Abstract
Kidney paired donation (KPD) can facilitate living donor transplantation for candidates with an incompatible donor, but requires waiting for a match while suffering the morbidity of dialysis. The balance between waiting for KPD versus desensitization or deceased donor transplantation relies on the ability to estimate KPD wait times. We studied donor/candidate pairs in the National Kidney Registry (NKR), a large multi-center KPD clearinghouse, between 10/2011-9/2015 using a competing risk framework. Among 1894 candidates, 52% were male, median age was 50 years, 66% were white, 59% had blood type O, 42% had PRA>80, and 50% obtained KPD through NKR. Median times to KPD ranged from 2 months for candidates with ABO-A and PRA 0, to over a year for candidates with ABO-O or PRA 98+. Candidates with PRA 80-97 and 98+ were 23% (95% CI: 6-37%) and 83% (78-87%) less likely to be matched than PRA 0 candidates. ABO-O candidates were 67% (61-73%) less likely to be matched than ABO-A candidates. Candidates with ABO-B or ABO-O donors were 31% (10-56%) and 118% (82-162%) more likely to match than those with ABO-A donors. Providers should counsel candidates about realistic, individualized expectations for KPD, especially in the context of their alternative treatment options.
INTRODUCTION
Kidney paired donation (KPD) can improve compatibility and facilitate transplantation between incompatible living donor/candidate pairs (1–5). However, there are other options for these candidates, including desensitization (6–9) as well as awaiting deceased donor kidney transplantation (DDKT). Although the Kidney Allocation System increased access to DDKT for highly sensitized candidates (10), waitlist mortality remains high, and some phenotypes do not benefit from the new allocation priorities (11). With these considerations in play, it is critical for providers and candidates to understand wait times for KPD so they can make informed treatment decisions.
Prior reports of wait times in small KPD programs suggested that their benefit may be limited to candidates with easy-to-match blood types and antibody profiles (12, 13). These findings were reported before the establishment of large multicenter KPD registries that have expanded rapidly in the past decade due to evidence that the transport of living donor kidneys is safe and feasible (14, 15). For example, the National Kidney Registry (NKR), the largest KPD registry in the US by volume, has facilitated over 2000 KPD matches since its inception in 2008, and continues to facilitate more matches each year (16, 17). Unfortunately, the only estimates of individual wait times in large KPD programs came from simulations performed by our group that suggested that almost all patients with panel-reactive antibody (PRA) <80% would match within a few months, but those with PRA >80 or blood type O would experience significantly longer wait times (18). However, these simulations were based on many assumptions, including an older paradigm of KPD (19). More recent reports suggest the landscape of living donor kidney transplantation has evolved differently than our simulations assumed: willingness to participate in KPD differs by race (20), and there is evidence of survival benefit of desensitization and incompatible transplantation as compared to remaining on the waitlist (8, 9). Thus, an up-to-date estimate of wait times in the current environment of KPD, based on observational data rather than simulation, is essential.
Using NKR data, we studied time to KPD for candidates and their paired donors, stratified by ABO blood type and PRA. To describe likelihood of KPD match, we modeled time to KPD in a competing risk framework accounting for alternative outcomes candidates faced while waiting in the registry, such as DDKT or death.
METHODS
Study Population
We studied kidney transplant candidate/potential donor pairs who registered in the National Kidney Registry (NKR) between October 2011 and September 2015. The registering potential donor (who entered the NKR with the candidate, but was incompatible with the candidate for direct donation) is referred to as a “paired donor” or “donor” for simplicity. Only 139 (7.3%) candidates registered with more than one potential donor. For those donors who underwent KPD within the NKR, the donor who participated in KPD was the donor listed. For other candidates, we listed the primary donor registered with the candidate. Recipients in a KPD chain who received a kidney facilitated by the chain without having their own incompatible donor (i.e. the last recipient in the chain who was drawn from the deceased donor waiting list and was never awaiting a match in KPD) were excluded. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Outcomes after Registration
Our primary outcome was KPD transplantation facilitated by NKR. We treated other removals from the NKR as competing events. Reasons for removal included: (1) identifying a compatible living donor outside of KPD, (2) transplantation via another KPD registry, (3) DDKT, (4) unavailability of incompatible paired donor (i.e. donor candidate no longer willing or able to donate), (5) candidate ineligibility (death or deteriorating condition) and (6) other/unspecified reason for removal.
