopathology and molecular diagnosis are combined.
Herein, we review these molecular discoveries, and the resulting lessons for the diagnosis of oligodendroglioma. We underscore the emerging ‘combined consensus’ definition of the entity of oligodendroglioma, incorporating the presence (and/or absence) of specific molecular genetic alterations alongside demographic data, clinical information and histopathologic review. Not discussed in this review are possible MRI correlates of oligodendroglioma histopathology and genetics, but several groups have pointed to apparent/associations between the location of oligodendrogliomas, their appearance on MRI and the presence (absence) of 1p/19q co-deletion and mutation of IDH1/2.
Oligodendroglioma histology & 1p/19q status
The therapeutic implications of the tight linkage between histopathologic appearance and molecular genetics in oligodendroglioma have been firmly established in a number of studies since the initial report [9]. It is worthwhile to review this existing evidence base as established by several completed and ongoing Phase III studies.
For anaplastic oligodendroglial tumors (WHO grade III), a histopathology of ‘pure’ anaplastic oligodendroglioma (AO) – versus the ‘mixed’ histology anaplastic oligoastrocytoma (AOA) – has proven to be an important distinction. In the EORTC 26951 study, a ‘pure’ AO histology identified tumors with a better prognosis, and predicted improved outcome after receipt of the chemotherapeutic regimen of procarbazine, CCNU (lomustine) and vincristine (PCV) when administered as adjuvant treatment in addition to a standard course of radiotherapy (RT). For patients with AO [15], overall survival (OS) associated with receipt of RT and PCV was significantly prolonged (3.5 vs 2.6 years compared with RT alone, a hazard ratio [HR]: 0.75; 95% CI: 0.60–0.95). However, there are limits to the predictive capacity of histology, since in the RTOG 9402 study of AO and mixed AOA [16], the median survival (though longer overall when compared to the EORTC cohort) was not different between patients receiving PCV plus RT (4.6 years) versus RT alone (4.7 years); HR: 0.79; 95% CI: 0.60–1.04.
Indeed, the molecular diagnosis of combined 1p/19q loss has proven to be a critical piece of this puzzle, identifying a cohort of tumors in both studies that responded to the addition of PCV to RT. The RTOG 9402 study observed an associated extension of median survival to 14.7 years (from 7.3 years) in this subgroup with combined 1p/19q loss; HR: 0.59; 95% CI: 0.37–0.95; p = 0.03. A similar finding was reported in the EORTC study, in which the median OS was not yet reached in the 1p/19q-deleted RT/PCV group, versus 9.3 years in the RT group; HR: 0.56; 95% CI: 0.31–1.03. An early report from the ongoing EORTC trial 22033–26033 of RT versus temozolomide (TMZ) chemotherapy in low-grade gliomas (grade II) has noted a similar prognostic association of chromosome 1p deletion with prolonged progressive-free survival (p = 0.0003; HR: 0.59; 95% CI: 0.45–0.78) and OS (p = 0.002; HR: 0.49; 95% CI: 0.32–0.77).
Thus, the glioma identified as ‘oligodendroglioma’ by histopathologic information alone is better-identified as a clinically actionable entity by the additional molecular scoring of 1p/19q status. Conversely, 1p/19q testing alone is not sufficient, as 1p/19q loss is not prognostic in non-oligodendroglial lesions [17]. With the explosion of molecular genetic discoveries within these tumors (detailed below), recent authors have attempted to explicitly render this distinction between the underlying neoplastic entity and the histopathologic diagnosis, using such consensus designations as ‘molecular oligodendroglioma’ [18], or ‘ICF glioma’ [19]. Oligodendrogliomas thus have served as the prototypic example of a molecularly refined clinicopathologic entity, underscoring the need for multiple lines of evidence to identify the clinically relevant neoplasm.
Genetic mutations as subclassifiers
In the last decade, molecular genetic sequencing methods have undergone a rapid technological advancement. As a result, several recurrent mutations have been identified in diffuse gliomas, allowing for a molecular re-evaluation of histologic subclassification systems.
