Abstract
In vitro skin permeation studies are commonly used in the risk assessment of toxic compound skin exposure. The present study examined the utility of transepidermal water loss (TEWL) and electrical conductance as barrier integrity tests before skin permeation studies in vitro using a large number of skin samples and fentanyl. TEWL and conductance of the skin samples were measured before the permeation experiments in Franz diffusion cells in vitro with a vapometer and low voltage application, respectively. The data were analyzed based on the in vitro permeation results and in vivo skin absorption information from the transdermal fentanyl product labels. The results showed poor correlations between TEWL and electrical conductance for the skin samples. Weak correlations between fentanyl delivery rate (flux × area) and TEWL and skin conductance were observed. For comparison, TEWL and conductance were also examined after skin perturbation with a syringe needle, and both TEWL and conductance values of the skin samples increased after the perturbation. The data suggest that either TEWL of 10 g/m2/h or skin conductance of 0.07 mS/cm2 can be used as exclusion criteria in skin integrity testing to remove skin samples with high permeabilities under the in vitro conditions studied.
Keywords: Skin, Skin integrity test, Skin permeation, Transepidermal water loss (TEWL), Skin electrical resistance
1. Introduction
Skin absorption is commonly evaluated in diffusion cell experiments in vitro. In these experiments, the skin is maintained between the donor and receptor diffusion chambers, and the permeation of a chemical from the donor chamber into and across the skin is measured. These skin permeation studies provide valuable information in risk assessment related to percutaneous absorption after skin exposure to hazardous chemicals (Flynn, 1990; Frasch et al., 2014), development of topical and transdermal formulations (Nicoli et al., 2006; Roy et al., 1996), evaluation and comparison of dermatological products (Bassani and Banov, 2016; Olivier et al., 2003), assessment of new topical and transdermal drug delivery technologies (Li et al., 2001; Tezel et al., 2004; Xu et al., 2009), and identification of the mechanisms of skin formulations such as permeation enhancers (Chantasart and Li, 2012; Charoenputtakun et al., 2015). These studies can also be used for quality control in product manufacturing (USP725, 2009) and bioequivalence assessment (FDA, 2016; Franz et al., 2009; Lehman and Franz, 2014).
To study skin absorption, human skin can be obtained from surgical procedures or cadavers. Mishandling of the skin samples during and after excision could lead to skin damage, which can compromise the skin permeation studies. Therefore, a skin barrier integrity test is usually performed before these skin absorption experiments to validate the integrity of skin samples and to avoid the use of damaged skin. The parameters commonly used in skin integrity tests include tritiated water permeation, skin conductivity (or transepidermal electrical resistance), and transepidermal water loss (TEWL). In the tritiated water permeation test, skin integrity is measured based on the permeability of skin to tritiated water by applying a small volume of tritiated water in the donor chamber of the diffusion cells. For example, skin samples with water permeation values > 1.6 μL/cm2 in 30 min are discarded (Franz et al., 2009). In the skin conductivity test, the electrical resistance of the skin is measured by the application of a small electrical potential across the skin sample. It has been suggested that skin conductivity can provide a rapid assessment of skin integrity (Davies et al., 2004; Fasano et al., 2002). Skin samples disrupted physically (iontophoresis and ultrasound) and chemically were also found to have lower electrical resistance than those before the disruption (Kopecna et al., 2017; Li et al., 1998; Song et al., 2002; Tang et al., 2001). In addition to tritiated water permeation and skin conductivity, TEWL has been used in skin integrity testing. Although TEWL is an established technique to evaluate the general barrier function of skin in vivo (Myer and Maibach, 2013), TEWL is not a well-accepted skin integrity test in diffusion cell experiments in vitro because the validity of this method to predict skin permeability is unclear. For example, the relationship between TEWL and skin permeability of tritiated water was previously investigated, and no correlation was observed between TEWL and water permeability under the conditions in the study (Chilcott et al., 2002).
In a recent study, skin permeation of fentanyl from transdermal delivery systems (TDS) was investigated with > 200 skin samples (Zhang, 2017). Both TEWL and skin conductance were used as the skin integrity tests. This large set of skin integrity test data have provided the opportunity to examine the effectiveness of TEWL and electrical conductance as skin integrity tests for in vitro skin permeation experiments. In addition, previous studies of skin integrity tests lacked in vivo human flux (or delivery rate) data for comparison. In the present study, in vivo human skin absorption information from the TDS product labels was available (50 μg/h fentanyl) to provide a reference point to evaluate these integrity tests. The objectives of the present study were to: (a) evaluate a possible relationship between skin integrity test parameters TEWL and skin electrical conductance, (b) examine the relationships between fentanyl flux (or delivery rate) and TEWL and skin conductance as barrier integrity tests, and (c) investigate the exclusion criteria of TEWL and skin conductance in skin integrity testing for the absorption of fentanyl from TDS as the model drug.
2. Experimental
2.1. Materials
Phosphate buffered saline (PBS) of pH 7.4 (consisting of 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride) was prepared by dissolving PBS tablets (MP Biomedicals, Solon, OH) in deionized water. Sodium azide (Acros Organics, Morris Plains, NJ) was added to PBS at 0.02% as bacteriostat. Fentanyl was the test drug and TDS used in the present study. Fentanyl TDS were Duragesic 50 μg/h (Janssen, Titusville, NJ) manufactured by Alza Corp, fentanyl transdermal system 50 μg/h from Apotex Corp (Weston, Florida) manufactured by Aveva Drug Delivery Systems, and fentanyl transdermal system 50 μg/h from Mylan (Canonsburg, PA).