Time to KPD
We defined time to KPD as the time from NKR registration to the recipient’s transplant date. Candidates who survived free of the defined outcomes were administratively censored on September 30, 2015 and were considered to be still waiting in the NKR. We used Kaplan-Meier curves to describe time to KPD within strata of candidate and donor ABO and candidate PRA categories. We used competing risk analysis as described by Fine and Gray to model likelihood of KPD transplant (21). This method allows modeling adjusted subhazard ratios within the subdistribution of the outcome of KPD transplant, with other outcomes considered competing events.
Statistical Analysis
Adjusted subhazard ratio (aSHR) confidence intervals are reported per the method of Louis and Zeger (22). We used a two-sided α of 0.05 to indicate a statistically significant difference. Statistical analysis was performed using Stata 14.2/MP for Linux (College Station, Texas). Figures were prepared using R 3.4 (the R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
During the study period, 1894 candidate/donor pairs registered in the NKR. The median (IQR) age of candidates was 50 years (39–60), 48% were female, and the median (IQR) body mass index (BMI) was 27 (23–31). The majority were Caucasian (66%) and 59% had blood type O. Candidate PRA ranged from 0%-100%; 42% had a PRA>80% and 79 (4.2%) reported PRA of 100%. Donors had a median (IQR) age of 45 years (35–53) and were predominantly female (62%) and ABO-A (47%, Table 1).
Table 1.
Characteristics of NKR registrants
| Candidate | Donor | |
|---|---|---|
| Age at registration, med (IQR) years | 50 (39-60) | 45 (35-53) |
| Female | 898 (48%) | 1168 (62%) |
| BMI, med (IQR) | 27 (23-31) | 26 (24-29) |
| Race | ||
| Caucasian | 1241 (66%) | 1310 (70%) |
| Black | 272 (14%) | 232 (12%) |
| Asian | 92 (5%) | 78 (4%) |
| Latino | 200 (11%) | 185 (10%) |
| Other | 79 (4%) | 79 (4%) |
| ABO | ||
| A | 450 (24%) | 888 (47%) |
| B | 278 (15%) | 333 (18%) |
| AB | 40 (2%) | 98 (5%) |
| O | 1126 (59%) | 573 (30%) |
| PRA | ||
| 0 | 524 (29%) | – |
| 1-79 | 528 (29%) | – |
| 80-97 | 323 (18%) | – |
| 98+ | 432 (24%) | – |
Outcomes after Registration
Among the 1894 candidates, 956 (50%) were eventually transplanted in an NKR-facilitated KPD, 102 (5.4%) were transplanted via another KPD registry, 178 (9.4%) underwent DDKT, 138 (7.3%) found a non-NKR mediated compatible living donor, 76 (4.0%) were removed due to donor ineligibility or unavailability, 129 (6.8%) were removed for other or unknown reasons, 90 (4.8%) died or were too sick for transplant, and 225 (12%) remained in the NKR at the end of the study (Figure 1). In all, 72.5% of the registered candidates ultimately received a kidney transplant during our study period.
Figure 1.