• IDH
Application of genome-scale sequencing techniques to malignant gliomas identified mutations in the isocitrate dehydrogenase gene IDH1 in a subset of glioblastomas [20]. On closer inspection, these IDH1-mutant tumors proved to be glioblastomas arising in younger patients from previously diagnosed lower-grade gliomas [20–23]. This discovery had immediate implications for the understanding of different WHO grades as discrete lesions, as low-grade gliomas retain the IDH1 gene mutation and continue to have an improved prognosis, despite histologic grade progression. Thus, IDH1 mutation occurs early within the evolutionary development of a distinct molecular etiologic class of glioma [24–26].
Approximately 90% of these alterations occur within a ‘hotspot’ of arginine to histidine heterozygous mutations in codon 132 of the IDH1 gene (R132H), with the remainder as noncanonical substitutions in IDH1 (e.g., R132C or R132S) or in the homologous gene IDH2 (R172K). All tumor-associated IDH mutants acquire a neomorphic enzymatic activity resulting in the overproduction and near-100-fold accumulation of the R enantiomer of 2-hydroxyglutarate (also known as ‘D’ enantiomer or D-2-HG) [27]. The high concentration of 2-HG competitively inhibits a large family of 2-oxoglutarate (also called α-ketoglutarate)-dependent dioxygenases, and is thought to promote tumorigenesis by impacting chromatin structure and the epigenetic control of gene expression associated with the glioma CpG island methylator phenotype [28–30]. Pharmacologic inhibition of this mutant enzymatic activity may offer a targeted therapeutic strategy for these gliomas [31]; this is an area of active investigation.
Currently, however, IDH mutation status has already proven useful as a diagnostic tool for gliomas. A true clinical-translational breakthrough came with the introduction of a mutation-specific antibody directed against the most common IDH1 mutation (R132H, seen in >90% of mutant cases) by Dr von Deimling's laboratory group, allowing for rapid large-scale immunohistochemical screening of formalin-fixed specimens and widespread diagnostic assessment of IDH R132H status as part of routine neuropathologic practice [15,32–36]. The antibody is now is widespread use clinically, and can now be used to distinguish reactive gliosis from a diffusely infiltrating, low cellularity, grade II glioma and to distinguish diffuse grade II gliomas from glioneuronal tumors like gangliogliomas, dysembryoblastic neuroepithelial tumor (DNET) or clear-cell ependymoma [37,38]. It is of course important to reiterate that this antibody does not detect noncanonical IDH1 mutations such as R132C, R132S, R132G, R132L or IDH2 mutations.
Despite their distinct histologic differences, both lower-grade astrocytomas and oligodendrogliomas have high rates of IDH gene mutations, exceeding 80% in most series. For oligodendrogliomas, the linkage between IDH gene mutation and histology is particularly tight; the few oligodendrogliomas that do not harbor IDH1 mutations contain IDH2 mutations [24]. Careful genomic rescoring of tissue bank specimens demonstrates that virtually 100% of 1p/19q whole-arm-lost oligodendrogliomas harbor IDH gene mutations [39]. Thus, for class definition, it may be reasonable to require the presence of IDH gene mutations to identify oligodendroglioma, and their absence may indicate alternative histologic ‘mimics’ (such as DNET or clear-cell ependymoma [37]). Conversely, it has been recognized for some time that TP53 mutations are commonly found in grade II and III astrocytic gliomas, but are rarely found in oligodendroglial tumors [40]. Such molecular analyses call into question the histopathologic identification of a ‘mixed’ oligoastrocytoma as a discrete entity, as opposed to cases with histologic manifestations drawn from an overlapping spectrum of two otherwise distinct molecular groups: 1p/19q-codeleted oligodendroglioma and TP53-mutant astrocytoma. This inverse relationship proves to be even sharper within the IDH-mutant subgroup of gliomas [41].