2.2. Human skin
Excised split-thickness human cadaver skin dermatomed from the posterior torso of eight skin donors, males and females between the ages of 45 and 70 years, was obtained from the New York Firefighters Skin Bank (New York, NY). The cadaver skin was stored at −80 °C before use and was thawed by immersing the tissue in PBS in a 600-mL beaker. The skin was then patted dry with Kimwipe and cut into appropriate sizes. The thickness of the split-thickness skin samples was 0.20 ± 0.10 mm (mean ± SD, n = 321; the thickness of some skin samples in the first three sets of experiments was not measured). Unused skin was wrapped in aluminum foil and stored in a freezer at −20 °C for later use. The use of human tissues was approved by the Institutional Review Board (IRB) at the University of Cincinnati.
2.3. Franz diffusion cell setup
Skin samples were mounted onto Franz diffusion cells (effective diffusional area ~0.7 cm2) with the dermis facing the receptor. The diffusion cells had a static water-jacket to maintain the temperature of the receptor chamber at ~34 °C, and the donor chamber was un-occluded with skin surface temperature at ~32 °C. The receptor compartment was filled with ~6 mL PBS and equipped with a magnetic stir bar. After the assembly of the diffusion cells with the skin samples, skin conductance and then TEWL measurements were performed. Skin conductance was used as the skin integrity test of all skin samples (344 samples), and TEWL was used in combination with skin conductance as the skin integrity tests to compare these two tests in a subset of the skin samples (289 samples out of the 344 samples). Skin permeation experiments were performed after the skin integrity tests.
2.4. Skin electrical conductance measurement
Skin electrical resistance was measured at room temperature using an electrical system that applied a low voltage (< 0.25 V) across the skin when the skin was immersed in PBS for ~15 min in the Franz diffusion cell (i.e., by filling the donor chamber with PBS) before the skin permeation experiments. The voltage was applied across the skin for a few seconds through two Ag/AgCl electrodes that were placed in the donor and receptor chambers in the Franz diffusion cell. The electrical system was constructed using a 1.5-V battery and fixed resistor in series with the skin. The voltages across the skin and the fixed resistor were determined by voltmeters (Fluke 73III Multimeter, Everett, WA), and the resulting electric current across the skin was calculated by the voltage across the fixed resistor and the resistance of the fixed resistor using Ohm’s law. Skin electrical resistance was then calculated using the voltage across the skin, the electric current, and Ohm’s law (resistance = voltage/current). The electrical conductance is the reciprocal of the resistance, and skin conductance normalized by the surface area was determined.
2.5. TEWL measurement
TEWL was measured using Delfin vapometer SWL 4392 (Kuopio, Finland). Before the collection of TEWL data, experiments were performed to assess the effect of the diffusion cell donor chamber cap on TEWL readings. This comparison is necessary because the vapometer is calibrated for in vivo measurements when it is in direct contact with the skin without the donor chamber cap. In the first set of the experiments, TEWL was measured with the skin in the Franz diffusion cell at room temperature by placing the vapometer on top of the donor chamber. The size of the vapometer chamber adapter opening was similar to that of the donor chamber opening that the adapter was fitted on the top of the donor chamber to seal the chamber from the environment. In each TEWL experiment, the measurement was repeated until TEWL reached a constant value. In general, TEWL began to approach a constant value after two to three measurements (see “Results and Discussion” section) when these TEWL measurements were taken ~15–25 min apart. After these measurements, the donor chamber cap was removed and the measurements were repeated by placing the vapometer directly on the skin surface. In the second set of experiments, the order was reversed that TEWL was measured by placing the vapometer directly on the skin first and then with the donor chamber cap on. The TEWL readings with and without the donor chamber cap were compared. With the donor chamber cap on the skin, the average TEWL readings were 19% ± 14% (mean ± SD, n = 6) lower than those without the donor chamber cap. Among these skin samples, three skin samples had TEWL < 10 g/m2/h. For the skin samples of TEWL < 10 g/m2/h, the difference between the TEWL values with and without the donor chamber cap was 8% ± 12%, (mean ± SD, n = 3). For the skin samples of TEWL > 10 g/m2/h, the difference between the TEWL values with and without the donor chamber cap was 30% ± 1% (mean ± SD, n = 3). For the purpose of the present study to identify skin samples with TEWL < 10 g/m2/h, the effect of the diffusion cell donor chamber cap on vapometer measurement was small.
In the skin permeation experiments, TEWL was measured using the vapometer at room temperature immediately before the permeation experiments (before applying TDS) when the skin surface was dry. The vapometer was placed on top of the Franz diffusion cell donor chamber to seal the donor chamber from the environment as described above. TEWL was measured, and the measurement was repeated until TEWL reached a constant value. Each TEWL measurement was taken ~15–25 min apart and the duration of the whole TEWL experiment was 1–1.5 h.
2.6. Skin permeation experiment
Skin permeation experiments with TDS were conducted in the Franz diffusion cell setup at ~32 °C as described in “Franz diffusion cell setup” section (Zhang, 2017). A punch-out (0.58 cm2) of the TDS was affixed to the skin surface in the donor chamber by the manual application of a light pressure. During the experiment, 0.5 mL samples were withdrawn from the receptor compartment at predetermined time intervals (e.g., 2, 4, 6, 8, and 10 h following TDS application for this part of the study). Immediately after sampling, an equal volume of fresh PBS was added back to the receptor to maintain a constant volume. The samples were stored at 4 °C until HPLC assay (usually assayed within one week). The fluxes were calculated by the cumulative amounts delivered across the skin divided by time and normalized to the surface area of skin exposed to TDS:
| (1) |
where J is the flux, Q is the cumulative amount, t is time, and ATDS is the skin area exposed to TDS (i.e., 0.58 cm2). The flux values were then multiplied by the surface areas of the respective TDS products to calculate the delivery rates of the TDS products:
| (2) |
where Rate is the TDS product delivery rate and Aproduct is the TDS product area (22.9, 21.5, and 13.1 cm2 for Alza, Aveva, and Mylan TDS, respectively). For the comparison of skin integrity using the TDS in the analyses, the drug delivery rates of the TDS products should be used because the brand name and generic TDS products have different flux performance due to the different formulations but have the same delivery rate at their respective TDS sizes (areas) according to their product labels. The extrapolation of TDS fluxes to their respective delivery rates allowed the use of the in vivo skin absorption information from the TDS product labels as the reference.