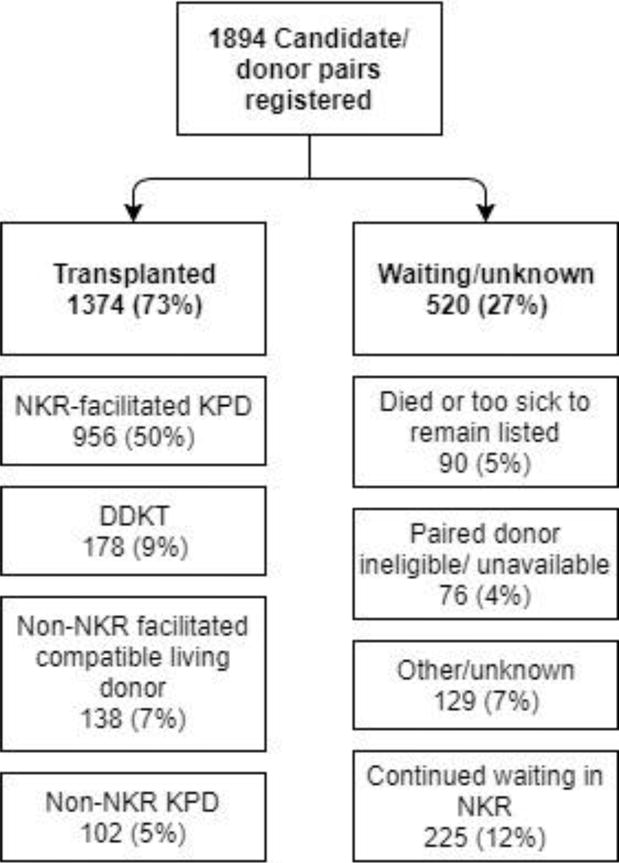
Candidate registry removal reasons
Of the 956 NKR-facilitated KPD matches, 25 (2.6%) were ABO incompatible. Of these, 8 were A2 to O, 1 A1 to O, 8 A to O without A subtyping available, 2 A2B to B, 1 AB to A, 1 AB to B without A subtyping available, 3 B to O, and 1 B to A.
Likelihood of NKR-facilitated KPD
Matching into a KPD transplant was the most frequent outcome among NKR registrants. At 3, 6, and 12 months, 32%, 38%, and 46% of candidates underwent NKR-facilitated KPD (Figure 2). More recent registration was also associated with a 17% increased chance of NKR KPD per year (aSHR: 1.101.171.24, p<0.001, Table 2). Each decade of candidate age was associated with a 5% increased chance of NKR KPD (1.0021.051.10, p=0.04). Relative to candidates with a PRA of 0%, candidates with a PRA between 80-97% had a 23% lower chance of matching into an NKR KPD (0.630.770.94, p=0.01), and candidates with PRA >97% had an 83% lower chance of NKR KPD (0.130.170.22, p<0.001, Table 2). Candidates with ABO O had a 67% lower chance of matching relative to ABO A candidates (0.270.330.39, p<0.001). Conversely, candidates whose donor had ABO blood type B or ABO O had a 31% and 118% higher chance of matching respectively (ABO B: 1.101.311.56, p<0.01; ABO O: 1.822.182.62, p<0.001, Table 2).
Figure 2. Stacked Plot of NKR Registrant Candidate Outcomes.
