Abstract
Objectives
Seizures can be provoked by systemic diseases associated with metabolic derangements, but the association between liver disease and seizures remains unclear.
Methods
We performed a retrospective cohort study using inpatient and outpatient claims between 2008 and 2015 from a nationally representative 5% sample of Medicare beneficiaries. The primary exposure variable was cirrhosis, and the secondary exposure was mild, non-cirrhotic liver disease. The primary outcome was seizure, and the secondary outcome was status epilepticus. Diagnoses were ascertained using validated ICD-9-CM codes. Survival statistics were used to calculate incidence rates, and Cox proportional hazards models were used to examine the association between exposures and outcomes while adjusting for seizure risk factors.
Results
Among 1,782,402 beneficiaries, we identified 10,393 (0.6%) beneficiaries with cirrhosis and 19,557 (1.1%) with mild, non-cirrhotic liver disease. Individuals with liver disease were older and had more seizure risk factors than those without liver disease. Over 4.6 ±2.2 years of follow-up, 49,843 (2.8%) individuals were diagnosed with seizures and 25 patients (0.001%) were diagnosed with status epilepticus. Cirrhosis was not associated with seizures (hazard ratio [HR], 1.1; 95% confidence interval [CI], 1.0-1.3), but there was an association with status epilepticus (HR, 1.9; 95% CI, 1.3-2.8). Mild liver disease was not associated with a higher risk of seizures (HR, 0.8; 95% CI, 0.6-0.9) or status epilepticus (HR, 1.1; 95% CI, 0.7-1.5).
Significance
In a large, population-based cohort, we found an association between cirrhosis and status epilepticus, but no overall association between liver disease and seizures.
Keywords: Status epilepticus, mild liver disease, cirrhosis, seizure, liver disease
Chronic liver disease imposes a heavy public health burden, leading to approximately 2 million outpatient visits and 750,000 hospitalizations annually in the United States alone1 and approximately 1 million deaths annually worldwide.2 Neurological phenomena such as asterixis, encephalopathy, coma, and seizures are not infrequently observed in acute and chronic liver disease. Adams and Foley reported seizures in up to 30% of their observed cohort of patients with liver disease.3 Many reports describe seizures in the setting of cerebral edema from acute liver failure,4, 5 and convulsions have also been variably reported in chronic liver patients.6 Finally, additional case reports exist of convulsive status epilepticus, epilepsia partialis continua, and electrographic seizures among patients with liver disease.7–9 However, because it is unknown whether liver disease is an independent risk factor for seizures, we examined the association between cirrhotic liver disease and, separately, mild non-cirrhotic chronic liver disease, and seizures.
Methods
Study Design
We performed a retrospective cohort study using inpatient and outpatient claims between 2008 and 2015 from a random 5% sample of Medicare beneficiaries. The U.S. federal government’s Centers for Medicare and Medicaid Services (CMS) provide health insurance to a large majority of U.S. residents once they reach 65 years of age. CMS makes available to researchers data on claims submitted by providers and hospitals in the course of Medicare beneficiaries’ clinical care.10 Claims data from hospitals include International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes and dates of hospitalization. Physician claims include Current Procedural Terminology (CPT) codes, the dates of service, and physicians’ specialty. Multiple claims for a given patient can be linked via a unique beneficiary identifier code, thus allowing for a comprehensive and longitudinal analysis of each beneficiary’s care over time. The Weill Cornell Medical College institutional review board approved our analysis of these data.
Patient Population
Following standard methods in the analysis of Medicare data, we included only beneficiaries ≥65 years of age with continuous coverage in traditional fee-for-service Medicare (both Parts A and B) for at least 1 year (or until death, if applicable) and no enrollment in a Medicare Advantage plan.11 We included beneficiaries only after 1 year of coverage eligibility to allow time for beneficiaries’ files to accrue claims that reflect their baseline comorbidities. Patients with a diagnosis of seizure before 1 year of coverage eligibility were excluded.