2.7. HPLC assay for permeation experiment
The samples from the skin permeation experiments were assayed using a Shimadzu HPLC system (Shimadzu Scientific Instruments, Addison, IL) with SPD-20A UV–Vis detector, SIL-20A autoinjector, and a Microsorb-MV100-5 C18 column (15 cm × 4.6 mm, 4.6 μm, Varian, Lake Forest, CA). The mobile phase was composed of 4 volumes of ammonium acetate solution (1 in 100) and 6 volumes of a mixture of methanol:acetonitrile:glacial acetic acid (400:200:0.6). The pH of the mobile phase was adjusted to 6.6 ± 0.1 with glacial acetic acid if necessary. The flow rate was 2.0 mL/min. The detection wavelength was 230 nm. Standard solutions of 0.5–50 μg/mL fentanyl were prepared in PBS to construct the calibration curve. Fentanyl USP standard was used to verify the assay method.
2.8. TEWL and skin conductance sensitivity test
At the end of the skin permeation experiments, a small subset of selected skin samples that had electrical conductance < 0.07 mS/cm2 and TEWL < 10 g/m2/h (skin integrity test criteria suggested later in the “Results and Discussion” section) were used in the sensitivity test. In this test, skin electrical conductance measurements and then TEWL measurements were performed immediately after TDS removal. When the skin was still dry in the Franz diffusion cells after the TEWL measurements, the skin was punctured with a 30-gauge syringe needle five times to create five holes. These holes were not visible by observation. After the physical perturbation, TEWL was measured followed by skin conductance measurements. TEWL and skin conductance after the puncture were compared with those before the puncture.
3. Results and discussion
3.1. TEWL and skin conductance
Fig. 1 shows the typical TEWL values obtained under the TEWL protocol in the present study. After the assembly of the diffusion cell, the first TEWL measurement usually had a higher value than those in the subsequent measurements. TEWL decreased with time in these measurements and approached a relatively constant value in the third or fourth measurements. The TEWL values used in the present study were the average values from this constant region (e.g., average of the last three measurements). The decrease of TEWL with time observed in the present study is consistent with the trend observed previously (Elkeeb et al., 2010; Saadatmand et al., 2017). Fig. 2 presents the data to examine a relationship between TEWL and skin conductance determined before the permeation experiments of all skin samples (total of > 200 skin samples from eight skin donors). Although there is a trend of higher TEWL from skin of higher electrical conductance, the correlation between TEWL and skin conductance is poor (r2 = 0.098).
Fig. 1.
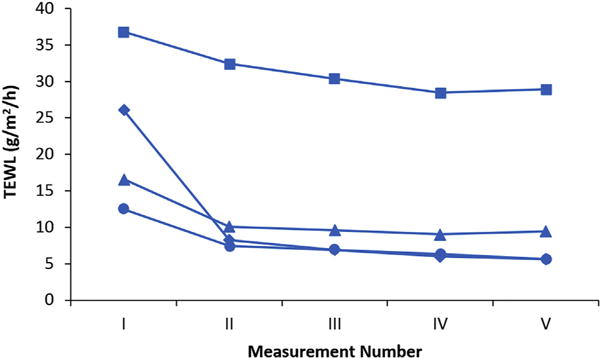
Representative plots of TEWL vs. measurement number (Measurements I to V). Data from four skin samples are shown individually (squares, diamonds, triangles, and circles).
Fig. 2.
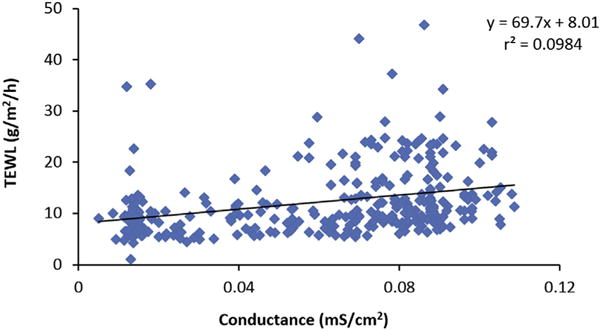
Relationship between TEWL and skin conductance of all skin samples. Each data point represents the TEWL and skin conductance measured before each skin permeation experiment. The line is the linear least squares line.
3.2. Fentanyl flux, TEWL, and skin conductance
Fig. 3 presents the results of the fentanyl permeation experiments and TEWL (measured before the experiments) to examine a relationship between these two parameters and to identify possible integrity test criteria using TEWL. The average fluxes of fentanyl between 6 and 10 h were obtained from the skin permeation experiments of fentanyl TDS and the delivery rates were calculated by the areas of the TDS products for the analysis (Eq. 2). The fluxes in this time interval were selected because they were close to their peak values for fentanyl TDS delivery. Fig. 4 presents the results of fentanyl delivery rate and skin electrical conductance (measured before the permeation experiments) in a similar analysis. The results in these figures show weak correlations between fentanyl delivery rate and TEWL and skin conductance (linear least squares regressions of r2 = 0.158 and 0.169, respectively). It should be noted that a caveat in the present skin study was the use of TDS, e.g., instead of a simple drug solution; possible effects of TDS formulations on the skin barrier were not investigated. Also, the present analyses combined the data from fentanyl TDS of Alza, Aveva, and Mylan as there was no noticeable difference between the delivery rate, TEWL, and skin conductance relationships for the Alza, Aveva, and Mylan TDS (results of the linear least squares regressions of each TDS are summarized in the figure captions).
Fig. 3.