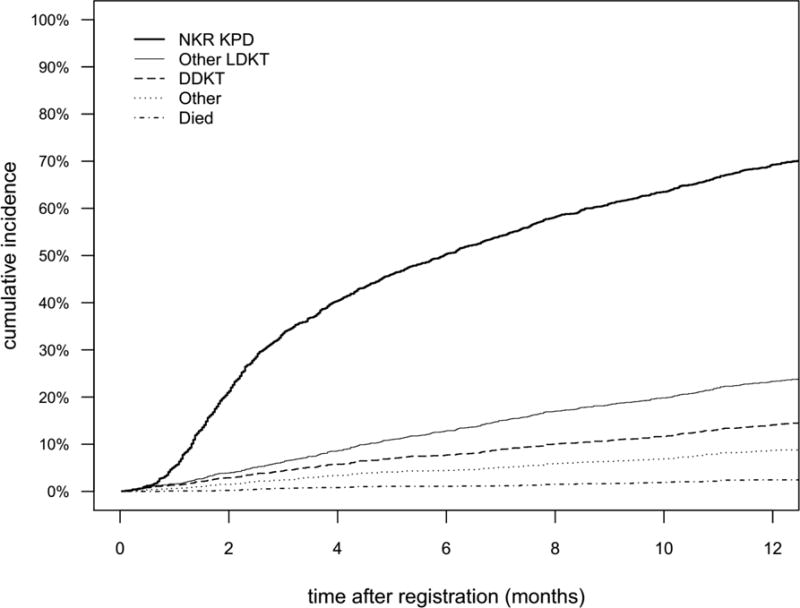
Other includes: other, unknown, and unavailable donor; Other LDKT includes other KPD and found compatible donor; Died includes too sick. The numbers in the graph represent the percentage of registrants who experienced that outcome at that time. For example, at 1 year, 46% of registrants were matched into an NKR KPD, and 9.2% received another LDKT, etc.
Table 2.
Factors associated with matching into a KPD through NKR
| Adjusted Subhazard Ratio | p-value | |
|---|---|---|
| Year of registration |
|
<0.001 |
| Candidate age (per 10 years) |
|
0.04 |
| Candidate PRA | ||
| 0 | 1 (ref) | |
| 1-79 |
|
0.08 |
| 80-97 |
|
0.01 |
| 98+ |
|
<0.001 |
| Candidate ABO | ||
| A | 1 (ref) | |
| B |
|
0.5 |
| AB |
|
0.6 |
| O |
|
<0.001 |
| Donor ABO | ||
| A | 1 (ref) | |
| B |
|
<0.01 |
| AB |
|
0.1 |
| O |
|
<0.001 |
Time to NKR-facilitated KPD
Median times to KPD ranged from 2 months for candidates with ABO type A and PRA 0 to over a year for candidates with blood type O or PRA 98+ (Figure 3). With respect to donor ABO type and candidate PRA, median times to KPD ranged from 2.5 months for candidates with PRA 0 and a donor with blood type AB, to over a year for candidates with PRA 98+ (Figure 4). With respect to candidate and donor ABO types, median times to KPD ranged from 2.3 months for candidates with blood type A and donors with blood type AB to over a year for candidates with blood type O (Figure 5).
Figure 3. Cumulative Incidence and Median Time to KPD in NKR by Candidate ABO Type and PRA.
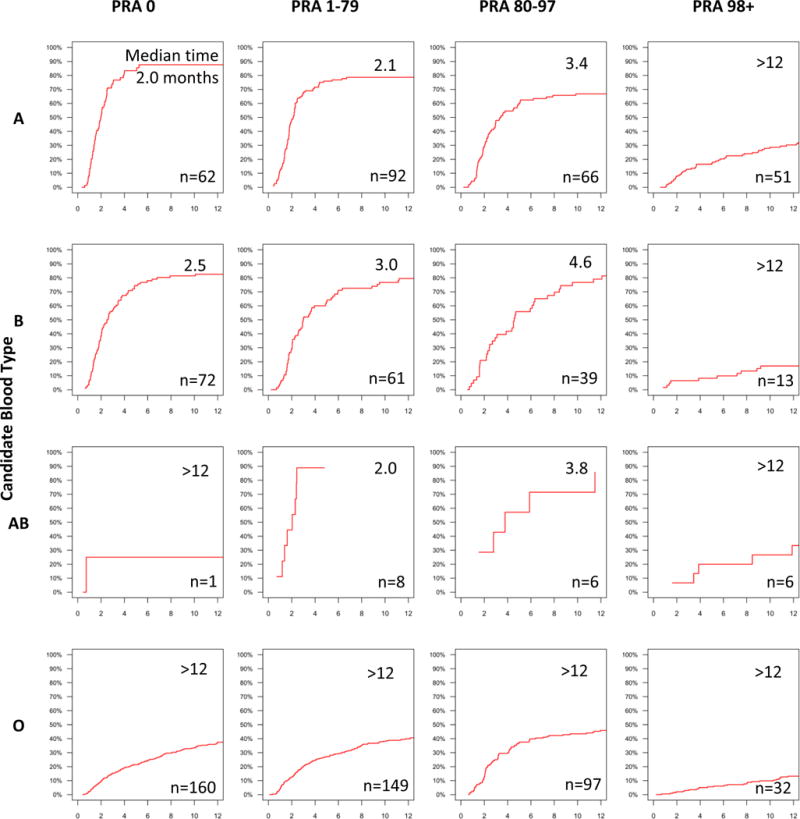
Median times to KPD, in months, and number of KPDs are shown within each cumulative incidence curve pane.
