Abstract
Rituximab is an anti-CD20 monoclonal antibody (mAb) used in the treatment of B-cell malignancies. Loss of surface CD20 antigen from the surface of target cells is thought to be one mechanism governing resistance to rituximab, but how this occurs is not completely understood. Two explanations for this have been proposed: antigenic modulation whereby mAb:CD20 complexes are internalised in a B-cell intrinsic process; and shaving, where mAb:CD20 complexes undergo trogocytic removal by effector cells such as macrophages. However, there are conflicting evidence as to which predominates in clinical scenarios and hence the best strategies to overcome resistance. Here we investigated the relative importance of modulation and shaving in the downregulation of surface mAb:CD20. We used both murine and human systems and treated ex-vivo macrophages with varying concentrations of non-Fc gamma receptor (FcγR)-interacting beads to achieve differential macrophage saturation states, hence controllably suppressing further phagocytosis of target cells. We then monitored the level and localisation of mAb:CD20 using a quenching assay. Suppression of phagocytosis with bead treatment decreased shaving and increased modulation suggesting that the two compete for surface rituximab:CD20. Under all conditions tested modulation predominated in rituximab loss whilst shaving represented an epiphenomenon to phagocytosis. We also demonstrate that the non-modulating, glycoengineered, type II mAb obinutuzumab caused a modest but significant increase in shaving compared to type II BHH2 human IgG1 wild-type mAb. Therefore shaving may represent an important mechanism of resistance when modulation is curtailed and glycoengineering mAb to increase affinity for FcγR may enhance resistance due to shaving.
Keywords: Rituximab, Obinutuzumab, shaving, modulation, phagocytosis
Introduction
The introduction of rituximab, a type I anti-CD20 mAb, revolutionised the treatment of B-cell associated haematologic malignancies and autoimmune pathologies. However, there is an absence of a clear consensus on both its mechanisms of B-cell depletion and resistance in patients. A large proportion of B-cell lymphomas are still unresponsive or resistant to treatment(1) with some patients demonstrating loss of CD20 from their target cell surface(2, 3)Two explanations have been proposed for CD20 loss: ‘modulation’- B-cell intrinsic internalisation of rituximab:CD20 complexes(4, 5) and trogocytosis (also known as shaving) from the surface of B-cells by effector cells(6–8). Both are thought to occur, but there is a lack of understanding about which might be more important for resistance, knowledge which would be critical in order to develop further CD20 based mAb therapy modalities and with implications for other depleting mAb.
Rituximab binds to CD20 through its variable region and elicits downstream immune effector functions via Fc:FcγR interactions(9). Although the identity of the FcγR expressing effector cells is still debated, a multitude of data supports a role for phagocytic monocytes or macrophages(4, 10, 11). A requirement for macrophages was similarly reported in the context of anti-CD30(12) and anti-CD40(13) antibody therapy in mouse lymphoma models, and recently in checkpoint blockade therapy such as anti-CTLA-4(14) and anti-PD-L1(15) against melanoma in murine models. Further to previous indications, recent in vivo evidence using intravital imaging suggests that hepatic Kupffer cells are responsible for the clearance of circulating CD20-expressing cells(16). Macrophages may thus be at least partially responsible for the efficacy of anti-CD20 mAbs. However, the ability of cells of the monocyte-macrophage lineage, via their FcγRs, to mediate the ‘shaving’ of rituximab:CD20 immune complexes from the surface of B-cells in vitro(6–8) and in vivo(17) has been reported. This shaving phenomenon has also been suggested to occur in vivo in rituximab recipients(18) and has thus been proposed as a mechanism to limit therapeutic efficacy. It has been implied that the body’s effector mechanisms may be saturated at high burdens of rituximab-opsonised B-cells and as a consequence, opsonised B-cells are processed by an alternative pathway, involving removal or shaving of rituximab-CD20 from B-cells by monocytes/macrophages(19). Although evidence for shaving was originally provided in 1976(20), there is a paucity of experimental data and published research providing a link between macrophage saturation status and shaving.
To better understand the relative contribution of shaving versus modulation to the loss of surface mAb:CD20 in the context of differential macrophage saturation states, we developed an in vitro assay, built upon a quenching assay used by us to study modulation(4). Contrary to previous observations,(18) we show that mAb:CD20 shaving is limited by macrophage saturation. Suppression of macrophage phagocytosis when fully loaded with beads led to concomitant decrease in shaving and increase in type I mAb mediated modulation suggesting that the two mechanisms of mAb:antigen loss compete. However, overall, more surface type I mAb:CD20 was removed by modulation. This was consistently observed with murine bone marrow derived macrophage (BMDM) and human monocyte derived macrophage (MDM), with both cell-lines and primary B-cells used as targets for phagocytosis. Additionally, type II anti-CD20 mAb, known to be less susceptible to modulation, were also susceptible to shaving from the B-cell surface, suggesting that the process of shaving may predominate in circumstances where modulation is not active.
Materials and Methods
Cell culture, clinical samples and ethics
Ethical approval was obtained by Southampton University Hospitals NHS Trust from Southampton and South West Hampshire Research Ethics Committee. Informed consent was provided in accordance with the Declaration of Helsinki. Chronic lymphocytic leukaemia (CLL) samples were released from the Human Tissue Authority licensed University of Southampton, Cancer Sciences Unit Tissue Bank and leukocyte cones from Southampton General Hospital National Blood Service. Human B cell lymphoma (Ramos) cell line were maintained at 37°C, 5% CO2 in RPMI 1640 (Gibco) supplemented with glutamine and pyruvate, penicillin-streptomycin and 10% v/v fetal calf serum (FCS). Additionally, Geneticin (Invitrogen) was used as a selection antibiotic at 20 µg/ml. Human monocytic leukaemia THP1 cell line was obtained from European Collection of Cell Cultures and maintained in supplemented RPMI 1640 (37°C, 5% CO2).
Bead labelling
Bovine serum albumin (BSA) or Alexa Fluor® 488 (A488)-lebellled BSA were dialysed in 50mM trisodium citrate (pH 4.2). 200μl of 3μm sulphate latex beads (Invitrogen) were centrifuged and 400μg of dialysed BSA – representative of saturating quantities – was added to packed beads, mixed and incubated for 1 hour at 4°C. BSA-coated beads were centrifuged, washed 3 times and re-suspended in a final volume of 400μl phosphate buffered saline (PBS).