Measurements
The primary exposure was cirrhosis, and the secondary exposure was mild, non-cirrhotic liver disease. The primary outcome was seizure, and the secondary outcome was status epilepticus. We used validated algorithms to identify patients with liver disease and seizure.12, 13 We measured cirrhotic liver disease by the following codes 571.2, 571.5, 572.2, 572.3, 572.4, 456.0, 456.1, 456.20, 456.21, and 567.23. We excluded the diagnosis code for ascites because it has poor specificity.13 To be classified as having cirrhosis, patients required at least one hospital discharge diagnosis or two outpatient diagnoses. We did not include patients with biliary cirrhosis in our analyses because of the diagnosis code’s low specificity13. Administrative claims data have been used to identify patients with cirrhosis with good reliability in multiple additional settings.13–15
We ascertained seizures using the following codes ICD-9-CM codes: 345.9, 345.3, 345.1, 345.8, 345.5, 345.4, 345.7, 345.0, 345.2, and 345.6. This code algorithm has previously shown to have a positive predictive value of 84% to 98% in adult patients.12 We defined secondary outcome as status epilepticus ICD-9-CM code 345.3. We identified mild liver disease using the following diagnosis codes: 070.22, 070.32, 070.33, 070.44, 070.54, 070.6, 070.9, 570, 570.0, 570.1, 570.3, 570.4, 570.5, 570.8, 570.9. 573.3, 573.4, 573.8, 573.9, and v427. The codes represent chronic etiologies of liver disease without cirrhosis: chronic infectious hepatitis, chronic non-infectious liver disorders such as non-alcoholic fatty liver disease, and other chronic liver conditions. This code schema was adapted from the definition of mild liver disease in the list of Charlson comorbidities.16
In addition to general medical comorbidities,16 we pre-specified several seizure risk factors to include among our covariates: cerebrovascular disease, central nervous system tumors, traumatic brain injury, and central nervous system infections. In terms of cerebrovascular disease, prior diagnoses of ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhages were ascertained using a validated ICD-9-CM algorithm.17
Statistical Analysis
Patients’ baseline characteristics were compared using the χ2 test and the t-test, as appropriate. Crude rates were reported using descriptive statistics with exact 95% confidence intervals (CI). Survival statistics were used to calculate incidence rates, and Cox proportional hazards regression analysis was used to evaluate the association between exposure and outcomes while adjusting for age, sex, race, and the aforementioned Charlson comorbidity index and seizure risk factors. All covariates were included in Cox proportional hazards regression models regardless of significance at the univariate level. Patients were censored at the time of an outcome, end of Medicare coverage, death, or on September 30, 2015. The threshold of statistical significance was set at p = 0.05. All statistical analyses were performed by H.K. using Stata/MP version 14 (College Station, TX, USA).
Results
Risk of Seizure in Patients with Cirrhosis
Among 1,782,402 beneficiaries, 10,393 (0.6%) patients had cirrhosis (mean age, 73.2 6.5 years; 48% female). Compared to patients without cirrhosis, patients with cirrhosis were more frequently male and more often had seizure risk factors such as traumatic brain injury and central nervous system infections (Table 1). During a mean of 4.6 2.2 years of follow-up, 49,843 (2.8%) had seizure and 25 patients (.001%) were diagnosed with status epilepticus. Patients with seizure had more risk factors for seizures (Table 2).
Table 1.
Characteristics of Patients, Stratified by Presence or Absence of Cirrhosis
| Characteristicsa | Cirrhosis (n = 10,393) | No Cirrhosis (n = 1,772,009) |
|---|---|---|
| Age, mean (SD), y | 73.2 (6.5) | 73.5 (7.8) |
|
| ||
| Female | 4984 (48) | 1,011,316 (57.1) |
|
| ||
| Raceb | ||
|
| ||
| White | 8,779 (84.5) | 1,525,335 (86.1) |
| Black | 663 (6.4) | 140,060 (7.9) |
| Other | 951 (9.2) | 106,614 (6) |
|
| ||
| A.fib | 2,139 (20.6) | 133,738 (7.6) |
|
| ||
| HTN | 8,151 (78.4) | 906,703 (51.2) |
|
| ||
| TBI | 445 (4.3) | 37,142 (2.1) |
|
| ||
| CNS infection | 26 (0.3) | 1,609 (0.1) |
|
| ||
| CNS tumors | 25 (0.2) | 2,914 (0.2) |
|
| ||
| Myocardial infarction | 846 (8.1) | 39,719 (2.2) |
|
| ||
| CHF | 2,589 (24.9) | 101,964 (5.8) |
|
| ||
| Peripheral vascular disease | 1,227 (11.8) | 72,275 (4.1) |
|
| ||
| Cerebral vascular disease | 1,633 (15.7) | 114,389 (6.5) |
|
| ||
| Dementia | 239 (2.3) | 23,202 (1.3) |
|
| ||
| Chronic kidney disease | 2,296 (22.1) | 78,491 (4.4) |
|
| ||
| Chronic pulmonary disease | 2,830 (27.2) | 171,492 (9.7) |
|
| ||
| Diabetes | 6,587 (63.3) | 334,250 (18.9) |
|
| ||
| Cancer | 2,228 (21.4) | 135,933 (7.7) |
|
| ||
| AIDS | 26 (0.3) | 684 (0.04) |
|
| ||
| Metastatic disease | 388 (3.7) | 13,178 (0.7) |
|
| ||
| Tobacco use | 973 (9.4) | 22,039 (1.2) |
|
| ||
| Alcohol use | 1,869 (18) | 44,188 (2.5) |
Abbreviations: IQR, interquartile range; SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Self-reported by patients or their surrogates.