Figure 4. Cumulative Incidence and Median Time to KPD in NKR by Paired Donor ABO Type and Candidate PRA.
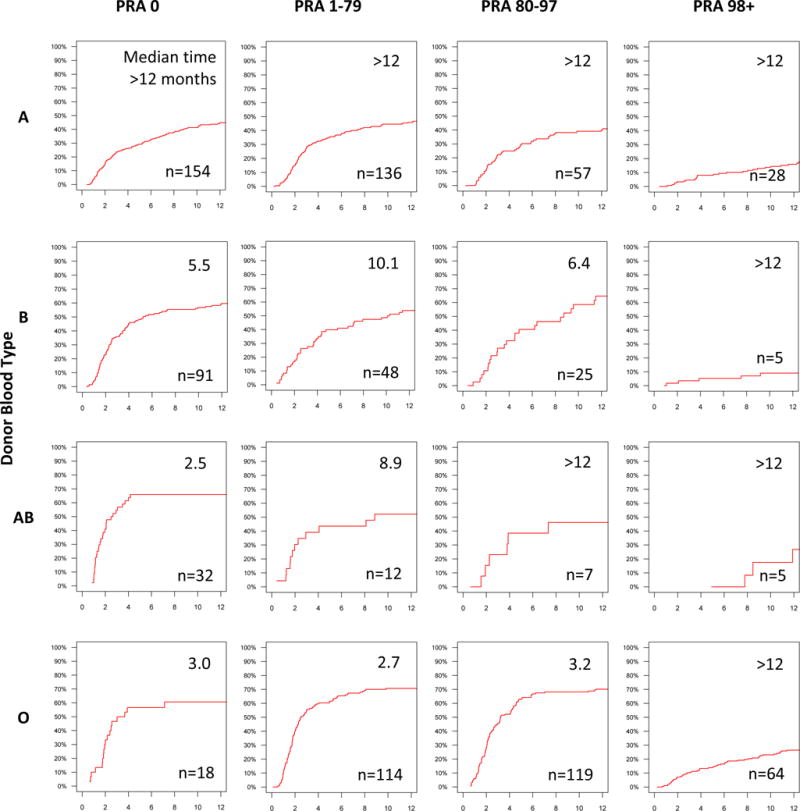
Median times to KPD, in months, and number of KPDs are shown within each cumulative incidence curve pane.
Figure 5. Cumulative Incidence and Median Time to KPD in NKR by Candidate and Donor ABO Type.