Generation and polarisation of murine bone-marrow derived macrophage (BMDM); and generation of human monocyte derived macrophage (MDM)
BMDM and MDM were generated as described(21, 22). For polarisation of mBMDM, cells were stimulated overnight with M1 stimuli, lipopolysaccharide (LPS): 50ng/ml, recombinant murine (rm) interferon gamma (IFN-γ): 2ng/ml (Peprotech) and M2 stimuli rm interleukin-4 (IL-4): 10ng/ml (Peprotech) and rmIL-13: 10ng/ml (Peprotech).
In vivo B cell deletion
Human CD20 transgenic BALB/c mice were intravenously (i.v) administered with 2.5μg, 25μg or 250μg Ritm2a or 250μg of an irrelevant antibody WR17m2a in 200μl PBS via tail vein. Blood was sampled at indicated time points and was labelled with CD19-APC (ebioscience) to determine remaining circulating B cells. To determine CD20 expression on B cells, cells were opsonised with excess antibody (10μg/ml) and stained with secondary goat anti-mouse IgG-FITC antibody (Jackson ImmunoResearch), and labelled with CD19-APC and B220-PerCP (ebioscience). Percentage of CD20 antigen expression on B cells was calculated in relation to MFI of CD20 on B cells in mice treated with control WR17 antibody.
Phagocytosis and quenching assay
BMDM were incubated with Trypsin-EDTA solution at 37°C for 15 mins whilst MDM were incubated with PBS on ice for 15 mins. Cells were harvested using a cell-scrapper, re-suspended at 5x105 cells/ml and plated at 100μl/well (5x104 cells) in flat-bottomed 96-well plates in complete media. BSA-coated 3μm beads were used to differentially saturate macrophages for 1 hour, gently washed with PBS and 100μl fresh media added. Murine splenic human CD20 transgenic B-cells(4, 21, 22), human Ramos B cell line(23) or primary B-CLL cells, labelled with PKH26 (Sigma), were opsonised with Alexa Fluor® 488 (A488)- Ritm2a or Rituximab, respectively, or an irrelevant antibody (anti-CD40 mAb Lob-7/6 or Herceptin, respectively; 10μg/ml, 30 minutes, 4°C); washed and added to relevant wells at a ratio of 5 targets: 1 macrophage effector (100μl/well; 2.5x105 cells). Opsonised B-cells were also plated in the absence of macrophages and incubated at 37°C (modulation positive control) or 4°C (modulation negative control) for overnight. Following incubation for ~16 hours, wells were harvested, washed and 2.5μl of anti-A488 quenching antibody (Life technologies) added to half of all samples. Following incubation on ice for 30 minutes, cells were washed and analysed by flow cytometry (FACS Scan, Calibur or Canto II). FACS data was analysed using FCS Express V3 software (De Novo Software™). After gating on B-cells and subtraction of background fluorescence, the calculations used for each level of macrophage saturation are as follows:
Confocal microscopy
Human MDM adhered to 1μ-Slide 8 well ibiTreat (ibidi) plates were stained with 1μM CellTracker™ Green CMFDA (5-Chloromethylfluorescein Diacetate) (Invitrogen), and loaded with 3μm BSA-beads for 1 hour or left unloaded. Primary B-CLL cells were labelled with 8μM of nuclear dye Hoechst 33342 (Sigma) at 37°C and subsequently opsonised with 10μg/ml Rituximab-pHrodo on ice, for 30 minutes each, with wash steps in between. Alternatively, MDMs were labelled with PKH26 (Sigma). Live cell imaging was implemented on addition of CLL cells or 3μm-BSA-A488 beads to MDMs, using a Leica SP5 Laser Scanning Confocal Microscope. Image J was used for analysis.
Statistics
Assays were set up in triplicate and repeated three times whenever possible. Statistical significance between groups was calculated using ANOVA with post-hoc correction (GraphPad Prism 6), and a p value <0.05 was considered significant at the 95% confidence interval. Asterisks denote statistical significance (* p<0.05; ** p<0.01; *** p<0.001; and ****p<0.0001).
Results
Quantitation of BMDM uptake of latex beads and target B-cells
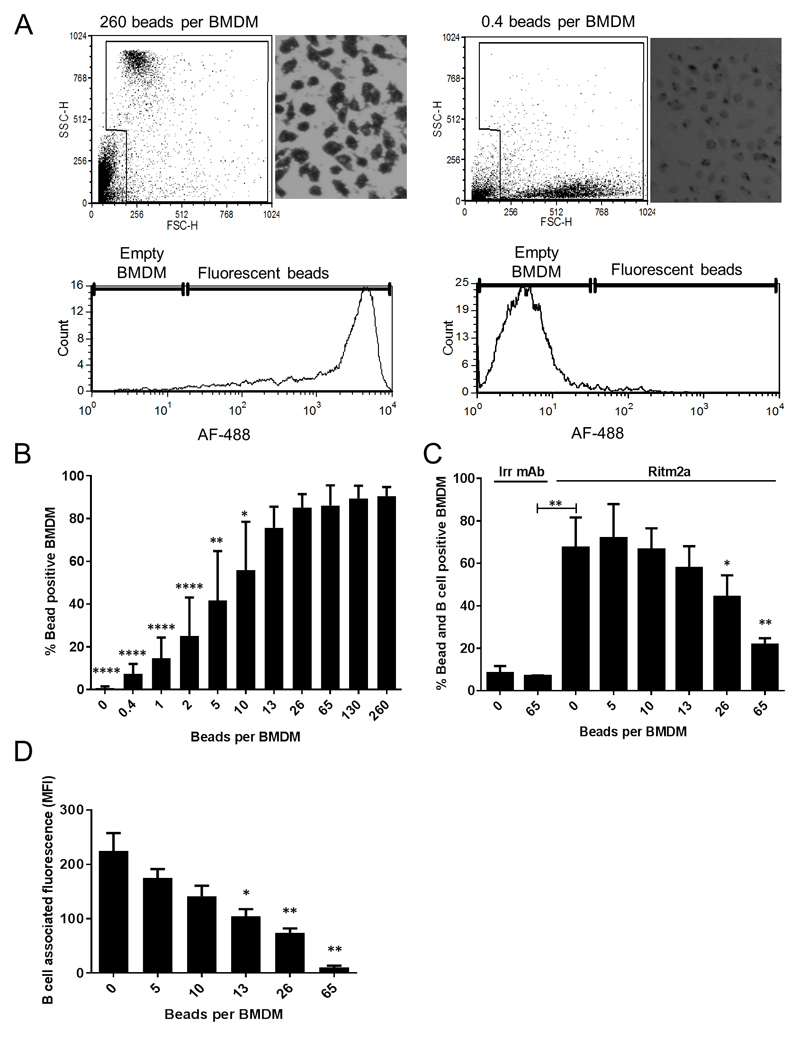
We first established a bead-loading assay to identify the number of beads required to saturate BMDM. Uptake of beads larger than 15μm has previously been shown to be FcγR-dependent(24). Therefore, to bypass the requirement for FcγR in the uptake of beads and to achieve maximal uptake by macrophages, BSA-coated 3μm beads were chosen as targets. BMDM were loaded with differential densities of A488-BSA labelled 3μm non-degradable latex beads. We observed by both microscopy and flow cytometry (Figure 1A and Supplemental figure 1A) that BMDM saturation occurred with bead density greater than 26:1 (beads:macrophage) (Figure 1B). We then assessed the interaction of bead loaded BMDM with PKH26 labelled human CD20 transgenic murine splenic B-cells that were opsonised with Ritm2a, a mouse IgG2a version of rituximab used by us previously(4, 21, 22), or an irrelevant (Irr) antibody (Figure 1C and Supplemental figure 2). As expected, ‘empty’ (0 beads) BMDM were able to efficiently phagocytose target B-cells while BMDM previously fully loaded with beads (65 beads) were unable to further phagocytose target cells, giving a negative correlation between bead saturation state and phagocytosis of Ritm2a opsonised B-cells (Figure 1C). B-cell associated fluorescence and hence the total number of B-cells phagocytosed by BMDM also decreased with increasing bead concentration (Figure 1D) suggesting that BMDM phagocytic capacity is limited and saturation prevents further uptake.