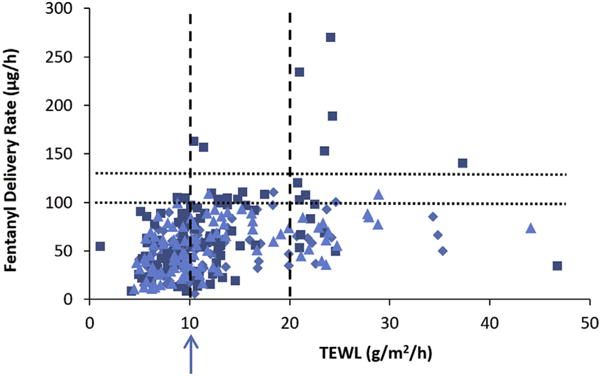
Fentanyl delivery rate (flux × area of TDS product) in skin permeation experiment vs. TEWL in skin integrity test before the experiment. Symbols: Alza TDS (squares), Mylan TDS (diamonds), Aveva TDS (triangles). Linear least squares regression equations: Alza: y = 2.8x + 33, r2 = 0.185; Aveva: y = 1.6x + 34, r2 = 0.213; Mylan: y = 1.4x + 32, r2 = 0.162; combined: y = 2.0x + 33, r2 = 0.158. The dotted lines indicate 100 and 130 μg/h values. The dashed lines indicate 10 and 20 g/m2/h values. The arrow indicates the TEWL value required to remove skin samples with > 130 μg/h.
Fig. 4.
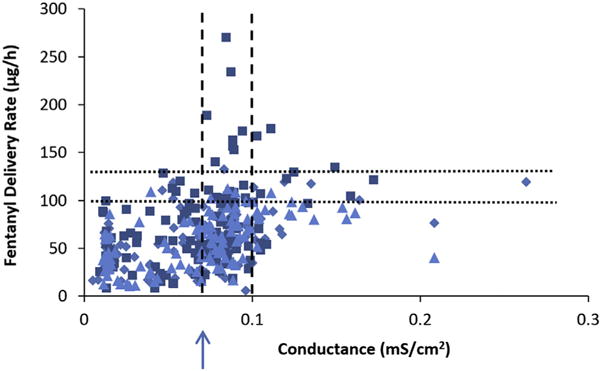
Fentanyl delivery rate (flux × area of TDS product) in skin permeation experiment vs. skin conductance in skin integrity test before the experiment. Symbols: Alza (squares), Mylan (diamonds), Aveva (triangles). Linear least squares regression equations: Alza: y = 539x + 37, r2 = 0.150; Aveva: y = 353x + 34, r2 = 0.258; Mylan: y = 317x + 36, r2 = 0.226; combined: y = 395x + 37, r2 = 0.169. The dotted lines indicate 100 and 130 μg/h values. The dashed lines indicate 0.07 and 0.1 mS/cm2 values. The arrow indicates the skin conductance value required to remove skin samples with > 130 μg/h.
3.3. TEWL and skin conductance as skin integrity tests
Fig. 5 shows the distribution of fentanyl delivery rates for the skin samples studied. The distribution suggests that skin with > 130 μg/h was not typical. For the skin integrity test, fentanyl delivery rates > 100 μg/h and 130 μg/h were chosen as the skin exclusion criteria in the present investigation. Skin samples with delivery rates > 130 μg/h fentanyl from the TDS were likely the outliers and not representative of normal skin barrier. Skin samples with delivery rates > 100 μg/h were considered to be higher than the normal range expected for human skin in vivo according to the delivery rate indicated in the TDS product labels. The delivery rate of 100 μg/h was two standard deviations above the delivery rate of 50 μg/h from the TDS product labels when 25 μg/h was assumed as the standard deviation. This is a reasonable assumption because the coefficient of variation (CV) of the delivery rate data for all skin samples was 56% in the present study and 56% CV of 50 μg/h is 28 μg/h, which is close to the assumption value. Statistically, 95% and 99.7% of the data lie within two and three standard deviations in a normal distribution (i.e., ~100 and 130 μg/h), respectively. In addition, the average flux at ~6–10 h after fentanyl TDS application (the flux measured in the present study) was the peak flux that was ~1.9–2.6 times the flux at 72 h (lowest flux near the end of the duration of TDS application) (Zhang, 2017). This decreasing flux during TDS product use suggests that the product label delivery rate of 50 μg/h is an average value over the entire duration of TDS use and the early delivery rate at ~6–10 h can be as much as 2 times greater than the 50 μg/h product label value. This supports the examination of 100 μg/h delivery rate as another exclusion criterion in the present investigation.
Fig. 5.
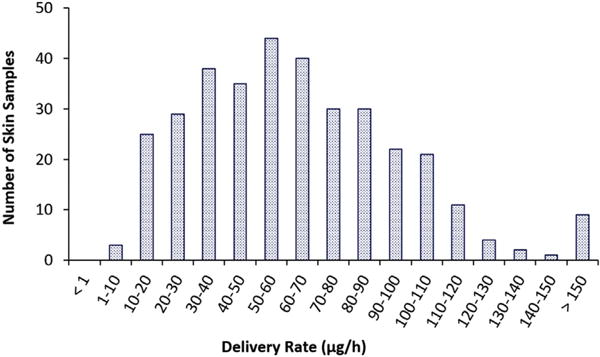
Number of skin samples within each fentanyl delivery rate range for all the skin samples studied. The numbers of skin samples are plotted against the delivery rate groups to illustrate the distribution.
The data in Figs. 3 and 4 suggest that the integrity test criteria of TEWL < 10 g/m2/h and conductance < 0.07 mS/cm2 (arrows in the figures) could exclude all skin samples with delivery rate > 130 μg/h and a majority of skin samples with delivery rate > 100 μg/h, albeit that there are many false positives of skin exclusion with delivery rates ~50 μg/h. Table 1 provides an analysis of the impact of the exclusion criteria on the skin permeation results. In general, the impact of skin exclusion based on TEWL and skin conductance on the means and standard deviations of fentanyl delivery rates is not significant. Changing the TEWL criterion from TEWL < 10 g/m2/h to TEWL < 20 g/m2/h, although leads to the inclusion of more skin samples of delivery rates > 100 μg/h, it would still exclude most of the skin samples of delivery rates > 100 μg/h (5% skin samples with > 100 μg/h) and decrease the number of exclusion false positives significantly (decrease by 4×). On the other hand, changing the skin conductance criterion from conductance < 0.07 mS/cm2 to < 0.10 mS/cm2 would reduce the ability of skin conductance to exclude skin samples of delivery rates > 100 μg/h (11% skin samples with > 100 μg/h). Skin conductance could be lowered to 0.05 mS/cm2 to achieve similar exclusion of skin samples with delivery rate > 100 μg/h as the TEWL method, but this would result in more false positives than the TEWL method. It should be pointed out that the percentage values in Table 1 are related to the sources and quality of the skin samples obtained in the present study and can be different from skin from other sources.