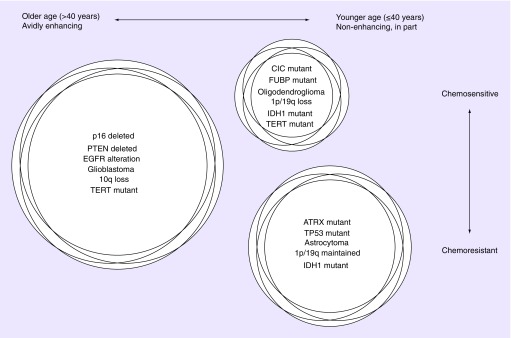
These clear-cut divisions allowed for the emerging outlines of a class-defining description of three ‘molecular groups’ of diffuse gliomas corresponding in large part to major histopathologic entities: IDH wild-type gliomas (characterizing the vast majority of primary glioblastomas), IDH-mutant, TP53-mutant astrocytic gliomas (consisting of lower- and intermediate-grade astrocytomas, and progressive/secondary glioblastomas) and IDH-mutant 1p/19q-codeleted oligodendroglial gliomas (consisting of mostly ‘pure’ oligodendrogliomas) [19] (Figure 1).
Figure 1. . Concurrent molecular genetic alterations identify glioma subgroups.
Three groups of adult diffuse gliomas are identified, each characterized by distinct and largely non-overlapping molecular genetic features. The most common group is typically found in older patients, with TERT promoter mutation in the absence of IDH gene alterations, and characteristic “glioblastoma” histology. The second most-common is found in younger adults, with concurrent IDH, TP53 and ATRX alterations and astrocytic histology. The least common, oligodendroglial tumors, are found in patients between the two extremes of age, with IDH and TERT promoter mutations, along with 1p/19q-codeletion. Oligodendroglioma and glioblastoma, to a lesser degree, have been proven to derive clinical benefit from treatment with chemotherapy. Astrocytic tumors do not have a similar evidence base.
• CIC/FUBP1
The acceleration of genomic sequencing technology then rapidly facilitated further ‘class-defining’ mutation identification. Focused analysis of 1p/19q-codeleted oligodendrogliomas resulted in the identification of inactivating mutations in the CIC gene on chromosome 19 and the FUBP1 gene on chromosome 1p [42–44].
CIC, which is most frequently found to be mutated in these tumors, is thought to function downstream of growth factor receptor signaling pathways by binding to DNA regulatory elements as a reversible repressor of target genes [45]. Notably, missense mutations in CIC in oligodendrogliomas cluster within the putative DNA binding domain. This tumor-suppressor spectrum of CIC mutational inactivation suggests that oligodendrogliomas may have a basal level of ‘released’ activation of these pathways, which might explain why growth factor receptor amplification events are rarely observed in this tumor type, as they might not provide additional selective growth advantage for the tumor cells.
FUBP1 is a little-studied DNA-binding protein, with unknown function in gliomagenesis. The relationship between CIC/FUBP1 inactivation and chemosensitivity, if any, is yet unknown and awaits further study.
• ATRX/TERT
Additional studies have identified mutations in the ATRX gene on the X chromosome in IDH-mutant astrocytic gliomas [19,46–48]. ATRX is a large protein that complexes with histones and heterochromatin at centromeres and telomeres, whose inactivation is associated with the alternative lengthening of telomeres (ALT) phenotype. As mentioned above, IDH-mutant astrocytic gliomas are typically TP53 mutant, and ATRX inactivation therefore serves as an additional identifying genetic alteration to positively categorize these lesions, and exclude a diagnosis of oligodendroglioma. Since immunohistochemical staining of ATRX can be used to identify inactivation of this protein [49], retained nuclear staining of ATRX in a tumor positive for IDH R132H immunohistochemical staining and the histologic appearance of oligodendroglioma offers a confirmatory molecular diagnostic approach to identify 1p/19q-codeleted, IDH-mutant, CIC-mutant oligodendroglioma.
Recent work has also identified TERT promoter somatic mutations in IDH-mutant, CIC-mutant oligodendrogliomas [50]; these mutations are associated with overexpression of TERT [51]. Interestingly, somatic TERT promoter mutations are also found in the vast majority of IDH wild-type glioblastomas, while they are virtually never seen in IDH-mutant TP53-mutant ATRX-mutant astrocytic gliomas. Thus, for the diffuse gliomas, TERT mutations and ATRX mutations are mutually exclusive. Oligodendrogliomas, therefore, now are recognized to contain at least four different recurrent molecular genetic correlates: IDH mutation, 1p/19q loss, CIC mutation and TERT promoter mutation.