Figure 1. BMDM phagocytosis of target B cells is dependent on BMDM saturation status.
(A) BMDM were loaded with AF488 labelled 0.4-260 beads per BMDM and the extent of phagocytosis was assessed by flow cytometry and microscopy. Histogram show AF488 intensity when BMDM are empty (0.4 beads per BMDM) or full (260 beads beads per BMDM). (B) Increasing the amount of bead per BMDM increased BMDM loading with plateaux above 26:1 (* denote statistical significance when compared to 260 beads per BMDM condition). (C) Differentially bead loaded BMDM were incubated together with target human CD20 transgenic murine splenic B cells opsonised with Ritm2a or an Irrevelant antibody (Irr) and percentage of bead and B cell positive BMDM, (* denote statistical significance when compared to 0 beads per BMDM condition) and (D) B cell associated fluorescence after subtraction of Irr-B cell fluorescence (* denote statistical significance when compared to 0 beads per BMDM condition) is shown. Means of 4-5 independent experiments, error bar: mean±SD, analysed by ANOVA.
Shaving is limited in the context of saturated BMDMs
Following optimisation of our bead loading assay, we investigated the relative contributions of modulation and shaving to loss of surface mAb:CD20 complexes. Assays were paired, as described previously(4), with one sample left unquenched for every sample quenched with anti-A488. This enabled calculations of percentage surface accessible CD20, which estimates how much mAb:CD20 is on the surface of remaining B-cells, or internalised due to modulation, at each particular level of macrophage saturation. Loss of mAb:CD20 complexes was also calculated by comparing tests as a percentage of total fluorescence from unquenched B-cell control; changes in total fluorescence values were compared to estimate shaving. Percentage of surface accessible CD20 was significantly lower for the 37°C control (Ritm2a opsonised B-cells only, after overnight incubation) than the 4°C control (Ritm2a opsonised B-cells only, at time 0) reflecting that modulation had taken place during overnight incubation (Figure 2A). However when fully loaded (65 beads) BMDMs were co-cultured with B-cells overnight the proportion of Ritm2a:CD20 on the cell surface was not different when compared to the 37°C ‘modulation’ control. We only observed a significant decline in percentage of surface accessible CD20 at lower levels of BMDM saturation (Figure 2A). Notably, B-cell percentage of total control fluorescence readings were significantly reduced under conditions of sub-saturated BMDM relative to both controls and saturated BMDM (Figure 2B) indicating that shaving is concomitant with phagocytosis and is limited in conditions of macrophage saturation.
Figure 2. The influence of BMDM saturation on shaving and modulation with type I and type II anti-CD20 mAbs.
BMDM were loaded with differential bead densities (0-65 bead/BMDM) and co-cultured with human CD20 transgenic murine splenic B cells opsonised with (A and B) type I Ritm2a or (C and D) type II BHH2m2a (10μg/ml), subjected to the combined B cell phagocytosis and quenching assay and analysed by flow cytometry. BMDM and B cell gates were set to exclude beads from B cell and percentage of surface accessible CD20 and percentage of control total fluorescence calculated as described in materials and methods. Means of 3-4 independent experiments, error bar ± SD, analysed by ANOVA, ns: not significant.
Only type I anti-CD20 mAb internalise but both type I and II shave
Although type II mAbs have been reported to be similarly subject to shaving(25), the shaving of CD20-type II mAb complexes by saturated macrophages has not been assessed. We(4, 22) and others(26) have shown that macrophages are capable of mediating the phagocytosis of type II antibody opsonised B-cells. The results above demonstrate that shaving occurs concomitantly to phagocytosis, and we hypothesised that type II antibody-opsonised B-cells would also remove/‘shave’ mAb:CD20 complexes in the context of non-saturated (empty), but not in saturated (full) macrophages. We used both Ritm2a and BHH2m2a antibodies at a concentration of 10μg/ml as they elicit phagocytosis optimally, display similar propensity to elicit ADCP and saturate all CD20 sites on B cells at this concentration (22). In line with previous studies, type II antibodies did not induce modulation to the same extent as type I antibodies in our control groups (Figure 2C). Notably, the percentage of surface accessible CD20 did not substantially differ throughout the groups cultured in the presence of macrophages that were loaded with differential concentrations of beads (Figure 2C) indicating that the majority of type II mAb:CD20 complexes were surface accessible in the face of ongoing phagocytosis. However, type II antibody-opsonised B-cells were equally susceptible to shaving in our assay (Figure 2D), as has been reported(25)as the percentage of control total fluorescence significantly differed between macrophages that were not saturated and saturated with beads. Taken together with previous results demonstrating the greater efficacy in vitro and in vivo of type II versus type I mAb(4, 22, 27) these data indicate a more dominant role for modulation in the limitation of type I antibody therapeutic efficacy.