Table 1.
Impact of exclusion criteria on the conclusion of the skin permeation experiments.
| Skin group/exclusion criterion | Number of skin samples (n) | Fentanyl delivery rate (μg/h)a | Maximum fentanyl delivery rate (μg/h) | Number of samples with delivery rate > 100 μg/h | Number of samples with delivery rate > 130 μg/h | Number of samples with delivery rate < 100 μg/h and excluded |
|---|---|---|---|---|---|---|
| All TEWL subset | 289 | 57 ± 33 | 270 | 22 (8%) | 7 (2%) | N/A |
| TEWL < 20 g/m2/h | 248b | 53 ± 27 | 163 | 12 (5%) | 2 (1%) | 31 (11%)c |
| TEWL < 10 g/m2/h | 145b | 45 ± 22 | 105 | 2 (1%) | 0 | 124 (43%)c |
| All conductance subset | 344 | 64 ± 36 | 270 | 47 (14%) | 12 (3%) | N/A |
| Conductance < 0.1 mS/cm2 | 300b | 60 ± 35 | 270 | 33 (11%) | 9 (3%) | 30 (9%)d |
| Conductance < 0.07 mS/cm2 | 157b | 49 ± 27 | 128 | 9 (6%) | 0 | 149 (43%)d |
| Conductance < 0.05 mS/cm2 | 102b | 45 ± 25 | 128 | 3 (3%) | 0 | 198 (58%)d |
Mean ± SD.
Number of skin samples that meet the criterion.
Based on total of 289 skin samples.
Based on total of 344 skin samples.
3.4. TEWL and skin conductance sensitivity test
Fig. 6 presents the data to test the abilities of TEWL and skin conductance to detect physical skin perturbation. When the skin was compromised after needle puncture, both TEWL and skin conductance increased for the skin samples: all data points are above the dotted lines in the plots. These results suggest that TEWL and skin conductance can both detect severe skin perturbation under the conditions in the present study. The effect of skin damages on skin resistance observed is also similar to those of pinhole damages in a previous study (White et al., 2013). However, despite the fact that the same number of holes was created in the skin samples, the TEWL and skin conductance values are quite variable for the damages (~9–30 g/m2/h and ~0.04–0.3 mS/cm2, respectively). The least damaged skin samples also showed relatively low TEWL and skin conductance values (~9 g/m2/h and ~0.04 mS/cm2, respectively). This suggests that (a) the damages caused by the procedure in some experiments were not as significant as the others, (b) the TEWL and skin conductance methods could not quantify such damages in the skin samples, and/or (c) some skin recovered quickly from the needle stick such as the closing of the holes due to hydration and swelling in the skin.
Fig. 6.
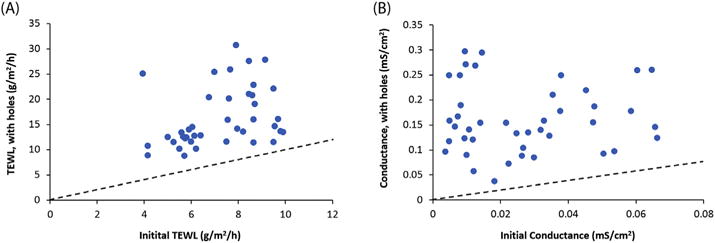
Effects of physical skin perturbation on (a) TEWL and (b) skin electrical conductance. TEWL and skin conductance of the samples after syringe-needle perturbation were plotted against their respective values before the perturbation. The dotted lines indicate equal values of TEWL or skin conductance before and after the perturbation.
3.5. Mechanisms of TEWL and skin conductance
TEWL and skin electrical conductance were both suggested to be indicators of the skin barrier in in vitro studies. For example, Netzlaff et al. investigated the effectiveness and limitations of TEWL as skin integrity test for in vitro permeation studies (Netzlaff et al., 2006). Fasano et al. and Davies et al. compared electrical resistance and tritiated water as skin integrity tests and suggested electrical resistance as a quick and reliable method (Davies et al., 2004; Fasano et al., 2002). Guth et al. compared TEWL, electrical resistance, and a 3H-labeled internal reference standard method for in vitro skin permeation studies (Guth et al., 2015). Heylings et al. examined TEWL and skin resistance to assess skin barrier function for irritation studies (Heylings et al., 2001). The present study used a large number of skin samples, model permeant fentanyl, and in vivo delivery rate of fentanyl product labels to examine TEWL and electrical conductance as integrity tests to control skin quality for in vitro permeation studies. The present results demonstrate a relationship between TEWL and skin conductance for the skin samples but the linear regression correlation between these two skin parameters is poor. It should be noted that the r2 value in Fig. 2 is similar to that of tritiated water permeability vs. TEWL reported in a previous study (Chilcott et al., 2002) but lower than that in another study (Hui et al., 2012). The lack of a good correlation between TEWL and skin conductance could be attributed to a number of factors. First, these two methods are likely to measure different skin barrier properties. Skin conductance measures ion transport across the skin, and hence, this method is related to skin permeation across the skin polar pathway (Li et al., 1999; Li and Peck, 2013; Tang et al., 2001). As such, skin conductance should be indicative of skin defect or damage related to these “pores” of the polar pathway with little or no correlation to permeation across the skin lipoidal pathway (i.e., for lipophilic permeants). This hypothesis is consistent with the lack of a skin permeability vs. electrical resistance correlation for a lipophilic permeant observed in previous studies (Chantasart et al., 2007; Ibrahim and Li, 2009). TEWL measures water permeation across the skin that is likely related to the skin lipoidal pathway; skin lipids are the predominant barrier for water permeation (Grubauer et al., 1989; Scheuplein, 1966), and lipid lamella have relatively high permeability to water with large surface area in the skin available for water permeation. TEWL therefore could be related to skin permeation across the lipoidal pathway. The suggested difference in mechanisms of skin permeation for water molecules and conducting ions is consistent with the lack of a correlation between tritiated water absorption and skin electrical resistance observed in a previous study (Ibrahim, 2009).
