Summary
Non-encephalopathic presentations of CNS thiamine deficiency may be difficult to diagnose. We describe neuro-otologic findings of Wernicke syndrome in 5 patients with vestibular manifestations. Diagnosis was confirmed by low serum levels, response to replacement, and brain MRI to exclude other causes. All had bilaterally abnormal horizontal head impulse vestibulo-ocular reflex (VOR) responses and pathologic gaze-evoked nystagmus, without encephalopathy. After thiamine replacement, 4 had total resolution of vestibular and oculomotor findings. Novel findings included 2 patients whose VOR function improved within minutes of IV repletion and 1 whose recovery was documented by serial quantitative recordings. Early diagnosis of Wernicke by examining vestibular reflexes and prompt IV treatment might prevent encephalopathy and other neurologic or systemic complications of thiamine depletion.
The complete Wernicke encephalopathy triad includes ophthalmoplegia, ataxia, and encephalopathy. In their seminal 1971 study, Victor, Adams, and Collins1 defined the clinical presentation and neuropathology of classic Wernicke in 232 cases. Altered mental status was present in 90%. Ocular findings were noted in 96% (228/232). Nystagmus was the most common ocular finding (87%, 198/228), and horizontal, bilateral gaze-evoked nystagmus the most common form, occurring in 97% (192/198). This form of nystagmus suggests failure of the oculomotor pathways for horizontal gaze-holding.2 Nystagmus was the only ocular feature in 65% (151/232). Vestibular reflexes were not tested clinically, as the bedside head impulse test of vestibulo-ocular reflex (VOR) function was not described by Halmagyi and Curthoys3 until 1988. Depending on the direction of the head impulse applied, this test can assess each component of the VOR,4 including the horizontal VOR (h-VOR) and vertical VOR (v-VOR) canal plane responses. These tests can now be readily applied to patients with thiamine deficiency at the bedside.
In animal models of thiamine deficiency, bilateral, symmetric lesions of the vestibular nuclei are a consistent finding.5 The most common human vestibular finding in Wernicke is bilateral vestibular hypofunction, occurring in 54/60 (90%) of previously reported cases.6–9 Although some of these apparently had normal mentation,7 most had comorbid encephalopathy.6,8,9 In the only 2 prior reported cases where high-acceleration bedside head impulse testing was performed, it revealed dissociated h-VOR deficits localizing to the horizontal semicircular canal afferents (presumably within the brainstem vestibular nuclei), leaving v-VOR responses unaffected.9 It appears VOR recovery may take weeks to months after IM followed by oral thiamine repletion.6 We report rapid improvement of the VOR gain after high-dose, IV thiamine treatment.
Vestibular findings in the pre-encephalopathy phase, despite their potential value to assist early diagnosis and enable early treatment before severe neurologic morbidity occurs, are not widely known. We sought to report our experience with 5 nonencephalopathic, thiamine-deficient patients presenting with predominantly vestibular symptoms and signs, including 2 novel clinical findings: a patient who presented with a central mimic of an acute peripheral vestibulopathy (pseudo-neuritis10) and 2 patients with dramatic and immediate resolution of h-VOR deficits in response to high-dose IV thiamine repletion.
METHODS
We conducted a retrospective chart review of 5 cases of thiamine deficiency presenting with vestibular findings to a single center (July 2008–October 2011). The study was approved by the University of Illinois College of Medicine at Peoria Institutional Review Board. All patients underwent clinical neurologic, neuro-ophthalmologic, and neurovestibular evaluation. VOR testing was performed by clinical head impulse testing in all 5, by video-nystagmography during caloric testing in 2, and by video head impulse testing11 in 1. The h-VOR response to high-dose IV thiamine was tested in 3 patients. Testing was incomplete because of the acute nature of the illness. Serum thiamine, folate, and vitamin B12 levels were measured. Brain MRI (with/without contrast enhancement) was obtained in all 5 patients within 24 hours of initial examination. Final diagnoses were confirmed by low or borderline serum thiamine levels, normal B12 levels, response to therapy, and exclusion of other diagnostic possibilities. All patients were followed for at least 3 months post treatment.