Reduced type I anti-CD20 mAb dosing does not mitigate modulation without impacting efficacy in vivo
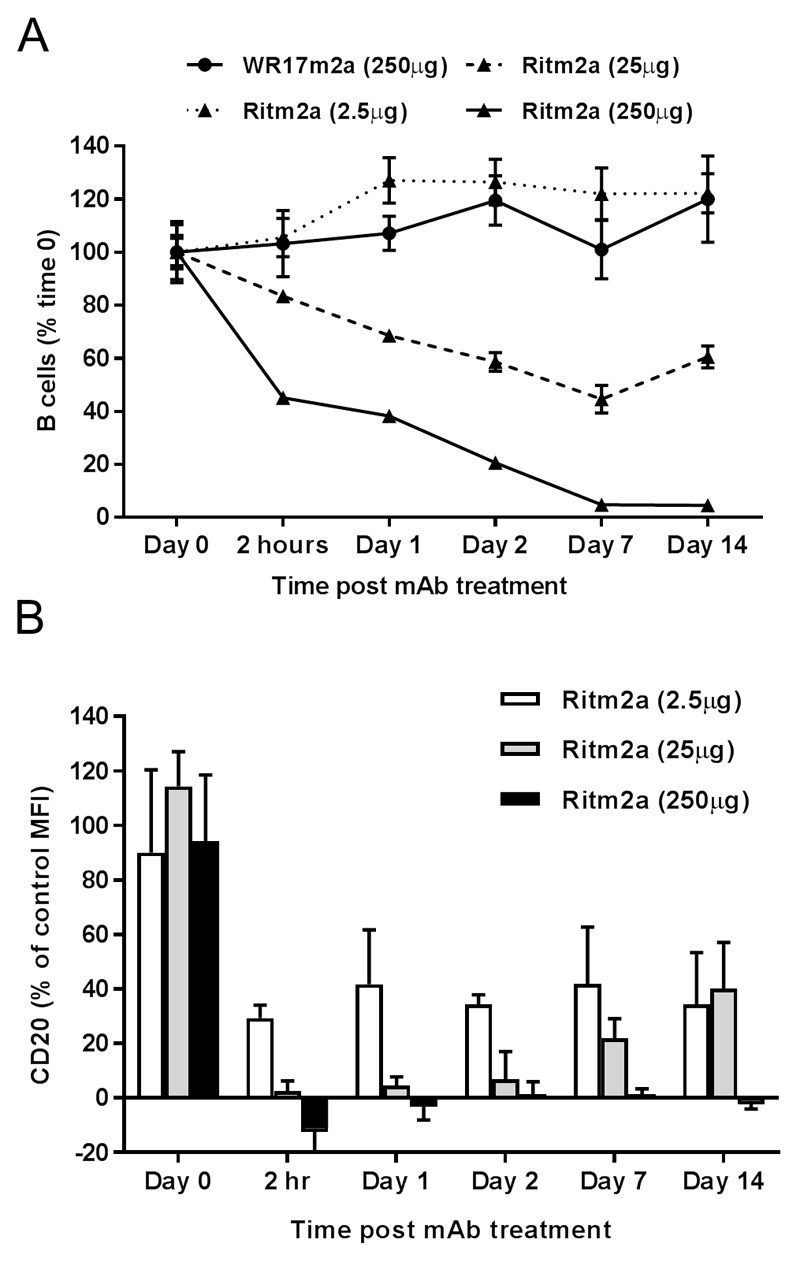
Given that our in vitro observations contradict those of Taylor and colleagues(18), we sought to confirm our observations regarding Ritm2a in vivo. We have previously reported the impact on in vivo human CD20 transgenic B cell depletion efficacy using lower doses of Ritm2a(4), but had not directly investigated the effects of lower dose administration on modulation. Here we set out to determine over a 100 fold range of mAb concentrations whether reducing type I mAb doses would lead to reduced modulation and more effective depletion. Ritm2a was administered to human CD20 transgenic mice by tail vein injection and the number of circulating B cells monitored over time. A single administration of 250μg of Ritm2a led to a rapid and sustained depletion of circulating B cells as previously reported (4, 21, 22), 25μg led to a small reduction in B cell number which began to recover after 7 days and 2.5μg had no effect (Figure 3A). At the same time we also determined the percentage of CD20 antigen present on the surface of remaining B cells by flow cytometry. These results demonstrate that at even at high doses of mAb where depletion was relatively effective a proportion of circulating B cells remain and these cells are negative for CD20 thereby explaining their resistance to depletion (Figure 3B). At reduced concentrations there is a concomitant increase in CD20 on remaining B cells but at 25μg of Ritm2a where only a minor effect on B cell numbers is seen the vast majority of CD20 is still lost from the remaining B cells and this CD20 is only regained as the B cell numbers begin to normalise (as previously observed [4] and in line with clearance of mAb from the circulation). Notably, at 2.5μg mAb administration approximately 60% of CD20 is still lost despite no effect on B cell numbers. These data support our contention that reducing mAb concentration to increase efficacy through reduced modulation is unlikely to be effective.
Figure 3. In vivo depletion of murine circulating B cells by type I anti-CD20 mAb Ritm2a.
Human CD20 transgenic BALB/c mice were i.v administered with 2.5μg, 25μg or 250μg Ritm2a or 250μg of an irrelevant antibody WR17m2a and (A) percentage of circulating B cells (expressed as 100% at time 0) and (B) % CD20 compared to controls on B cells assessed by flow cytometry from tail blood at indicated time points. n=3 in each group (representative of two independent experiments).