Epigenetics
Epigenetic alterations are changes that affect cell behavior through events other than direct DNA sequence changes such as mutations and amplifications. Examples include nonsequence modifications to DNA itself, such as DNA methylation, as well as changes in DNA-related proteins, such as post-translational modification of histones. Because much of the control of DNA expression is mediated at an epigenetic level, epigenetic alterations can have tumorigenic effects, including in gliomas [52]. In adult glioblastoma, for example, methylation of the MGMT promoter occurs in approximately half of cases and is associated with improved response to alkylating agent chemotherapy [53]. In pediatric glioblastomas, mutations occur in histone genes (H3F3A) or in genes that regulate histones (ATRX and DAXX), implying that deregulation of histone function is critical to pediatric gliomagenesis [54]. As mentioned above, mutation of the IDH genes appears to be a causative factor in the global DNA methylation phenotype in gliomas known as the glioma-CpG island methylator phenotype (G-CIMP) [29,30].
Few studies have explored epigenetic alterations in oligodendrogliomas. MGMT promoter methylation has been documented in many oligodendrogliomas [55] and hypermethylation can be found in the promoter regions of many tumor-related genes in oligodendrogliomas [56]. A subset of oligodendrogliomas have widespread changes in DNA methylation akin to G-CIMP [57]. Since IDH mutations are near-universal in oligodendrogliomas, extensive methylation abnormalities in these tumors may be related to their mutant IDH status.
It therefore remains possible that some of the biological effects reflected in 1p/19q loss and the associated molecular genetic alterations such as IDH1 mutation result from alterations in epigenetic regulatory mechanisms, similar to what has been noted in other tumor types in which pathogenic changes may be related to promoters, regulatory enhancers and noncoding lincRNAs [52]. For example, loss of distal enhancers on 1p/19q could lead to changes in transcriptional regulation via loss of interactions between regulatory sites and target promoters, which could account for the tumor-promoting phenotypes seen with methylation of the NHE-1 gene on chromosome 1 [58]; similarly, the 1q/19p fusion could create novel interactions between enhancers and promoters. At the present time, however, these possibilities have not yet been evaluated in oligodendrogliomas.
Modern management & future directions
The clustering of recurrent molecular abnormalities within these groups has allowed for diffuse glioma diagnosis based on a consensus of identifiers, or ‘molecular group’ (Figure 1). While each molecular test has imperfect sensitivity and specificity, the tendency for overlap with other mutations can be combined with histologic information and clinical/demographic details to draw the borders between these tumor categories more sharply. Indeed, these diagnostic pursuits drive the modern management of a patient at initial presentation with a diffuse glioma. A firm consensus ‘molecular group’ diagnosis provides a stronger rationale for the subsequent adjuvant treatment of patients, allowing clinicians to use a more evidenced-based approach. It is tempting to envision molecular diagnostic approaches that will score for the two complementary hotspot recurrent mutations (IDH1 and TERT) to facilitate rapid and specific glioma subclassifications on even small biopsy specimens.
Still, there remain many areas of ongoing uncertainty, and prior studies can be reinterpreted with an eye toward molecular groups, to highlight the areas in need of a strengthening evidence base to guide treatment. As a critical first step, molecular retrospective review of previous glioma studies could be undertaken with the above-described immunohistochemical and PCR-based sequencing techniques, which are amenable to efficient scoring of formalin-fixed paraffin-embedded tissues from clinical trial specimen banks.
For oligodendrogliomas, such a re-examination of the RTOG and EORTC studies outlined above suggests that adjuvant PCV, when combined with radiation therapy, provides a substantial survival benefit to the 1p/19q-codeleted, IDH-mutant, CIC-mutant subgroups. It is possible that the administration of PCV chemotherapy following radiation therapy to patients with grade II oligodendrogliomas with 1p/19q codeletion, IDH mutation and CIC mutation may provide similar clinical benefit.