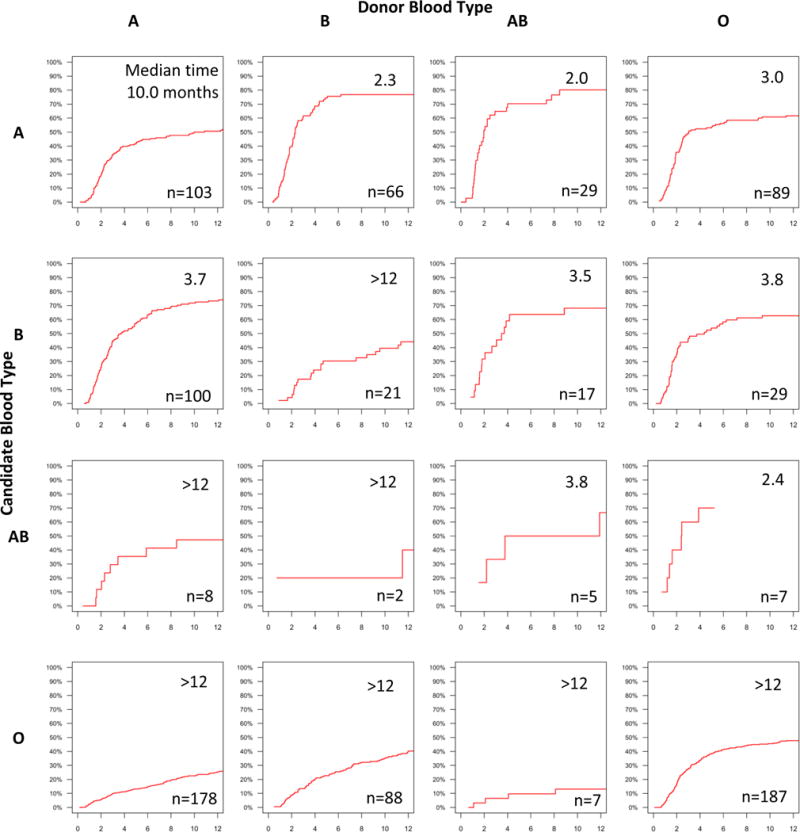
Median times to KPD, in months, and number of KPDs are shown within each cumulative incidence curve pane.
Time to Removal from the NKR for DDKT or Other KPD Match
For the 178 (9.4%) candidates who left the registry due to DDKT, the median (IQR) time to removal from the registry was 10.3 months (4.3-20.0). For the 102 (5.4%) candidates who left the registry due to matching in another KPD program, the median (IQR) time to removal from the registry was 8.3 months (4.9-18.4).
DISCUSSION
In this study of almost 2000 candidate-donor pairs in the largest multicenter KPD clearinghouse in the United States, we found that 50% received an NKR-facilitated KPD transplant, 22% received a living donor or deceased donor kidney transplant outside of NKR, and 5% died waiting. Candidates with PRA 0 and blood type A experienced the shortest median wait time to KPD at 2 months, while candidates with blood type O, PRA 98+, and those with donors with blood type A all experienced median wait times of more than a year.
The general trends in wait times identified in this observational study are consistent with the trends described by our early simulation-based estimates: candidates with higher PRA and candidates with blood type O experienced longer wait times to KPD match (18). However, these new observational data suggest that KPD wait time is more varied than we previously simulated: more than a third of registered candidates left the NKR for alternate reasons that were not KPD. Prior simulations either could not account for these competing risks, or had to assume that non-KPD facilitated transplants were solely due to failure to match (18, 23). Furthermore, there is variability in living donor quality which might affect acceptance of matches in KPD programs (24), and these results provide insight into matches which were accepted and proceeded to transplantation. The benefit of these new real-world observational data over prior simulations is the ability to capture behavior, real compatibility testing, and the contribution of each of the competing risks experienced by candidates.
Some limitations of our study warrant further discussion. First, although our study analyzed the largest KPD registry in the US, KPD, in general, remains underutilized (1, 12, 25), and therefore these wait times and outcomes may not be applicable to future KPD opportunities if KPD expands substantially. As these data were reported by centers registering their candidates and paired donors as part of an administrative database, we do not have additional clinical granularity beyond the covariates reported, although the data available to us are considered highly reliable in that they were required to facilitate KPD matches for the patients; similarly, we were not limited by any missing values of those covariates we report, as registration to the NKR system cannot be completed until all fields are entered.