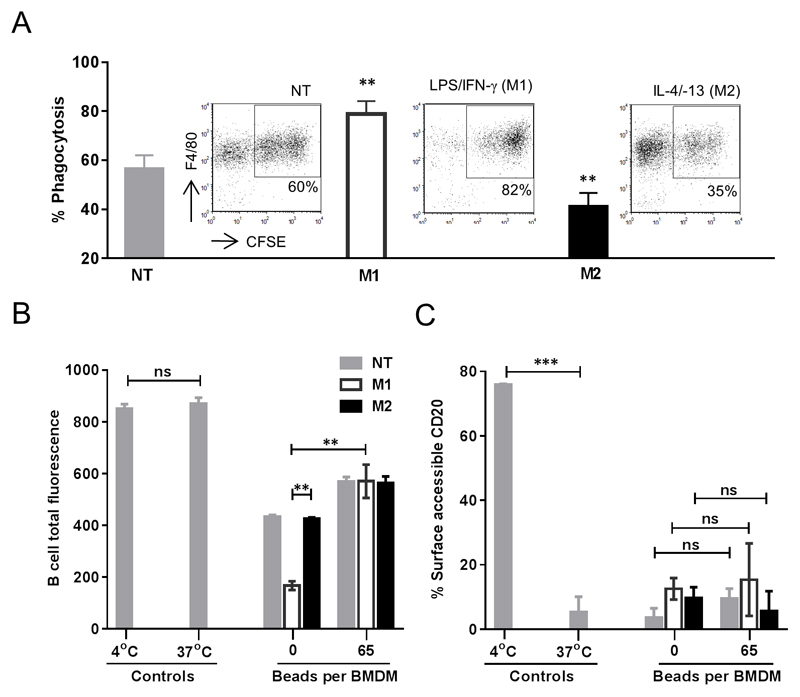
BMDM activation state does not alter bead-loading, but M1-polarised BMDMs demonstrate enhanced phagocytosis and accelerated shaving
An additional question we asked was how BMDM activation state, reflecting to some extent different in vivo macrophage activation phenotypes(28), influences the propensity for mAb:CD20 shaving or modulation. We stimulated BMDM with the archetypal classical activation (M1) and alternative activation (M2) reagents LPS/IFN-γ and IL-4/-13 respectively and assessed the ability of skewed BMDMs to uptake 3μm beads. Skewing did not alter the ability of macrophages to be loaded with beads, although a slight increase in M1 stimulated macrophages was observed (Supplemental figure 3). We then co-cultured empty or fully loaded, pre-skewed macrophages with opsonised target cells. In consensus with our published observations(29), M1-skewed empty macrophages displayed augmented phagocytosis of target cells in comparison to non-treated (NT) and M2-skewed BMDMs (Figure 4A). As seen in the above experiments, approximately 25% loss of B-cell total fluorescence was observed in the context of non-saturated macrophages, compared to saturated macrophages, and this was substantially accentuated (~70%) with M1 skewing compared to non-polarised and M2 macrophages (Figure 4B). However, there was no change in percentage of surface accessible CD20 with skewing (Figure 4C). This further supports the contention that shaving is an epiphenomenon to phagocytosis as in M1 skewed conditions, where phagocytosis was substantially enhanced, shaving was also augmented.
Figure 4. M1 macrophages accentuate loss of B cell total fluorescence under non-saturating conditions.
(A) BMDM stimulated with M1 and M2 stimulus LPS/IFN-γ and IL-4/-13 respectively for overnight were harvested and plated at 5x104 cells/well and assessed for their ability to phagocytose target cells by ADCP assay. Flow cytometry plots in the inset: Target cells were labelled with CFSE and BMDM with F4/80. CFSE+APC+ events indicate phagocytic macrophages. (B) BMDM were differentially saturated with beads and subjected to the combined B cell phagocytosis and quenching assay and analysed by flow cytometry where BMDMs and B cell gates were set to exclude contaminating beads and cells of contrasting lineages and B cell total fluorescence and (C) percentage surface accessible CD20 calculated as described in materials and methods. Means of 3-4 independent experiments, error bar± SD, analysed by ANOVA, ns: not significant.
Modulation removes more surface CD20 from Ramos cells
Following our murine experiments, we then extended our observations to human macrophages and targets. Previous investigations of shaving in B-cell malignancies predominantly adopted the monocytic THP-1 cell line in their models(6, 8). We observed that the THP-1 cell-line was poorly phagocytic (Supplemental figure 4D), and therefore opted for primary macrophages derived from peripheral blood precursors in our assays. Once again, we established the bead-loading assay conditions required to differentially saturate MDM and identified low, medium and high bead density as approximately 7, 50 and 93 beads per macrophage. As observed with murine BMDM, MDM when saturated at high bead density were unable to mediate further phagocytosis (Supplemental figure 4A and B). Analysis of geometric mean fluorescence intensity (MFI) of these MDM showed a positive correlation between MFI and the concentration of beads (Supplemental figure 4C).
We then examined the impact of shaving and modulation on surface rituximab:CD20 levels. We have previously shown that FcγRIIB enhances the internalisation of mAb on the surface of B cells(23). We therefore stably transfected modulation resistant Ramos cells with physiological levels of FcγRIIB, opsonised with AF488 labelled rituximab, co-cultured them overnight with MDMs treated with low, medium and high bead density or not-treated (NT) MDM and analysed using flow cytometry. As seen with the BMDM assay, the percentage of rituximab:CD20 on the cell surface was significantly lower for the 37°C control (rituximab-opsonised Ramos cells only, after overnight incubation) than the 4°C control (rituximab-opsonised Ramos cells only, at time 0) reflecting that modulation had taken place during overnight incubation (Figure 5A). However when MDMs were co-cultured with Ramos cells overnight, the proportion of CD20-rituximab on the cell surface was not different at any of the bead concentrations when compared to the 37°C control. To confirm that MDMs were undergoing phagocytosis and shaving on rituximab-opsonised Ramos cells and bead treatments modified such interaction, the amount of fluorescently-tagged mAb taken up by MDMs was assessed (Figure 5B). Results indicated that bead treatment affected the amount of rituximab:CD20 taken up by MDMs and not surprisingly, NT MDM ‘shaved’ more rituximab:CD20 than MDM treated with high concentration of beads. This indicated that bead treatment does modify interactions between MDM and rituximab-opsonised B-cells and bead treatment suppressed phagocytosis.
Figure 5. Loss of surface CD20-Rituximab from Ramos cells can be attributed to modulation rather than shaving.
Human MDM pre-loaded with differential bead densities were co-cultured with target Ramos cells (effector:target ratio of 1:2) opsonised with 10μg/ml Herceptin-AF488 or Rituximab-AF488 for 16-18 hours. (A) Percentage of total rituximab:CD20 on Ramos cell surface following mAb opsonisation (4°C control), 16-18 hours incubation alone (37°C control), and 16-18 hours co-culture with MDMs (with or without beads pre-loaded). Quantification was performed by measuring decreases in fluorescence levels following quenching assay using Anti-A488 rabbit IgG1. (B) Quantification of rituximab:CD20 uptake through phagocytosis and shaving in MDM treated with different concentrations of beads following 16-18 hours co-culture with Ramos cells. (C) Level of rituximab:CD20 in Ramos cells normalised to that of the 4°C and 37°C controls averaged. Loss in Ramos cell fluorescence levels in conditions that were co-cultured with MDMs reflect level of shaving by MDM. (D) Percentage of CD20 modulated from the surface of Ramos cells cultured alone or co-cultured with MDMs for 16-18 hours. Data are composite of 4 independent experiments using 4 different MDM donors, error bars: mean +/- SD, analysed by ANOVA, ns: not significant.