Second, the assumption of a correlation between TEWL and skin permeability to water is questionable. A previous study investigating these two skin properties has suggested little correlation between TEWL and tritiated water permeability (Chilcott et al., 2002). A possible explanation is that TEWL does not only measure skin permeation of water from the receptor chamber but also water desorption from the skin in the Franz diffusion cell. Water on the skin and moisture in the skin can contribute to TEWL, making it difficult to measure transdermal water permeation accurately using TEWL before steady-state water permeation from the receptor is attained. It should be pointed out that a main difference between the experimental procedure in the present and previous studies was the wait time of the TEWL measurements. In the present study, the duration of the entire TEWL measurements was 1 to 1.5 h, which was more than a magnitude longer than that in the previous study (within 1 min) (Chilcott et al., 2002). Despite the long wait time, steady-state water permeation cannot be independently verified in the present TEWL study.
Another difference between the TEWL and skin conductance methods in the present study was the conditions under which the skin was assessed. The skin surface was relatively dry and exposed to room conditions when TEWL was measured whereas the skin was immersed in PBS and was hydrated when skin conductance was measured. It is well known that skin hydration can enhance skin permeation (Li et al., 2016). Skin defects (damages) can behave differently under normal skin condition with a dry surface (partially hydrated state) vs. its hydrated state.
3.6. Impact of skin integrity tests TEWL and skin conductance
Fentanyl has a moderate molecular size (MW = 336.5 Da) and is lipophilic according to its octanol/water partition coefficient (Log Koct = 4.05 (EPA, 2012)). It is a weak base and cationic at physiological pH, and hence has moderate octanol/water distribution coefficients under the physiological condition. The water solubility and skin permeability coefficient of fentanyl at pH 7.4 is 1.36 mg/mL and 1.4 × 10−2 cm/h, respectively (Zhang, 2017). The data in the present study show weak correlations of fentanyl delivery rate vs. TEWL and skin conductance, in contrast to the good correlation between skin permeability of polar solutes and skin resistance shown previously (Li and Peck, 2013). The low r2 values of the regression analyses of fentanyl delivery rate vs. TEWL and skin conductance could be due to a number of factors. First, the skin areas assessed by TEWL and skin conductance were not the same as the skin area available for drug delivery under TDS (i.e., not the entire skin surface in the donor chamber was covered by the TDS), which can introduce variability in the data analyses resulting in the low r2 values observed in the present study. Second, the skin conditions under TDS in the presence of transdermal formulations such as permeation enhancers, under immersion in PBS during skin conductance measurements, and with normal exposure to the environment during TEWL measurements are different, and this could lead to variability. In addition, the mechanisms of water permeation in TEWL, ion permeation in skin conductance measurements, and fentanyl permeation across the skin are likely different. Fentanyl is a cation at the physiological pH and may utilize both the lipoidal and polar pathways in skin permeation. Considering the nature of the regression analyses with these varying factors, the low r2 values of the correlations are not surprising.
An important outcome of the present study is the examination of the exclusion criteria of TEWL and skin conductance in barrier integrity tests for skin permeation in vitro using fentanyl as the model drug, a weak base with molecular weight similar to most commonly encountered drugs in dermatological products. The relationships between fentanyl delivery rate, TEWL, and skin conductance were analyzed to identify the exclusion criteria of TEWL and conductance to remove outliers in skin permeation studies. Other factors that could affect the quality of the skin samples include the thickness of skin samples, age and ethnicity of the skin donors, the body sites from which the skin was excised, and the storage conditions of the skin samples. For example, although the main barrier for skin permeation is the stratum corneum and not the dermis, the thickness of skin samples (i.e., mostly from dermis thickness) could reflect the skin dermatome process that could affect the quality of the skin samples. The skin quality and dermatome process could also be affected by the body sites of skin excision. Skin permeability, TEWL, and skin conductance could be related to the sources of the skin samples such as age and ethnicity of the skin donors. The specific effects associated with these factors were not investigated because the objective of the present study was to examine the skin conductance and TEWL methods to exclude skin samples of high permeability independent of these factors. These factors are not generally controlled in the designs of skin permeation experiments due to the intent to include all human population sample groups and limited sources of human skin. In addition, the outliers are likely related to skin damages in the process of skin procurement and sample handling. Future studies are required to investigate the effects of different factors on TEWL, skin conductance, and skin permeability of drugs with different physicochemical properties.
In the assessment of the exclusion criteria, the relationships between fentanyl delivery rate, TEWL, and skin conductance and the analyses of Table 1 suggest that either skin conductance of 0.07 mS/cm2 (i.e., resistance of 15 kohm cm2) or TEWL of 10 g/m2/h can be used as the exclusion criteria to remove most outliers in the in vitro skin permeation study. The exclusion of skin samples using these criteria had a small effect on the mean delivery rates of the data set. This implies that when a large number of skin samples is used in a permeation study, the exclusion criteria in these skin integrity tests would likely not have a large impact on the conclusion of the study. However, the use of these criteria can be important when the number of skin samples used in an in vitro permeation study is small; the permeation results can be significantly affected when a small sample group (e.g., n = 4) is studied and one of the skin samples is an outlier. In practice, TEWL and skin conductance tests are more convenient and less time-demanding compared to tritiated water test, which could be an advantage.