RESULTS
Patient demographic and clinical features are listed in the table. All 5 were at high risk for nutritional deficiency. No patients were taking metronidazole, which may produce a syndrome similar to Wernicke.12 All patients had normal orientation and sensorium, had a normal Mini-Mental State Examination score (30/30), and were able to provide a coherent medical history and cooperate with the neurologic examination. One presented with an acute vestibular syndrome characterized by acute, persistent, vertigo, with severe vomiting and gait ataxia for 48 hours, mimicking vestibular neuritis or stroke (case 2). The others presented with subacute, progressive symptoms dominated by postural instability (difficulty standing), gait unsteadiness, falls, and oscillopsia. Case 3 had severe lymphedema precluding examination of posture and gait.
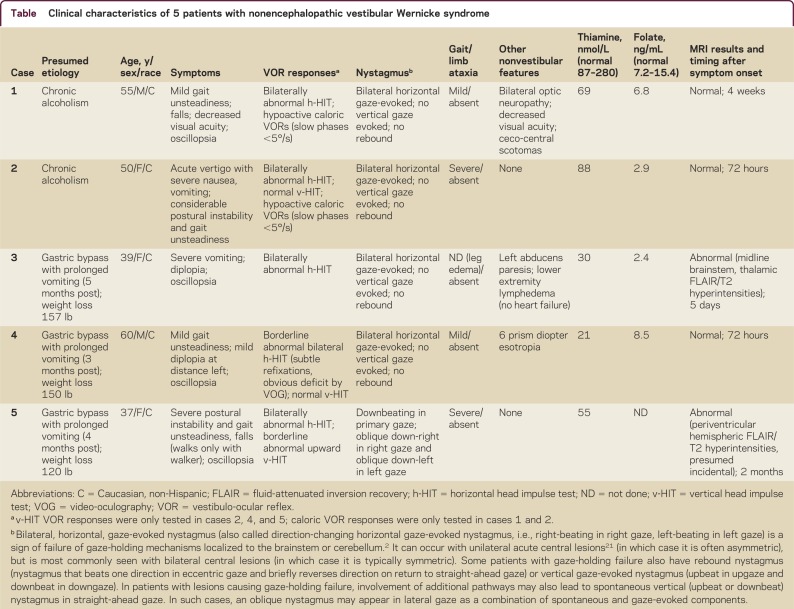
Table Clinical characteristics of 5 patients with nonencephalopathic vestibular Wernicke syndrome
Pathologic horizontal gaze-evoked nystagmus (right-beating in right gaze and left-beating in left gaze) and bilaterally abnormal horizontal head impulse tests were found in all 5 patients. In 3 where v-VOR responses were tested (cases 2, 4, 5), 2 (cases 2, 4) clearly had dissociated loss of h-VOR function with spared v-VOR function (video 1 at Neurology.org/cp). One patient (case 5) with primary gaze downbeat nystagmus appeared clinically to have decreased upward VOR gain; however, without quantitative analysis, this might simply have reflected a normal downward nystagmus fast phase. Brain MRI performed when the patients were first examined was normal in 3 patients (cases 1, 2, 4). Case 3 had areas of increased fluid-attenuated inversion recovery/T2 signal in the midline thalami, upper midbrain, and pons, consistent with Wernicke. Case 5 had nonspecific hemispheric leukoaraiosis.
We suspected thiamine deficiency in all 5 patients; 2 had a history of chronic alcoholism. Two bypass patients had a duodenal switch (case 3, 5 months, and case 4, 3 months prior to the clinical onset of thiamine deficiency). In 1 bypass patient (case 5), we had no detailed record of the specific surgical procedure performed 4 months prior to presentation. Weight loss ranged from 120 to 157 pounds. Case 3 had a 10-day history of daily, frequent vomiting due to a postprocedure stomach kink before the onset of neurologic symptoms. Case 4 had nausea and vomiting right after the procedure, which he attributed to the vitamin supplements he was prescribed, so he chose to discontinue taking the supplements. Case 5 had recurrent, frequent vomiting beginning 1 month after the procedure. We obtained baseline serum levels of thiamine in all cases and ordered a head MRI, which was obtained within 24 hours after initial evaluation in all 5 cases.
We began thiamine replacement following clinical examination. Thiamine levels were low or borderline in all 5 and folate levels were also low in 3 of 4 who were tested. Cases 1 and 2 received only oral thiamine replacement (100 mg daily). Cases 4 and 5 received an IV dose of 500 mg thiamine in 100 mL of normal saline given over a 5-minute period, followed by 100 mg/day orally. Case 3 had oral replacement followed by IV thiamine after partial improvement. Four had complete resolution of clinical findings and 1 had a partial response (case 5).