The data emerging from the ongoing grade II glioma study RTOG 9802 suggest this ‘molecular grouping’ perspective may be appropriate. In outcomes recently announced, patients with grade II gliomas (defined as high risk – either patient aged >40 years, or subtotal resection), PCV chemotherapy in addition to radiation therapy was associated with a median survival extension to 13.3 years from 7.8 years. Intriguingly, the patients in this study were not molecularly stratified, and it would not be expected that even the majority of the tumors were 1p/19q-deleted oligodendrogliomas – for instance, in the ‘low-risk’ observation arm of this same study, only 50 of 111 patients were categorized as ‘pure oligodendroglioma’ histologically [59].
These results raise the possibility that the mechanistic driver of PCV responsiveness may be the presence of IDH mutation, which is common to both oligodendroglial and astrocytic low-grade gliomas. A recent molecular reanalysis of the RTOG 9402 data may support this hypothesis, as AO and AOA patients having an IDH mutation derived a substantial survival benefit from PCV chemotherapy [60]. However, similar analyses of patients from the EORTC 26951 study did not reveal that IDH mutation was predictive in an otherwise histologically similar cohort [61,62]. It is hoped that future appropriately designed clinical trials, with attention to molecular genomic alterations, will resolve these questions.
Footnotes
Financial & competing interests disclosure
This work was supported by the Burroughs–Wellcome Career Award (DPC). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neurooncology. 2013;15(Suppl. 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey P, Bucy P. Oligodendrogliomas of the brain. J. Pathol. Bacteriol. 1929;32:735–754. [Google Scholar]
- 4.Burger PC, Rawlings CE, Cox EB, McLendon RE, Schold SC, Jr, Bullard DE. Clinicopathologic correlations in the oligodendroglioma. Cancer. 1987;59(7):1345–1352. doi: 10.1002/1097-0142(19870401)59:7<1345::aid-cncr2820590719>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann. Neurol. 1988;23(4):360–364. doi: 10.1002/ana.410230408. [DOI] [PubMed] [Google Scholar]
- 6.Cairncross JG, Macdonald DR, Ramsay DA. Aggressive oligodendroglioma: a chemosensitive tumor. Neurosurgery. 1992;31(1):78–82. doi: 10.1227/00006123-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin. Cancer Res. 2001;7(4):839–845. [PubMed] [Google Scholar]
- 8.Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am. J. Pathol. 1994;145(5):1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 9.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 10.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79(7):1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Prayson RA, Agamanolis DP, Cohen ML, et al. Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J. Neurol. Sci. 2000;175(1):33–39. doi: 10.1016/s0022-510x(00)00274-4. [DOI] [PubMed] [Google Scholar]
- 12.Nigro JM, Takahashi MA, Ginzinger DG, et al. Detection of 1p and 19q loss in oligodendroglioma by quantitative microsatellite analysis, a real-time quantitative polymerase chain reaction assay. Am. J. Pathol. 2001;158(4):1253–1262. doi: 10.1016/S0002-9440(10)64076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller CE, Wang H, Zhang W, Fuller GN, Perry A. High-throughput molecular profiling of high-grade astrocytomas: the utility of fluorescence in situ hybridization on tissue microarrays (TMA-FISH) J. Neuropathol. Exp. Neurol. 2002;61(12):1078–1084. doi: 10.1093/jnen/61.12.1078. [DOI] [PubMed] [Google Scholar]
- 14.Hatanpaa KJ, Burger PC, Eshleman JR, Murphy KM, Berg KD. Molecular diagnosis of oligodendroglioma in paraffin sections. Lab. Invest. 2003;83(3):419–428. doi: 10.1097/01.lab.0000059948.67795.ef. [DOI] [PubMed] [Google Scholar]
- 15.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 16.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol. 2000;18(3):636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 18.Wiestler B, Capper D, Holland-Letz T, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126(3):443–451. doi: 10.1007/s00401-013-1156-z. [DOI] [PubMed] [Google Scholar]
- 19.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am. J. Pathol. 2009;174(4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller M, Wick W, von Deimling A. Isocitrate dehydrogenase mutations: a challenge to traditional views on the genesis and malignant progression of gliomas. Glia. 2011;59(8):1200–1204. doi: 10.1002/glia.21130. [DOI] [PubMed] [Google Scholar]