In conclusion, we have characterized the time to KPD in the largest multicenter KPD clearinghouse in the US. We have also identified donor and candidate characteristics associated with longer time to KPD, particularly candidate blood type O, PRA 98+ and donor blood type A. Finally, we explored the variety of other reasons that candidate-donor pairs left the registry. These observational data offer insight into the likelihood and timeline of KPD match, facilitating informed dialogue between providers and individual candidates about KPD and the candidate’s specific options, which might include incompatible transplantation.
Acknowledgments
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the US Government. Funding for this study was provided in part by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), the American College of Surgeons, and the National Kidney Registry (NKR). This study was supported by grants number F32DK109662 (PI: Holscher), F32DK113719 (PI: Jackson), F32AG053025 (PI: Haugen), F32DK405600 (PI: DiBrito), K01DK101677 (PI: Massie), R01DK098431 (PI: Segev), and K24DK101828 (PI: Segev) from the NIDDK. Dr. Holscher is supported by the American College of Surgeons Resident Research Scholarship.
Abbreviations
- aSHR
adjusted subhazard ratio
- BMI
body mass index
- DDKT
deceased donor kidney transplant
- IQR
interquartile range
- KPD
kidney paired donation
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NKR
National Kidney Registry
- PRA
panel reactive antibody
Footnotes
DR COURTENAY M HOLSCHER (Orcid ID: 0000-0002-5808-5954)
MR. ALVIN G. THOMAS (Orcid ID: 0000-0003-4911-8192)
DR CHRISTINE HAUGEN (Orcid ID: 0000-0001-5884-2604)
DR SOMMER E. GENTRY (Orcid ID: 0000-0003-4530-8917)
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Gentry SE, S D, Simmerling M, Montgomery RA. Expanding kidney paired donation through participation by compatible pairs. Am J Transplant. 2007;7(10):2361–2370. doi: 10.1111/j.1600-6143.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 2.Gentry SE, M R, Swihart BJ, Segev DL. The roles of dominos and nonsimultaneous chains in kidney paired donation. Am J Transplant. 2009;9(6):1330–1336. doi: 10.1111/j.1600-6143.2009.02622.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari P, C L, Ta J, Woodroffe C, DʼOrsogna L, Holdsworth R. Providing Better-Matched Donors for HLA Mismatched Compatible Pairs Through Kidney Paired Donation. Transplantation. 2016 doi: 10.1097/TP.0000000000001196. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RA, Z A, Ratner LE, Segev DL, Hiller JM, Houp J, Cooper M, Kavoussi L, Jarrett T, Burdick J, Maley WR, Melancon JK, Kozlowski T, Simpkins CE, Phillips M, Desai A, Collins V, Reeb B, Kraus E, Rabb H, Leffell MS, Warren DS. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA. 2005;294(13):1655–1663. doi: 10.1001/jama.294.13.1655. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari P, H P, Cohney SJ, Woodroffe C, Fidler S, D’Orsogna L. ABO-incompatible matching significantly enhances transplant rates in kidney paired donation. Transplantation. 2013;96(9):821–826. doi: 10.1097/TP.0b013e3182a01311. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. The New England journal of medicine. 2011;365(4):318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 7.Segev DL, Simpkins CE, Warren DS, King KE, Shirey RS, Maley WR, et al. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5(10):2570–2575. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 8.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. The New England journal of medicine. 2016;374(10):940–950. doi: 10.1056/NEJMoa1508380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14(7):1573–1580. doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 10.Massie AB, Luo X, Lonze BE, Desai NM, Bingaman AW, Cooper M, et al. Early Changes in Kidney Distribution under the New Allocation System. Journal of the American Society of Nephrology: JASN. 2016;27(8):2495–2501. doi: 10.1681/ASN.2015080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant. 2017;17(Suppl 1):21–116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segev DL, K L, Gentry SE, Montgomery RA. Utilization and outcomes of kidney paired donation in the United States. Transplantation. 2008;86(4):502–510. doi: 10.1097/TP.0b013e3181812f85. [DOI] [PubMed] [Google Scholar]