4. Conclusion
To evaluate TEWL and electrical conductance as barrier integrity tests before skin permeation experiments in vitro, the present study investigated the relationships between TEWL and skin conductance and transdermal delivery rate of fentanyl TDS as the model drug. Under the conditions studied, the correlation between TEWL and skin conductance was poor. The data of the fentanyl TDS suggest weak correlations between drug delivery rate and TEWL and drug delivery rate and skin conductance with linear regression r2 ~ 0.15–0.17. With > 200 skin samples studied and using the in vivo drug delivery rate provided by the TDS product labels, the present analyses suggest that either skin conductance of 0.07 mS/cm2 (i.e., resistance of 15 kohm cm2) or TEWL of 10 g/m2/h could be used as exclusion criteria in skin integrity testing before the in vitro skin permeation experiments. These criteria could allow the removal of most skin outliers (i.e., skin samples with high drug fluxes) in the permeation experiments although a significant number of good skin samples (false positives) was also excluded. With the large number of skin samples studied, the impact of TEWL and skin conductance exclusion criteria on the skin permeation results is not significant. However, the use of these criteria can be important when the number of skin samples used in an in vitro permeation study is small.
Acknowledgments
The authors thank Drs. Sam G Raney, Priyanka Ghosh, and Jinsong Hao for helpful discussion. Funding for this project was made possible, in part, by the U.S. Food and Drug Administration (FDA) through a cooperative agreement (Research Award U01 FD004942). The views expressed in this paper do not reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
References
- Bassani AS, Banov D. Evaluation of the percutaneous absorption of ketamine HCl, gabapentin, clonidine HCl, and baclofen, in compounded transdermal pain formulations, using the Franz finite dose model. Pain Med. 2016;17:230–238. doi: 10.1111/pme.12899. [DOI] [PubMed] [Google Scholar]
- Chantasart D, Li SK. Structure enhancement relationship of chemical penetration enhancers in drug transport across the stratum corneum. Pharmaceutics. 2012;4:71–92. doi: 10.3390/pharmaceutics4010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantasart D, Sa-Nguandeekul P, Prakongpan S, Li SK, Higuchi WI. Comparison of the effects of chemical permeation enhancers on the lipoidal pathways of human epidermal membrane and hairless mouse skin and the mechanism of enhancer action. J Pharm Sci. 2007;96:2310–2326. doi: 10.1002/jps.20865. [DOI] [PubMed] [Google Scholar]
- Charoenputtakun P, Li SK, Ngawhirunpat T. Iontophoretic delivery of lipophilic and hydrophilic drugs from lipid nanoparticles across human skin. Int J Pharm. 2015;495:318–328. doi: 10.1016/j.ijpharm.2015.08.094. [DOI] [PubMed] [Google Scholar]
- Chilcott RP, Dalton CH, Emmanuel AJ, Allen CE, Bradley ST. Transepidermal water loss does not correlate with skin barrier function in vitro. J Invest Dermatol. 2002;118:871–875. doi: 10.1046/j.1523-1747.2002.01760.x. [DOI] [PubMed] [Google Scholar]
- Davies DJ, Ward RJ, Heylings JR. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol in Vitro. 2004;18:351–358. doi: 10.1016/j.tiv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Elkeeb R, Hui X, Chan H, Tian L, Maibach HI. Correlation of transepidermal water loss with skin barrier properties in vitro: comparison of three evaporimeters. Skin Res Technol. 2010;16:9–15. doi: 10.1111/j.1600-0846.2009.00406.x. [DOI] [PubMed] [Google Scholar]
- EPA. EPI Suite — Estimation Program Interface. 2012 https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface.
- Fasano WJ, Manning LA, Green JW. Rapid integrity assessment of rat and human epidermal membranes for in vitro dermal regulatory testing: correlation of electrical resistance with tritiated water permeability. Toxicol in Vitro. 2002;16:731–740. doi: 10.1016/s0887-2333(02)00084-x. [DOI] [PubMed] [Google Scholar]
- FDA. Draft Guidance on Acyclovir, Guidance for Industry ANDA Submissions. 2016 https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM428195.pdf.
- Flynn GL. Physicochemical determinants of skin absorption. In: Gerrity TR, Henry CJ, editors. Principles of Route to Route Extrapolation for Risk Assessment. Elsevier; New York: 1990. pp. 93–127. [Google Scholar]
- Franz TJ, Lehman PA, Raney SG. Use of excised human skin to assess the bioequivalence of topical products. Skin Pharmacol Physiol. 2009;22:276–286. doi: 10.1159/000235828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch HF, Dotson GS, Bunge AL, Chen CP, Cherrie JW, Kasting GB, Kissel JC, Sahmel J, Semple S, Wilkinson S. Analysis of finite dose dermal absorption data: implications for dermal exposure assessment. J Expo Sci Environ Epidemiol. 2014;24:65–73. doi: 10.1038/jes.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89–96. [PubMed] [Google Scholar]
- Guth K, Schafer-Korting M, Fabian E, Landsiedel R, van Ravenzwaay B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol in Vitro. 2015;29:113–123. doi: 10.1016/j.tiv.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Heylings JR, Clowes HM, Hughes L. Comparison of tissue sources for the skin integrity function test (SIFT) Toxicol in Vitro. 2001;15:597–600. doi: 10.1016/s0887-2333(01)00069-8. [DOI] [PubMed] [Google Scholar]