Table 2.
Characteristics of Patients Stratified by Incidence of Seizure
| Characteristicsa | Seizure (n = 49,843) | No Seizure (n = 1,732,559) |
|---|---|---|
| Age, mean (SD), y | 74.6 (7.5) | 73.4 (7.8) |
|
| ||
| Female | 28,371 (56.9) | 987,929 (57) |
|
| ||
| Raceb | ||
|
| ||
| White | 40,295 (80.8) | 1,493,819 (86.2) |
| Black | 6,872 (13.8) | 133,851 (7.7) |
| Other | 2,676 (5.4) | 104,889 (6.1) |
|
| ||
| A.fib | 5,430 (10.9) | 130,447 (7.5) |
|
| ||
| HTN | 30,375 (60.9) | 884,479 (51.1) |
|
| ||
| TBI | 2,936 (5.9) | 34,651 (2) |
|
| ||
| CNS infection | 172 (0.4) | 1,463 (0.1) |
|
| ||
| CNS tumors | 487 (0.98) | 2,452 (0.1) |
|
| ||
| Myocardial infarction | 2,100 (4.2) | 38,465 (2.2) |
|
| ||
| CHF | 5,212 (10.5) | 99,341 (5.7) |
|
| ||
| Peripheral vascular disease | 3,567 (7.2) | 69,935 (4) |
|
| ||
| Cerebral vascular disease | 9,877 (19.8) | 106,145 (6.1) |
|
| ||
| Dementia | 1,979 (4) | 21,462 (1.2) |
|
| ||
| Chronic kidney disease | 4,041 (8.1) | 76,746 (4.4) |
|
| ||
| Chronic pulmonary disease | 7,962 (16) | 166,360 (9.6) |
|
| ||
| Diabetes | 14,173 (28.4) | 53,333 (18.9) |
|
| ||
| Cancer | 4,750 (9.5) | 133,411 (7.7) |
|
| ||
| AIDS | 46 (0.09) | 664 (0.04) |
|
| ||
| Metastatic disease | 536 (1.1) | 13,030 (0.8) |
|
| ||
| Tobacco use | 1,163 (2.3) | 21,849 (1.3) |
|
| ||
| Alcohol use | 2,381 (4.8) | 43,676 (2.5) |
Abbreviations: IQR, interquartile range; SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Self-reported by patients or their surrogates.
The annual seizure incidence rate was 0.61% (95% CI, 0.60-0.61%) in those without cirrhosis and 1.17% (95% CI, 1.03-1.33%) in those with cirrhosis. Patients with cirrhosis had a higher risk of seizure in an unadjusted model (hazard ratio [HR], 1.7; 95% CI, 1.5-2.0) and after adjustment for demographic characteristics (HR, 1.8; 95% CI, 1.6-2.0). However, after adjustment for demographic characteristics, general medical comorbidities, and the pre-specified seizure risk factors of cerebrovascular disease, central nervous system tumors, traumatic brain injury, and central nervous system infections, there was no longer a significant association (HR, 1.1; 95% CI, 1.0-1.3; P = 0.06). There remained a significant association between cirrhosis and status epilepticus in models adjusting for demographics and comorbidities (HR, 1.9; 95% CI, 1.3-2.8) (Figure 1).
Figure 1.