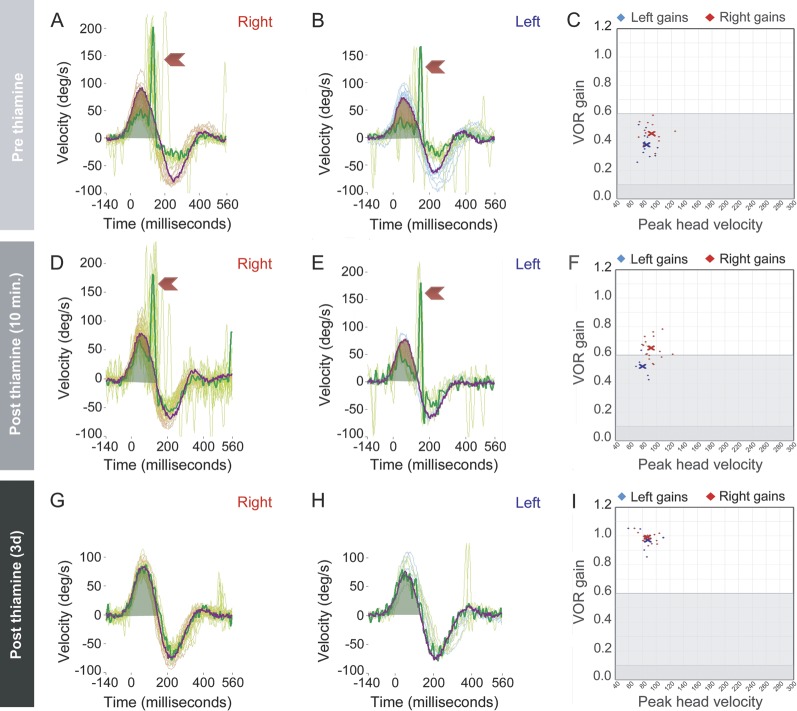
Two of 3 improved dramatically after IV thiamine. Case 3 with initial abducens palsy and abnormal MRI had partial improvement of the ophthalmoplegia after oral thiamine but remained nauseated with horizontal nystagmus and a bilaterally positive horizontal head impulse test. Her horizontal head impulse VOR normalized immediately after thiamine infusion (video 2) and she reported improvement of her symptoms in the ensuing hours. Case 4 had bilateral, horizontal gaze-evoked, direction-changing nystagmus; a subtle, 8-prism diopter esotropia in left gaze during cross-cover testing; and truncal ataxia; he also had mostly covert, corrective saccades13 (video 3, figure). Covert corrective saccades in VOR-deficient subjects occur during the head rotation in a head impulse test of the VOR, rather than after the head comes to rest; when corrective saccades are covert, clinical head impulse testing (without quantitative recording equipment) can be falsely negative.13 Following IV thiamine infusion, Case 4's quantitatively measured h-VOR improved immediately and normalized by day 3 (figure); horizontal nystagmus and truncal ataxia resolved the next morning. Case 5 did not have immediate improvement in her clinical findings with IV replacement, but improved gradually over the ensuing weeks; recovery was incomplete with residual nystagmus. In all patients, the h-VOR normalized.
Quantitative head impulse testing in a patient with nonencephalopathic vestibular Wernicke syndrome
Figure. Rows show 3 time points for a single patient (case 4) before IV thiamine (row 1), 10 minutes after IV thiamine (row 2), and 3 days after IV thiamine (row 3). Column A (panels A, D, G) shows right-sided and B (panels B, E, H) shows left-sided vestibular reflex responses. In each of these panels, multiple impulses are displayed with a single impulse highlighted. The orange trace corresponds to head velocity and the green trace to eye velocity. Green shading denotes the area under the curve of the eye trace during the impulse. Red shading (panels A, B, D, E) denotes the difference in area under the curve between the head trace and the eye trace. This red shading represents reduced vestibulo-ocular reflex (VOR) gain, also shown as abnormally low results in the VOR gain plots (panels C, F). Red chevrons in these same panels point to rapid corrective (“refixation”) saccades that are compensatory for the deficient vestibular response that fails to keep the eyes on target. Because these saccades occur during the head impulse (as opposed to after the head has come completely to rest), they are more difficult to detect clinically (compare video 3 to videos 1 and 2). These are known as “covert” corrective saccades since they occur before the head comes to rest, rather than afterwards, and are therefore hidden to the naked-eye interpretation of the head impulse VOR response. Panels G and H show no corrective saccades because VOR gains have completely normalized post treatment (panel I).