Furthermore, we monitored total (surface and cytoplasmic) rituximab:CD20 on Ramos cells to assess the effect of shaving on B-cells. In the presence of NT MDM, but not high bead density MDM, Ramos had significantly less total rituximab:CD20 following overnight incubation compared to the 37°C control (Figure 5C) implying that MDM are capable of trogocytic removal of CD20-rituximab from target B-cells and suppression of phagocytosis can influence the level of shaving. Subsequently the amount of rituximab:CD20 internalised by modulation was quantified. The level of modulation in Ramos cells was higher in the 37°C control than those co-cultured with NT MDMs (Figure 5D). Interestingly, the level of modulation of the 37°C control was no different to Ramos cells that were co-cultured with MDMs treated with high concentration of beads suggesting that modulation and shaving compete for surface rituximab:CD20 and that more is removed through modulation and this effect is potentiated when phagocytosis is suppressed.
Modulation drives loss of surface rituximab:CD20 on CLL cells but surface obinutuzumab:CD20 loss occurs predominantly through shaving
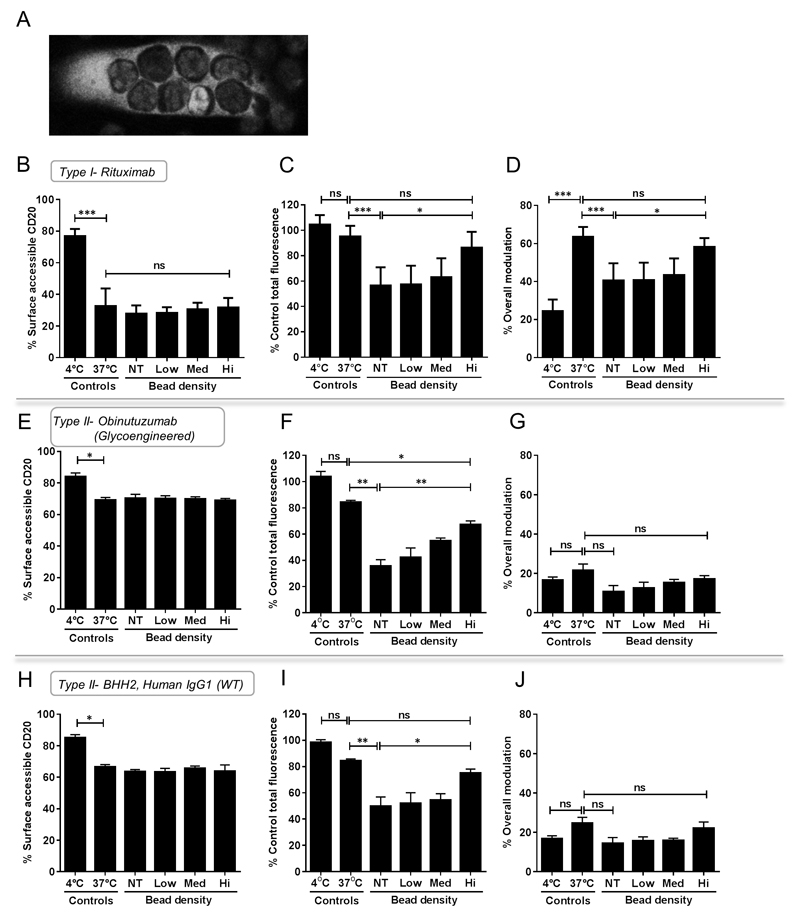
Having established with the Ramos cell-line, which is a poor phagocytic target, that we had observed the same overall effects although more muted than for murine BMDM we sought to confirm the relative contribution of shaving vs modulation of CD20 from the surface of primary human CLL cells by type I and II anti-CD20 antibodies. We used archetypal type I anti-CD20 rituximab and two type II mAb - obinutuzumab (GA101), a humanized glyocengineered Fc mAb(27) and BHH2 the human IgG1 wild-type parental mAb for GA101. We used CLL samples from patients in 5 independent co-culture experiments with MDMs from 5 different donors (Example of CLL phagocytosis by MDM is shown in Figure 6A). As seen with Ramos cells, the level of surface accessible rituximab:CD20 on the CLL cell surface in the presence of MDMs were not statistically significant at different bead density when compared to 37°C control (Figure 6B). However, the total level of rituximab:CD20 in CLL cells was significantly reduced in the presence of non-treated ‘empty’ MDMs when compared to the 37°C control but not MDMs treated with high concentration of beads (Figure 6C), consistent with earlier observations that suppression of phagocytosis can influence the level of trogocytic removal of rituximab:CD20. Furthermore, the level of overall modulation significantly decreased in the presence of non-treated MDM which was not the case with MDM treated with high concentration of beads (Figure 6D). Taken together, these results indicate that, like with Ramos cells, modulation is also the dominant cause of the loss rituximab:CD20 from the surface of CLL cells.
Figure 6. Relative contribution of shaving and modulation for loss of CD20 from CLL cells with type I and II anti-CD20 mAbs.
(A) Confocal microscopy of Rituximab-pHrodo opsonised Hoescht33342 labelled B-CLL cells seen ingested by celltracker green labelled MDM. (B-D) Primary CLL samples were opsonised with RTX-AF488, (E-G) Obinutuzmab-AF488 or (H-J) BHH2-AF488 at 10µg/ml for 30 minutes on ice before being cultured alone or co-cultured with MDMs pre-loaded with different concentrations of beads for 16-18 hours at 37°C. Cells were cultured in 96-well plates at an effector:target ratio of 1:5. Proportion of surface accessible mAb:CD20, percentage of total mAb:CD20 and percentage of mAb:CD20 modulated from the surface of CLL cells normalised to that of the 4°C and 37°C controls is shown. Data composite of 3-5 independent experiments, error bars mean± SD, analysed by ANOVA, ns: not significant.