- 13.Roodnat JI, vd W J, Claas FH, Ijzermans J, Weimar W. Persistently low transplantation rate of ABO blood type O and highly sensitised patients despite alternative transplantation programs. Transpl Int. 2012;25(9):987–993. doi: 10.1111/j.1432-2277.2012.01526.x. [DOI] [PubMed] [Google Scholar]
- 14.Segev DL, V J, Berger JC, Hiller JM, Hanto RL, Leeser DB, Geffner SR, Shenoy S, Bry WI, Katznelson S, Melcher ML, Rees MA, Samara EN, Israni AK, Cooper M, Montgomery RJ, Malinzak L, Whiting J, Baran D, Tchervenkov JI, Roberts JP, Rogers J, Axelrod DA, Simpkins CE, Montgomery RA. Transporting live donor kidneys for kidney paired donation: initial national results. Am J Transplant. 2011;11(2):356–360. doi: 10.1111/j.1600-6143.2010.03386.x. [DOI] [PubMed] [Google Scholar]
- 15.Allen R, P H, Clayton PA, Woodroffe C, Ferrari P. Outcomes of kidney paired donation transplants in relation to shipping and cold ischaemia time. Transpl Int. 2016;29(4):425–431. doi: 10.1111/tri.12719. [DOI] [PubMed] [Google Scholar]
- 16.Smith GC, T T, Kerr PG, Chadban SJ. Prospective psychosocial monitoring of living kidney donors using the Short Form-36 health survey: results at 12 months. Transplantation. 2004;78(9):1384–1389. doi: 10.1097/01.tp.0000140967.34029.f1. [DOI] [PubMed] [Google Scholar]
- 17.Veale J, H G. The National Kidney Registry: transplant chains–beyond paired kidney donation. Clin Transpl. 2009:253–264. [PubMed] [Google Scholar]
- 18.Segev DL, G S, Melancon JK, Montgomery RA. Characterization of waiting times in a simulation of kidney paired donation. Am J Transplant. 2005;5(10):2448–2455. doi: 10.1111/j.1600-6143.2005.01048.x. [DOI] [PubMed] [Google Scholar]
- 19.Massie AB, G S, Montgomery RA, Bingaman AA, Segev DL. Center-level utilization of kidney paired donation. Am J Transplant. 2013;13(5):1317–1322. doi: 10.1111/ajt.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segev DL, P N, Troll MU, Wang NY, Montgomery RA, Boulware LE. Willingness of the United States general public to participate in kidney paired donation. Clin Transplant. 2012;26(5):714–721. doi: 10.1111/j.1399-0012.2012.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94(446):496–509. [Google Scholar]
- 22.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics (Oxford, England) 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray M, W W, Song PX, Leichtman AB, Rees MA, Ashby VB, Eikstadt R, Goulding A, Kalbfleisch JD. Planning for Uncertainty and Fallbacks Can Increase the Number of Transplants in a Kidney-Paired Donation Program. Am J Transplant. 2015;15(10):2636–2645. doi: 10.1111/ajt.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massie AB, Leanza J, Fahmy LM, Chow EK, Desai NM, Luo X, et al. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant. 2016;16(7):2077–2084. doi: 10.1111/ajt.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melcher ML, B C, Baxter-Lowe LA, Delmonico FL, Gentry SE, Leishman R, Knoll GA, Leffell MS, Leichtman AB, Mast DA, Nickerson PW, Reed EF, Rees MA, Rodrigue JR, Segev DL, Serur D, Tullius SG, Zavala EY, Feng S. Dynamic challenges inhibiting optimal adoption of kidney paired donation: findings of a consensus conference. Am J Transplant. 2013;13(4):851–860. doi: 10.1111/ajt.12140. [DOI] [PubMed] [Google Scholar]