DISCUSSION
Our results suggest that thiamine deficiency should be considered in patients with nutritional deprivation and unexplained acute or subacute vestibular symptoms, even absent encephalopathy. Our series includes a rare case of a patient with Wernicke encephalopathy with an acute, 48-hour presentation closely mimicking vestibular neuritis or posterior fossa stroke.14 Early recognition is critical given that hypoglycemia and hypovolemia can also produce dizziness, vertigo, and ataxia.15 Patients seen in emergency departments may be erroneously treated with a glucose infusion or bolus, risking dangerous exacerbation of the underlying thiamine depletion.16
VOR testing is rarely performed in routine emergency practice. The head impulse test of VOR function revealed bilateral h-VOR failure in all 5 cases, presumably from direct involvement of the vestibular nuclei in the brainstem. This was true even in the case with an acute vertigo presentation; since vertigo is usually seen in patients with asymmetric vestibular lesions, we might speculate that this lesion began unilaterally and evolved to an asymmetric, bilateral lesion. Most bilateral vestibular failure is slowly progressive over months to years, including that seen in the cerebellar ataxia, neuropathy, and vestibular areflexia (CANVAS) syndrome.17,18 Acute bilateral vestibular failure, particularly without hearing loss, is rarely seen except in gentamicin and related aminoglycoside ototoxicity.19 Gentamicin damages vestibular hair cells and usually impairs the VOR in all 3 dimensions.20 Thus, loss of bilateral h-VOR function with spared v-VOR function could be a fairly specific predictor of Wernicke in patients presenting with acute vestibular symptoms, particularly those who lack recent exposure to antibiotics. Furthermore, bilateral, horizontal, gaze-evoked, direction-changing nystagmus should not accompany gentamicin toxicity, while this finding is routinely present in Wernicke (83%, 192/232).1 Further prospective study of these clinical signs is needed.
In one case where VOR abnormalities at the bedside were more difficult to detect clinically (case 4, video 3), use of a commercially available video-oculography device to measure the head impulse VOR helped identify the sign (figure). Rapid improvement of the VOR following parenteral thiamine, seen in 2 of our patients, may be a useful marker to gauge therapeutic effect and should be studied further. Quantification of VOR responses might assist in determining adequacy of thiamine repletion.
Oscillopsia likely resulted from nystagmus, bilateral VOR failure, or a combination of the two. The truncal ataxia found in our cases is likely the result of bilateral central vestibular failure in the brainstem, given the absence of a proprioceptive deficit or limb ataxia. A contribution from midline cerebellar dysfunction (due to Wernicke or alcohol cerebellopathy in cases 1 and 2) could also have been present.
Previous authors have suggested that the vestibulopathy and gaze-holding failure found in Wernicke disease is due to direct damage to the medial vestibular nuclei and nearby nucleus prepositus hypoglossi in the medulla.8 The bilaterally impaired h-VOR and bilateral gaze-holding nystagmus and the lack of overt signs of cerebellar dysfunction seen in our patients support this assertion. In particular, true gaze-holding failure cannot result from isolated peripheral vestibular dysfunction.2 Acute injury to the peripheral vestibular system produces unidirectional nystagmus that may increase with gaze in the direction of the fast phase, but should not change direction with gaze in the opposite direction14 (also see table footnote). Nevertheless, we are unaware of any neuropathologic studies of the vestibular labyrinth or nerve in Wernicke patients. Accordingly, we cannot exclude the possibility of combined central and peripheral lesions in our patients, as seen in the slowly progressive neurodegenerative syndrome known as CANVAS.18
Based on the aforementioned clinical features, we believe that our patients had bilateral brainstem lesions, most likely in the dorsal medulla and pons in the region of the medial vestibular nuclei. However, only 1 of 5 patients' images (case 3) showed subtle increased signal changes involving the typical periventricular and periaqueductal locations of the dorsomedial brainstem and diencephalon—notably not located in the region of the vestibular nuclei. Hemispheric white matter hyperintensities in case 5 were probably incidental, but did not aid diagnosis given the high prevalence of leukoaraiosis in patients with acute dizziness.21 Lack of radiologic evidence for brainstem lesions in the vestibular nuclei or 8th nerve fascicle in all 5 cases presumably indicates Wernicke neuropathology is subradiographic in these earlier/milder vestibular presentations.