- 27.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 33.Camelo-Piragua S, Jansen M, Ganguly A, Kim JC, Louis DN, Nutt CL. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509–511. doi: 10.1007/s00401-009-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loussouarn D, Le Loupp AG, Frenel JS, et al. Comparison of immunohistochemistry, DNA sequencing and allele-specific PCR for the detection of IDH1 mutations in gliomas. Int. J. Oncol. 2012;40(6):2058–2062. doi: 10.3892/ijo.2012.1404. [DOI] [PubMed] [Google Scholar]
- 36.Camelo-Piragua S, Jansen M, Ganguly A, et al. A sensitive and specific diagnostic panel to distinguish diffuse astrocytoma from astrocytosis: chromosome 7 gain with mutant isocitrate dehydrogenase 1 and p53. J. Neuropathol. Exp. Neurol. 2011;70(2):110–115. doi: 10.1097/NEN.0b013e31820565f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capper D, Reuss D, Schittenhelm J, et al. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. 2011;121(2):241–252. doi: 10.1007/s00401-010-0770-2. [DOI] [PubMed] [Google Scholar]
- 38.Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564–574. doi: 10.1111/j.1750-3639.2011.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2 . Neurology. 2010;74(23):1886–1890. doi: 10.1212/WNL.0b013e3181e1cf3a. [DOI] [PubMed] [Google Scholar]
- 40.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005;64(6):479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 41.Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am. J. Pathol. 2010;177(6):2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333(6048):1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J. Pathol. 2012;226(1):7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahm F, Koelsche C, Meyer J, et al. CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol. 2012;123(6):853–860. doi: 10.1007/s00401-012-0993-5. [DOI] [PubMed] [Google Scholar]
- 45.Ajuria L, Nieva C, Winkler C, et al. Capicua DNA-binding sites are general response elements for RTK signaling in drosophila. Development. 2011;138(5):915–924. doi: 10.1242/dev.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heaphy CM, De Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kannan K, Inagaki A, Silber J, et al. Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3(10):1194–1203. doi: 10.18632/oncotarget.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124(5):615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen DN, Heaphy CM, de Wilde RF, et al. Molecular and morphologic correlates of the alternative lengthening of telomeres phenotype in high-grade astrocytomas. Brain Pathol. 2013;23(3):237–243. doi: 10.1111/j.1750-3639.2012.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl Acad. Sci. USA. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arita H, Narita Y, Fukushima S, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267–276. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
- 52.Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339(6127):1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 54.Rheinbay E, Louis DN, Bernstein BE, Suva ML. A tell-tail sign of chromatin: histone mutations drive pediatric glioblastoma. Cancer Cell. 2012;21(3):329–331. doi: 10.1016/j.ccr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Kuo LT, Kuo KT, Lee MJ, et al. Correlation among pathology, genetic and epigenetic profiles, and clinical outcome in oligodendroglial tumors. Int. J. Cancer. 2009;124(12):2872–2879. doi: 10.1002/ijc.24303. [DOI] [PubMed] [Google Scholar]
- 56.Hong C, Bollen AW, Costello JF. The contribution of genetic and epigenetic mechanisms to gene silencing in oligodendrogliomas. Cancer Res. 2003;63(22):7600–7605. [PubMed] [Google Scholar]
- 57.Van Den Bent MJ, Gravendeel LA, Gorlia T, et al. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin. Cancer Res. 2011;17(22):7148–7155. doi: 10.1158/1078-0432.CCR-11-1274. [DOI] [PubMed] [Google Scholar]
- 58.Blough MD, Al-Najjar M, Chesnelong C, et al. DNA hypermethylation and 1p loss silence NHE-1 in oligodendroglioma. Ann. Neurol. 2012;71(6):845–849. doi: 10.1002/ana.23610. [DOI] [PubMed] [Google Scholar]
- 59.Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J. Neurosurg. 2008;109(5):835–841. doi: 10.3171/JNS/2008/109/11/0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 2014;32(8):783–790. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European organization for research and treatment of cancer brain tumor group. Clin. Cancer Res. 2010;16(5):1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 62.Van Den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]