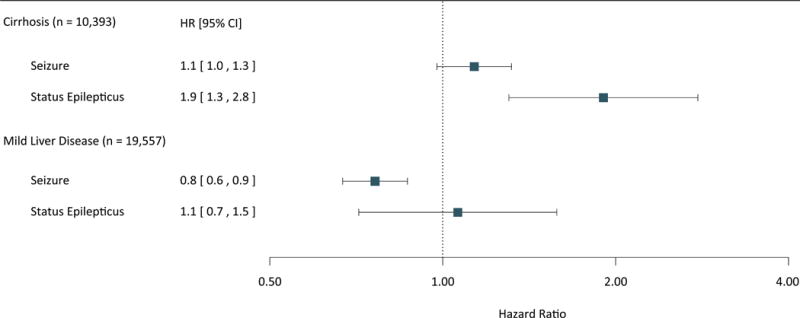
Risk of Seizures and Status Epilepticus in Patients with Liver Disease
Hazard ratios and 95% confidence intervals for seizure and status epilepticus in patients with liver disease.
Risk of Seizure in Patients with Mild Liver Disease
We identified 19,557 patients with mild liver disease. The annual incidence of seizure was the same among patients with mild liver disease 0.6% (95% CI, 0.5-0.7%] versus the general population (0.6%; 95% CI, 0.6-0.61%]. We found no association between mild liver disease and an increase risk of seizures in an unadjusted model (HR, 0.9; 95% CI, 0.8-1.0; P = 0.13) or a model adjusted for demographics and comorbidities (HR, 0.7; 95% CI, 0.6-0.8). There was also no association between mild liver disease and status epilepticus in the adjusted model (HR, 1.1; 95% CI, 0.7-1.5) (Figure 1).
Discussion
In a large, nationally representative sample of Medicare beneficiaries, we generally found no association between liver disease and seizures. Of the four associations that we tested, we found an association between liver disease and increased seizure risk only in the case of cirrhosis and status epilepticus.
Prior reports have described seizures and status epilepticus as manifestations of liver disease, more often in acute liver failure and less often chronic liver failure.4–9 The relationship between liver disease and seizure should be interpreted cautiously as EEG interpretation may change among electroencephalographers. On the other hand, other literature suggests an increased GABAergic tone in patients with acute and chronic liver failure as a result of increased pregnenolone, and the progesterone metabolites tetrahydroprogesterone (allopregnanolone) and tetrahydrodeoxycorticosterone (THDOC)18, which would argue against the hypothesis that liver disease might cause seizures. However, there is also an increase proinflammatory cytokines in this population, which may lead to an increase epileptogenesis19, 20. Amidst this uncertainty, our population-based study suggests that, on the whole, liver disease is not an independent risk factor for seizures. The one exception is the association that we found between cirrhosis and status epilepticus. This could represent a true link indicating that cirrhosis can increase the risk of seizures that are prolonged and severe. For example, liver cirrhosis may reduce the seizure threshold in individuals with other seizure risk factors and thus create a favorable milieu for status epilepticus. However, it may also be a spurious association due to detection bias from more frequent hospitalizations in patients with cirrhosis, or it may be a chance finding given the multiple hypotheses we tested, the generally negative association between cirrhosis and seizures, and the very small number of patients with status epilepticus. Additionally, the observed association may reflect misinterpretation of triphasic waves or other generalized periodic patterns as status epilepticus in the setting of severe encephalopathy. The association between cirrhosis and status epilepticus therefore requires further study before any definitive inferences can be made.
Our study has multiple limitations. First, we used ICD-9-CM codes to identify patients with seizures and liver disease, which may have resulted in misclassification of our exposure and outcome. We think this is unlikely because we used previously validated codes for liver disease and seizures12, 13, and we have previously found robust associations with other conditions in patients with cirrhosis and seizures,21, 22 Second, we lacked data on the characteristics of EEG and neuroimaging findings, as well as data on medication use, so we cannot account for the confounding effects of anti-epileptic or epileptogenic drugs that may have been used in our patient population. Third, our analysis was based on Medicare beneficiaries and may not be generalizable to younger populations.
In conclusion, our population-based study suggests that although metabolic derangements often cause seizures, and although liver disease is often association with metabolic derangements and neurological deficits such as encephalopathy, there is generally no independent association between liver disease and seizures.
Summary.
Metabolic derangements often cause seizures, the association between liver disease and seizures is unclear.
In our population-based study, we found an association between cirrhosis and status epilepticus.
We found no association between liver disease and seizures.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Acknowledgments
None.