Four of 5 patients recovered without neurologic sequelae, so this vestibular presentation of Wernicke may be reversible with prompt treatment. The rapid improvement probably reflects an early stage of thiamine deficiency, with normalization of neuronal metabolism after high-dose parenteral replacement. From the one case with quantitative measures, the rapid improvement within minutes was partial, rather than complete (figure). A partial response could be sufficient to help normalize the appearance of the clinical head impulse, so without quantitative recordings in the other patients who had dramatic improvement clinically (e.g., case 3, video 2), we cannot be sure that the immediate, dramatic improvement was complete. The failure of oral replacement in one patient to produce a clear benefit and the subsequent success of parenteral therapy suggests that, even in these milder (nonencephalopathic) cases, immediate IV repletion should probably be the first course of action. The benefit of high-dose IV thiamine in this clinical setting is probably both therapeutic and diagnostic, given that, absent encephalopathy, the diagnosis might remain uncertain pending delayed return of thiamine levels. This diagnostic role of IV therapy is particularly relevant given that MRI may be normal or nondiagnostic in these patients.
The frequent co-occurrence of folate deficiency without B12 deficiency or anemia in our series is interesting but of unclear significance. Folate deficiency can contribute to thiamine deficiency by reducing intestinal absorption.22 Whether folate deficiency was partly responsible for the clinical phenotype (e.g., optic neuropathy23 in case 1) is unknown, because optic neuropathy has been reported as a consequence of thiamine deficiency absent folate deficiency.24 Measuring folate levels and insuring adequate repletion may be important diagnostic and therapeutic steps in this clinical context.
Based on these findings and discussion, patients with thiamine deficiency may present with predominantly vestibular symptoms and signs without encephalopathy. Head impulse VOR responses in these patients could be an important bedside marker for diagnosis, response to therapy, or prognosis. A pattern of bilateral vestibular failure (especially dissociated h-VOR loss) plus bilateral, horizontal, gaze-evoked, direction-changing nystagmus may be a helpful sign to identify Wernicke syndrome in patients with acute dizziness, vertigo, or ataxia. Further prospective study of these signs in larger patient samples is warranted. Frontline providers evaluating patients with acute vestibular syndrome or acute ataxia should consider the diagnosis of thiamine deficiency; in cases with a high index of suspicion for malnutrition, empiric parenteral thiamine may be indicated.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
J.C. Kattah has served as a consultant for Biogen, Bayer, and Questcor and his laboratory records the head impulse test (HIT) with a Device Unit loaned by Otometrics Incorporated. S.S. Dhanani reports no disclosures. J. H. Pula has served as a consultant for Bayer, Biogen, Questcor, and TEVA. G. Mantokoudis receives research support from the Swiss National Science Foundation. A.S. Saber Therani reports no disclosures. D.E. Newman-Toker has received honoraria for travel, speaking, or both from Society to Improve Diagnosis in Medicine, Korean Balance Society, Neuro-otology Society of Australian, and Janssen Pharmaceuticals; received payment from Lammico for recording CME video about dizziness and stroke misdiagnosis; receives research support from AHRQ; and GN Otometrics has loaned his team the ICS Impulse video-oculography device for research purposes. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/01.CPJ.0000435749.32868.91.
ACKNOWLEDGMENT
The authors thank David S. Zee, MD, Johns Hopkins University School of Medicine, for critical review of the manuscript.
Supplementary Material
Correspondence to: kattahJ@uic.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/01.CPJ.0000435749.32868.91.
Footnotes
Supplemental Data: neurology.org/cp
Correspondence to: kattahJ@uic.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/01.CPJ.0000435749.32868.91.
REFERENCES
- 1.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome: a clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. 1971;7:1–206. [PubMed] [Google Scholar]
- 2.Leigh RJ, Zee DS. The Neurology of Eye Movements, 4th ed. New York: Oxford University Press; 2006.