Finally, using type II anti-CD20 mAb, which are known to be less susceptible to modulation(4), the importance of shaving on surface CD20-mAb level could be examined with minimal influence confounded by modulation. The proportion of obinutuzumab:CD20 or BHH2:CD20 on the surface of CLL cells following overnight incubation without MDMs displayed little change, consistent with the lower susceptibility of type II anti-CD20 mAb to modulate (Figure 6E and 6H). The presence of MDMs, whether non-treated or treated, led to a decrease in total obinutuzumab:CD20 and BHH2:CD20 levels in CLL cells (Figure 6F and 6I). Such decrease was less pronounced in MDMs treated with high concentration of beads compared to those non-treated. Notably, the total obinutuzumab:CD20 levels were significantly lower (p=0.0218), albeit it modestly, than BHH2:CD20 levels in NT ‘empty’ MDMs (Figure 5F vs 5I) showing that glycoengineering Fc approaches to enhance FcγR binding affinity can augment shaving. Not surprisingly, only a minor percentage of obinutuzumab:CD20 or BHH2:CD20 was lost through modulation in the presence of MDM, whether non-treated or treated with high concentration of beads (Figure 6G and 6J). Taken together, these results suggest that in the absence of modulation, surface mAb:CD20 is preferentially lost through shaving although this may not limit phagocytosis.
Discussion
It has been proposed that in the presence of a large tumour burden and following a standard dose of rituximab, macrophages rapidly become saturated which favours shaving over the phagocytosis of mAb-opsonised target cells(19). Here we developed an assay using protein-coated 3μm latex beads to differentially saturate macrophages, independently of FcγR, to reflect conditions of varying macrophage saturation and demonstrate that shaving is largely an epiphenomenon occurring in tandem to phagocytosis rather than a mechanism of resistance.
In our assay, using murine BMDM, we observed a decrease in B-cell phagocytosis with increased bead loading. B-cell modulation controls; negative at 4°C and positive at 37°C revealed that a significant proportion of surface CD20 was modulated after overnight culture. In comparison to the controls, modulation was shown to be affected by BMDM bead-loading. Little surface CD20 was accessible on the B-cells plated with empty BMDMs, indicating a large amount of modulation to have taken place, while a greater proportion of surface CD20 was accessible on those B-cells plated with saturated BMDMs. Furthermore, measurement of total mAb fluorescence, indicative of antibody left available on non-phagocytosed B-cells was seen to increase with BMDM saturation, suggesting that with increasing BMDM loading, less shaving occurs. These results contradict those of Williams et al(18) who suggested that upon saturation of the mononuclear phagocyte system (MPS) shaving increases and reduced dosing promotes clearance of target cells. Administration of lower doses of antibody did not improve the efficacy of B cell deletion, strengthening the notion that reduced mAb concentration may not increase efficacy by reducing modulation. Much data supports that the shaving reaction occurs in response to rituximab therapy both in vitro and in vivo, however little work has been carried out to test if this is in response to saturated macrophages. Our data shows that shaving does occur but more likely as a result of ongoing phagocytosis by non-saturated BMDMs than as a consequence of macrophage saturation. Beum et al also found that shaving of rituximab:CD20 complexes from CLL cells can occur with the THP-1 human monocytic cell-line(6, 8). The authors suggested a similar explanation to the above, namely that shaving occurs in response to standard rituximab doses causing high rituximab plasma concentrations and MPS saturation. However, actual THP-1 saturation was not investigated, and the shaving observed may be a result of ongoing phagocytosis as opposed to saturation. We provide evidence in line with this by differentially skewing macrophages. In comparison to NT and M2-skewed macrophages, non-saturated M1-polarised macrophages were responsible for an accelerated decline in B-cell total fluorescence on a background of concomitantly enhanced phagocytosis. Furthermore, we have shown that monocytic/macrophage cell-lines including THP-1 are comparably less phagocytic than MDM. It is not known how phorbol myristate acetate (PMA) used to stimulate THP-1 cells used in the studies of Beum et al(6, 8) affects the phenotype of these cells. Therefore to avoid the methodological challenges all together, use of MDM may be more relevant and likely to have a closer resemblance to their in vivo counterparts. However, our results may allow over-interpretation of shaving as it is difficult to formally rule out the selection of CD20 low targets. To address this concern we established this assay using a 5:1 excess of target to effector cells. In this setting and at the end of the assay a larger proportion of the target cells remained with lower MFI than existed in the control untreated population. This largely discounts the selection of lower CD20 expressers escaping phagocytosis preferentially.
Our studies with the type II anti-CD20 mAb obinutuzumab (glycoenigneered for enhanced FcγR interaction) and BHH2 (wild-type human IgG1) showed that shaving removed a substantially greater amount of obinutuzumab:CD20 compared to that when rituximab or BHH2 were used suggesting that trogocytic removal/shaving of antibody-antigen complexes may be a limiting factor to mAb that are less susceptible to modulation, and that glycoengineering could hinder the efficacy of type II anti-CD20 mAb immunotherapy.
In the last two decades, remarkable progress has been made in the development of antineoplastic mAbs with more than 14 approved by the US Food and Drug Administration and many others undergoing clinical trials. However, it is evident that resistance mechanisms beyond patient selection factors, such as modulation and shaving may hinder the full potential of these therapeutic agents. Whether or not FcγR mediated shaving forms a principal mechanism of tumouricidal activity of therapeutic antibodies is hugely debated with conflicting evidence. Contrary to previous findings(30), it was recently shown that neutrophils do not phagocytose CLL B-cell targets opsonised with anti-CD20 antibodies, but rather mediate shaving(31).The ability of macrophages to carry out shaving and phagocytosis of antibody-opsonized HER2-overexpressing breast cancer cells has also been demonstrated, and with antibody engineering approaches, the tumoricidal effects of shaving may also be enhanced(32). However, this may not be the case with some antibodies; anti-PD1 antibodies are captured from the T cell surface by macrophages within minutes and this macrophage accrual of anti-PD-1 does not involve transfer of cell membrane components or trogocytosis, which has been described for rituximab(33). Although our results do not immediately contest the existence and relative contribution of the shaving phenomenon, how other effectors like NK cells and neutrophils are limited by anti-CD20-mAb complex shaving or modulation, and how the predominant mechanism varies between patients and disease types are yet to be investigated. Through an increased understanding of resistance, future research in this area may benefit patients, not only receiving CD20 based treatments, but also other direct targeting and checkpoint antibodies, which may gain approval in the near future. Studies with such antibodies will provide more information about the importance of modulation to the loss of antibody efficacy. Ultimately, the use of similar well-validated assays, alongside in vivo models and human cells assays, for other mAbs and indications will be important to gauge potential mechanisms of action and resistance prior to clinical trials and/or regulatory body approval.