Source of Funding: Dr. Kamel receives funding support from NIH/NINDS (grants K23NS082367, R01NS097443, and U01NS095869) as well as the Michael Goldberg Research Fund.
Role of the Sponsor: No funding organization had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
DR. AYHAM ALKHACHROUM (Orcid ID: 0000-0003-0352-5913)
Author Contributions: Hooman Kamel had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Alkhachroum, Rubinos, Parikh, Merkler, Kummer and Kamel.
Acquisition of data: Kamel.
Analysis and interpretation of data: Alkhachroum, Rubinos, Parikh, Merkler, Kummer and Kamel.
Drafting of the manuscript: Alkhachroum.
Critical revision of the manuscript for important intellectual content: Rubinos, Parikh, Merkler, Kummer, Reynolds, Chatterjee, Claassen and Kamel.
Statistical analysis: Kamel.
Administrative, technical, or material support: Kamel.
Study supervision: Kamel.
Conflicts of Interest and Financial Disclosures: Dr. Kamel serves as Deputy Editor of JAMA Neurology.
References
- 1.Bachir NM, Larson AM. Adult liver transplantation in the United States. Am J Med Sci. 2012;343:462–469. doi: 10.1097/MAJ.0b013e3182308b66. [DOI] [PubMed] [Google Scholar]
- 2.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. The Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 3.Adams RD, Foley JM. The neurological disorder associated with liver disease. Res Publ Assoc Res Nerv Ment Dis. 1953;32:198–237. [PubMed] [Google Scholar]
- 4.Shawcross DL, Wendon JA. The neurological manifestations of acute liver failure. Neurochem Int. 2012;60:662–671. doi: 10.1016/j.neuint.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s Diagnosis of Stupor and Coma. Fourth. New York: Oxford University Press; 2007. [Google Scholar]
- 6.Weissenborn K, Bokemeyer M, Krause J, et al. Neurological and neuropsychiatric syndromes associated with liver disease. AIDS. 2005;19:S93–98. doi: 10.1097/01.aids.0000192076.03443.6d. [DOI] [PubMed] [Google Scholar]
- 7.Eleftheriadis N, Fourla E, Eleftheriadis D, et al. Status epilepticus as a manifestation of hepatic encephalopathy. Acta Neurol Scand. 2003;107:142–144. doi: 10.1034/j.1600-0404.2003.02092.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Ueda H, Kida Y, et al. Hepatic encephalopathy with status epileptics(sic): A case report. World J Gastroenterol. 2006;12:1793–1794. doi: 10.3748/wjg.v12.i11.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficker DM, Westmoreland BF, Sharbrough FW. Epileptiform Abnormalities in Hepatic Encephalopathy. J Clin Neurophys. 1997;14:230–234. doi: 10.1097/00004691-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Centers for medicare and medicaid services. Medicare limited dataset files. https://www.cms.gov/research-statistics-data-and-systems/files-for-order/limiteddatasets/. Accessed july 14, 2017 [online]
- 11.Walkey AJ, Hammill BG, Curtis LH, et al. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kee VR, Gilchrist B, Granner MA, et al. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):183–193. doi: 10.1002/pds.2329. [DOI] [PubMed] [Google Scholar]
- 13.Nehra MS, Ma Y, Clark C, et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg D, Lewis J, Halpern S, et al. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 18.Ahboucha S, Gamrani H, Baker G. GABAergic neurosteroids: the “endogenous benzodiazepines” of acute liver failure. Neurochem Int. 2012;60:707–714. doi: 10.1016/j.neuint.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Barker-Haliski ML, Löscher W, White HS, et al. Neuroinflammation in epileptogenesis: Insights and translational perspectives from new models of epilepsy. Epilepsia. 2017;58(Suppl 3):39–47. doi: 10.1111/epi.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295–2304. doi: 10.1007/s00018-005-5089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh NS, Navi BB, Schneider Y, et al. Association Between Cirrhosis and Stroke in a Nationally Representative Cohort. JAMA Neurol. 2017;74:927–932. doi: 10.1001/jamaneurol.2017.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reznik ME, Merkler AE, Mahta A, et al. Long-term risk of seizures in adult survivors of sepsis. Neurology. 2017;89:1476–1482. doi: 10.1212/WNL.0000000000004538. [DOI] [PMC free article] [PubMed] [Google Scholar]