- 3.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 4.Halmagyi GM, Aw ST, Cremer PD, Curthoys IS, Todd MJ. Impulsive testing of individual semicircular canal function. Ann NY Acad Sci. 2001;942:192–200. doi: 10.1111/j.1749-6632.2001.tb03745.x. [DOI] [PubMed] [Google Scholar]
- 5.Cogan DG, Witt ED, Goldman-Rakic PS. Ocular signs in thiamine-deficient monkeys and in Wernicke's disease in humans. Arch Ophthalmol. 1985;103:1212–1220. doi: 10.1001/archopht.1985.01050080124032. [DOI] [PubMed] [Google Scholar]
- 6.Ghez C. Vestibular paresis: a clinical feature of Wernicke's disease. J Neurol Neurosurg Psychiatry. 1969;32:134–139. doi: 10.1136/jnnp.32.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goor C, Endtz LJ, Muller Kobold MJ. Electro-nystagmography for the diagnosis of vestibular dysfunction in the Wernicke-Korsakow syndrome. Clin Neurol Neurosurg. 1975;78:112–117. doi: 10.1016/s0303-8467(75)80018-7. [DOI] [PubMed] [Google Scholar]
- 8.Furman JM, Becker JT. Vestibular responses in Wernicke's encephalopathy. Ann Neurol. 1989;26:669–674. doi: 10.1002/ana.410260513. [DOI] [PubMed] [Google Scholar]
- 9.Choi KD, Oh SY, Kim HJ, Kim JS. The vestibulo-ocular reflexes during head impulse in Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 2007;78:1161–1162. doi: 10.1136/jnnp.2007.121061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cnyrim CD, Newman-Toker D, Karch C, Brandt T, Strupp M. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis.”. J Neurol Neurosurg Psychiatry. 2008;79:458–460. doi: 10.1136/jnnp.2007.123596. [DOI] [PubMed] [Google Scholar]
- 11.Weber KP, MacDougall HG, Halmagyi GM, Curthoys IS. Impulsive testing of semicircular-canal function using video-oculography. Ann NY Acad Sci. 2009;1164:486–491. doi: 10.1111/j.1749-6632.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 12.Zuccoli G, Pipitone N, Santa Cruz D. Metronidazole-induced and Wernicke encephalopathy: two different entities sharing the same metabolic pathway? AJNR Am J Neuroradiol. 2008;29:E84. doi: 10.3174/ajnr.A1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology. 2008;70:454–463. doi: 10.1212/01.wnl.0000299117.48935.2e. [DOI] [PubMed] [Google Scholar]
- 14.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. 2011;183:E571–E592. doi: 10.1503/cmaj.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malouf R, Brust JC. Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol. 1985;17:421–430. doi: 10.1002/ana.410170502. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran TB, O'Hare B, Phelan D. Shoshin beri-beri precipitated by intravenous glucose. Crit Care Resusc. 2002;4:31–34. [PubMed] [Google Scholar]
- 17.Zingler VC, Cnyrim C, Jahn K. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann Neurol. 2007;61:524–532. doi: 10.1002/ana.21105. [DOI] [PubMed] [Google Scholar]
- 18.Szmulewicz DJ, Waterston JA, MacDougall HG. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis. Ann NY Acad Sci. 2011;1233:139–147. doi: 10.1111/j.1749-6632.2011.06158.x. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed RM, Hannigan IP, MacDougall HG, Chan RC, Halmagyi GM. Gentamicin ototoxicity: a 23-year selected case series of 103 patients. Med J Aust. 2012;196:701–704. doi: 10.5694/mja11.10850. [DOI] [PubMed] [Google Scholar]
- 20.Carey JP, Minor LB, Peng GC, la Santina CC, Cremer PD, Haslwanter T. Changes in the three-dimensional angular vestibulo-ocular reflex following intratympanic gentamicin for Meniere's disease. J Assoc Res Otolaryngol. 2002;3:430–443. doi: 10.1007/s101620010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40:3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyumpa AM. Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr. 1980;33:2750–2761. doi: 10.1093/ajcn/33.12.2750. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CT, Miller NR, Wray ML. Optic neuropathy from folic acid deficiency without alcohol abuse. Ophthalmologica. 2002;216:65–67. doi: 10.1159/000048300. [DOI] [PubMed] [Google Scholar]
- 24.Spinazzi M, Angelini C, Patrini C. Subacute sensory ataxia and optic neuropathy with thiamine deficiency. Nat Rev Neurol. 2010;6:288–293. doi: 10.1038/nrneurol.2010.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.