Supplementary Material
Acknowledgments
We would like to thank Roche Pharmaceutical Research and Early Development, for providing material and advice on the manuscript.
Financial Support: Funding was provided by CRUK A12343, CRUK Southampton Centre grant C349999/A18087, Southampton ECMC grant C24563/A15581 and by Bloodwise 12021. RJS was funded through an iCASE studentship with Promega from the BBSRC (BB/K011502/1).
Footnotes
Authorship and conflict of interest
LND, C-YH, RJS, AM, KC, JXB, MCT, PN, ATV conducted experiments, analysed and interpreted data, H.T CC, JHK and FF contributed critical reagents, primary cells and material support, SAB conceived the project. LND and SAB supervised the study and wrote the manuscript. SAB is a consultant for Astex Pharmaceuticals and has received research funding from Bioinvent International. Other authors do not have conflicts of interest to declare.
References
- 1.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. Journal of immunology (Baltimore, Md. : 1950) 2004;172:3280–3288. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 3.Jilani I, O'Brien S, Manshuri T, Thomas DA, Thomazy VA, Imam M, Naeem S, Verstovsek S, Kantarjian H, Giles F, Keating M, et al. Transient down-modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood. 2003;102:3514–3520. doi: 10.1182/blood-2003-01-0055. [DOI] [PubMed] [Google Scholar]
- 4.Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS, Dixon SV, Kim H, Cox KL, Kerr JP, et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 5.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, Beers SA, French RR, Cox KL, Davies AJ, Potter KN, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 6.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. Journal of immunology (Baltimore, Md. : 1950) 2006;176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 7.Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. Journal of immunology (Baltimore, Md. : 1950) 2008;181:2916–2924. doi: 10.4049/jimmunol.181.4.2916. [DOI] [PubMed] [Google Scholar]
- 8.Beum PV, Peek EM, Lindorfer MA, Beurskens FJ, Engelberts PJ, Parren PW, van de Winkel JG, Taylor RP. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. Journal of immunology (Baltimore, Md. : 1950) 2011;187:3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 9.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature medicine. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 10.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. The Journal of experimental medicine. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, Haas KM, Tedder TF. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood. 2008;112:1205–1213. doi: 10.1182/blood-2008-01-135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oflazoglu E, Stone IJ, Gordon KA, Grewal IS, van Rooijen N, Law CL, Gerber HP. Macrophages contribute to the antitumor activity of the anti-CD30 antibody SGN-30. Blood. 2007;110:4370–4372. doi: 10.1182/blood-2007-06-097014. [DOI] [PubMed] [Google Scholar]
- 13.Oflazoglu E, Stone IJ, Brown L, Gordon KA, van Rooijen N, Jonas M, Law CL, Grewal IS, Gerber HP. Macrophages and Fc-receptor interactions contribute to the antitumour activities of the anti-CD40 antibody SGN-40. British journal of cancer. 2009;100:113–117. doi: 10.1038/sj.bjc.6604812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer cell. 2015;28:285–295. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, Bousso P. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. The Journal of clinical investigation. 2013;123:5098–5103. doi: 10.1172/JCI70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Rituximab-CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. Journal of immunology (Baltimore, Md. : 1950) 2007;179:4263–4271. doi: 10.4049/jimmunol.179.6.4263. [DOI] [PubMed] [Google Scholar]
- 18.Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, Hamil SH, Eggleton JC, Taylor RP. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. Journal of immunology (Baltimore, Md. : 1950) 2006;177:7435–7443. doi: 10.4049/jimmunol.177.10.7435. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RP, Lindorfer MA. Antigenic modulation and rituximab resistance. Seminars in hematology. 2010;47:124–132. doi: 10.1053/j.seminhematol.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin FM, Jr, Griffin JA, Silverstein SC. Studies on the mechanism of The phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. The Journal of experimental medicine. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, Ahuja A, Shlomchik MJ, Cragg MS, Glennie MJ. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tipton TR, Roghanian A, Oldham RJ, Carter MJ, Cox KL, Mockridge CI, French RR, Dahal LN, Duriez PJ, Hargreaves PG, Cragg MS, et al. Antigenic modulation limits the effector cell mechanisms employed by type I anti-CD20 monoclonal antibodies. Blood. 2015;125:1901–1909. doi: 10.1182/blood-2014-07-588376. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan AT, Iriyama C, Beers SA, Chan CH, Lim SH, Williams EL, Shah V, Roghanian A, Frendeus B, Glennie MJ, Cragg MS. Inhibitory FcgammaRIIb (CD32b) becomes activated by therapeutic mAb in both cis and trans and drives internalization according to antibody specificity. Blood. 2013 doi: 10.1182/blood-2013-04-490821. [DOI] [PubMed] [Google Scholar]
- 24.Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. Journal of cell science. 1992;101(Pt 4):907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen AE, Jungersen MB, Pedersen CD. Monocytes mediate shaving of B-cell-bound anti-CD20 antibodies. Immunology. 2011;133:239–245. doi: 10.1111/j.1365-2567.2011.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafiq S, Butchar JP, Cheney C, Mo X, Trotta R, Caligiuri M, Jarjoura D, Tridandapani S, Muthusamy N, Byrd JC. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. Journal of immunology (Baltimore, Md. : 1950) 2013;190:2702–2711. doi: 10.4049/jimmunol.1202588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, Ferrara C, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 29.Dahal LN, Dou L, Hussain K, Liu R, Earley A, Cox KL, Murinello S, Tracy I, Forconi F, Steele AJ, Duriez P, et al. STING activation reverses lymphoma-mediated resistance to antibody immunotherapy. Cancer research. 2017 doi: 10.1158/0008-5472.CAN-16-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golay J, Da Roit F, Bologna L, Ferrara C, Leusen J, Rambaldi A, Klein C, Introna M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122:3482–3491. doi: 10.1182/blood-2013-05-504043. [DOI] [PubMed] [Google Scholar]
- 31.Valgardsdottir R, Cattaneo I, Klein C, Introna M, Figliuzzi M, Golay J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B-cells opsonized with anti-CD20 antibodies. Blood. 2017;129:2636–2644. doi: 10.1182/blood-2016-08-735605. [DOI] [PubMed] [Google Scholar]
- 32.Velmurugan R, Challa DK, Ram S, Ober RJ, Ward ES. Macrophage-Mediated Trogocytosis Leads to Death of Antibody-Opsonized Tumor Cells. Mol Cancer Ther. 2016;15:1879–1889. doi: 10.1158/1535-7163.MCT-15-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.