- Hui X, Elkeeb R, Chan H, Maibach HI. Ability to estimate relative percutaneous penetration via a surrogate maker – trans epidermal water loss. Skin Res Technol. 2012;18:108–113. doi: 10.1111/j.1600-0846.2011.00541.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim SA. PhD Dissertation. University of Cincinnati; Cincinnati, Ohio: 2009. A Structure-Enhancement Relationship and Mechanistic Study of Chemical Enhancers on Human Epidermal Membrane Based on Maximum Enhancement Effect (Emax) Ch 8. [Google Scholar]
- Ibrahim SA, Li SK. Effects of solvent deposited enhancers on transdermal permeation and their relationship with Emax. J Control Release. 2009;136:117–124. doi: 10.1016/j.jconrel.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecna M, Machacek M, Prchalova E, Stepanek P, Drasar P, Kotora M, Vavrova K. Galactosyl pentadecene reversibly enhances transdermal and topical drug delivery. Pharm Res. 2017;34:2097–2108. doi: 10.1007/s11095-017-2214-3. [DOI] [PubMed] [Google Scholar]
- Lehman PA, Franz TJ. Assessing topical bioavailability and bioequivalence: a comparison of the in vitro permeation test and the vasoconstrictor assay. Pharm Res. 2014;31:3529–3537. doi: 10.1007/s11095-014-1439-7. [DOI] [PubMed] [Google Scholar]
- Li SK, Peck KD. Passive and iontophoretic transport through the skin polar pathway. Skin Pharmacol Physiol. 2013;26:243–253. doi: 10.1159/000351926. [DOI] [PubMed] [Google Scholar]
- Li SK, Suh W, Parikh HH, Ghanem AH, Mehta SC, Peck KD, Higuchi WI. Lag time data for characterizing the pore pathway of intact and chemically pretreated human epidermal membrane. Int J Pharm. 1998;170:93–108. [Google Scholar]
- Li SK, Ghanem AH, Peck KD, Higuchi WI. Pore induction in human epidermal membrane during low to moderate voltage iontophoresis: a study using AC iontophoresis. J Pharm Sci. 1999;88:419–427. doi: 10.1021/js980331y. [DOI] [PubMed] [Google Scholar]
- Li SK, Ghanem AH, Teng CL, Hardee GE, Higuchi WI. Iontophoretic transport of oligonucleotides across human epidermal membrane: a study of the Nernst-Planck model. J Pharm Sci. 2001;90:915–931. doi: 10.1002/jps.1043. [DOI] [PubMed] [Google Scholar]
- Li X, Johnson R, Kasting GB. On the variation of water diffusion coefficient in stratum corneum with water content. J Pharm Sci. 2016;105:1141–1147. doi: 10.1016/S0022-3549(15)00173-2. [DOI] [PubMed] [Google Scholar]
- Myer K, Maibach H. Stratum corneum evaluation methods: overview. Skin Res Technol. 2013;19:213–219. doi: 10.1111/srt.12011. [DOI] [PubMed] [Google Scholar]
- Netzlaff F, Kostka KH, Lehr CM, Schaefer UF. TEWL measurements as a routine method for evaluating the integrity of epidermis sheets in static Franz type diffusion cells in vitro. Limitations shown by transport data testing. Eur J Pharm Biopharm. 2006;63:44–50. doi: 10.1016/j.ejpb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Penna E, Padula C, Colombo P, Santi P. New transdermal bioadhesive film containing oxybutynin: in vitro permeation across rabbit ear skin. Int J Pharm. 2006;325:2–7. doi: 10.1016/j.ijpharm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Olivier JC, Rabouan S, Couet W. In vitro comparative studies of two marketed transdermal nicotine delivery systems: Nicopatch and Nicorette. Int J Pharm. 2003;252:133–140. doi: 10.1016/s0378-5173(02)00637-3. [DOI] [PubMed] [Google Scholar]
- Roy SD, Gutierrez M, Flynn GL, Cleary GW. Controlled transdermal delivery of fentanyl: characterizations of pressure-sensitive adhesives for matrix patch design. J Pharm Sci. 1996;85:491–495. doi: 10.1021/js950415w. [DOI] [PubMed] [Google Scholar]
- Saadatmand M, Stone KJ, Vega VN, Felter S, Ventura S, Kasting G, Jaworska J. Skin hydration analysis by experiment and computer simulations and its implications for diapered skin. Skin Res Technol. 2017;23:500–513. doi: 10.1111/srt.12362. [DOI] [PubMed] [Google Scholar]
- Scheuplein RJ. Analysis of permeability data for the case of parallel diffusion pathways. Biophys J. 1966;6:1–17. doi: 10.1016/S0006-3495(66)86636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Li SK, Peck KD, Zhu H, Ghanem AH, Higuchi WI. Human epidermal membrane constant conductance iontophoresis: alternating current to obtain reproducible enhanced permeation and reduced lag times of a nonionic polar permeant. Int J Pharm. 2002;232:45–57. doi: 10.1016/s0378-5173(01)00910-3. [DOI] [PubMed] [Google Scholar]
- Tang H, Mitragotri S, Blankschtein D, Langer R. Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J Pharm Sci. 2001;90:545–568. doi: 10.1002/1520-6017(200105)90:5<545::aid-jps1012>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Tezel A, Dokka S, Kelly S, Hardee GE, Mitragotri S. Topical delivery of anti-sense oligonucleotides using low-frequency sonophoresis. Pharm Res. 2004;21:2219–2225. doi: 10.1007/s11095-004-7674-6. [DOI] [PubMed] [Google Scholar]
- USP725. Topical and Transdermal Drug Products—Product Performance Tests. The United States Pharmacopeial Convention Pharmacopeial Forum. 2009;35:1–12. [Google Scholar]
- White EA, Orazem ME, Bunge AL. Characterization of damaged skin by impedance spectroscopy: mechanical damage. Pharm Res. 2013;30:2036–2049. doi: 10.1007/s11095-013-1052-1. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ibrahim SA, Higuchi WI, Li SK. Ion-exchange membrane assisted transdermal iontophoretic delivery of salicylate and acyclovir. Int J Pharm. 2009;369:105–113. doi: 10.1016/j.ijpharm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. PhD Dissertation. University of Cincinnati; Cincinnati, Ohio: 2017. Evaluation of Heat Effects on Transdermal Delivery Systems Using In Vitro Permeation Test Strategy. [Google Scholar]


