SUMMARY
Deficits in social engagement are diagnostic of multiple neurodevelopmental disorders, including autism and schizophrenia [1]. Genetically tractable animal models like zebrafish (Danio rerio) could provide valuable insight into developmental factors underlying these social impairments, but this approach is predicated on the ability to accurately and reliably quantify subtle behavioral changes. Similarly, characterizing local molecular and morphological phenotypes requires knowledge of the neuroanatomical correlates of social behavior. We leveraged behavioral and genetic tools in zebrafish to both refine our understanding of social behavior and identify brain regions important for driving it. We characterized visual social interactions between pairs of adult zebrafish, and discovered that they perform a stereotyped orienting behavior that reflects social attention [2]. Furthermore, in pairs of fish, the orienting behavior of one individual is the primary factor driving the same behavior in the other individual. We used manual and genetic lesions to investigate the forebrain contribution to this behavior and identified a population of neurons in the ventral telencephalon whose ablation suppresses social interactions, while sparing other locomotor and visual behaviors. These neurons are cholinergic and express the gene encoding the transcription factor Lhx8a, which is required for development of cholinergic neurons in the mouse forebrain [3]. The neuronal population identified in zebrafish lies in a region homologous to mammalian forebrain regions implicated in social behavior such as the lateral septum [4]. Our data suggest that an evolutionarily conserved population of neurons controls social orienting in zebrafish.
Graphical abstract
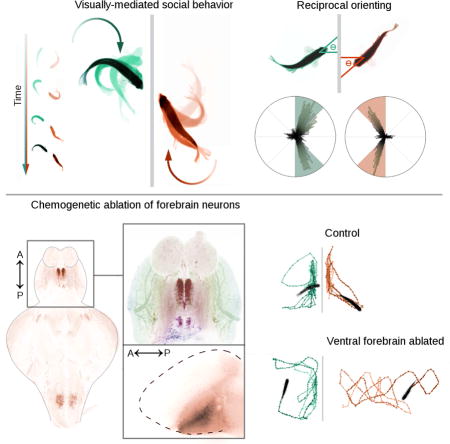
RESULTS & DISCUSSION
Social interactions in zebrafish
Adult zebrafish are highly motivated to aggregate, shoal, and school in both the wild and the laboratory [5]. Because of this natural sociability, zebrafish are an increasingly popular model for understanding the underlying genetic and developmental mechanisms that affect social behavior. When housed in pairs, zebrafish exhibit behaviors like parallel swimming and turning toward their partner [6]. Zebrafish are known to vary their behavior based on parameters of the social stimulus such as number, location, and velocity of conspecifics [7,8], but the extent to which these social interactions are driven by the behavior of the stimulus conspecific is largely undescribed. Existing metrics such as distance from conspecifics may be insensitive to disruptions in more subtle components of social interactions, such as the importance of behavioral stimuli. To address this shortcoming, we designed a strictly visual assay to induce social behavior consisting of separate tanks divided by a panel of electrochromic film, which can be electronically switched from opaque to transparent (Figure 1A). We identified a stereotyped orienting pattern in adult fish, similar to behavior previously described in juveniles [9], that occurs when an individual fish is presented with a social stimulus, a fish in the neighboring tank (Figures 1B-C and Video S1). We found that in these fish dyads, orienting between 45-90° relative to the electrochromic film divider transiently increases when the social stimulus is visible (p < .001, n = 112), returning to baseline within 5 min (Fig. 1D). These results suggest that zebrafish interact with a conspecific by orienting their body axis, and that in our assay they habituate to the social stimulus. Previous work suggests this behavior can reflect social attention in zebrafish, supporting the relevance of orienting in our assay [2,6]. Similarly, orienting metrics are highly correlated to average distance from the divider, a commonly used measure of social preference in other studies of fish social interactions (Figure 1E; R2 = .562, p < .001, n =112) [10].
Fig 1. Automated analysis of social orienting in wild-type and drug-treated zebrafish.
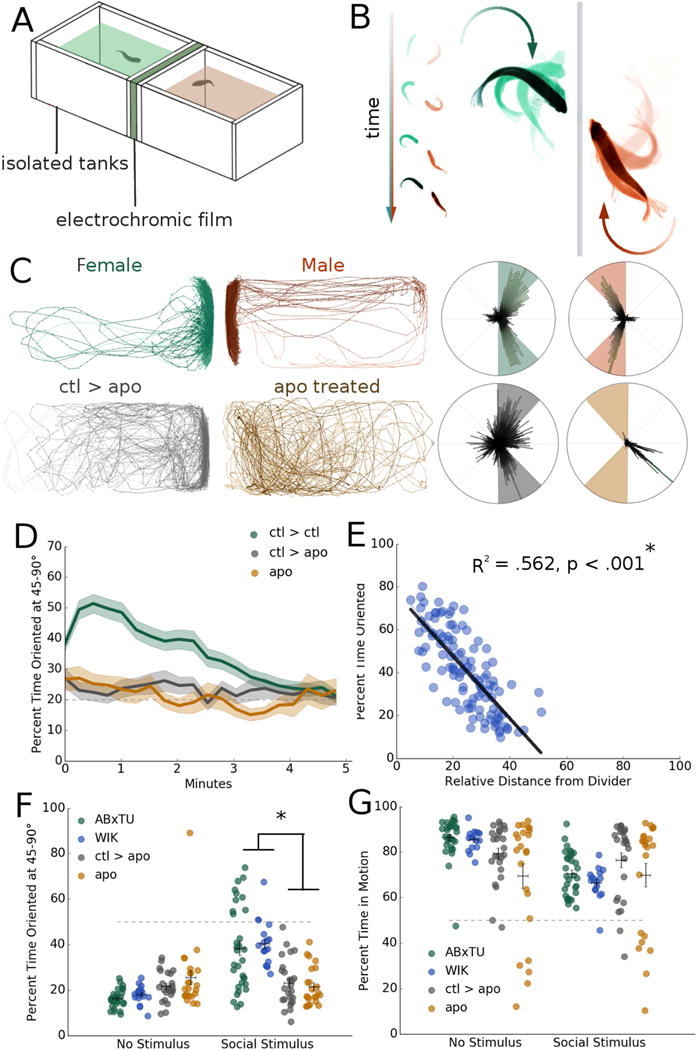
A. Schematic of dyad assay apparatus, consisting of two isolated 7″ (length) × 3.5″ (width) × 2.5″ (depth) tanks separated by a panel of electrochromic film.
B. Orienting behavior in a pair of isolated zebrafish.
C. Representative traces and polar histograms for a male and female ABxTU dyad and a control (ABxTU) animal (ctl>apo) paired with an impaired apomorphine-treated zebrafish (apo treated; apo). ABxTU animals paired with one another significantly increase their orienting behavior when exposed to another fish (*p < .001, n = 112) but not when paired with an apomorphine-treated fish that serves as a suboptimal social stimulus (p = .516, n =23).
D. Percent time oriented over 5 minute period for zebrafish paired with a normal social stimulus (ctl > ctl), zebrafish paired with a suboptimal stimulus (ctl > apo), and zebrafish treated with apomorphine (apo).
E. Correlation between test fish’s orienting behavior and relative distance from the divider. Relative distance is expressed in terms of minimum to maximum distance, 0-100%. Orienting behavior is significantly correlated with distance from the divider. R2 = .562, *p < .001, n = 112 linear regression.
F. Average percent time oriented at 45-90° for male and female ABxTU, male and female WIK pairs, ctl>apo and apo zebrafish before and after presentation of a social stimulus. *p < .05, repeated measures mixed model ANOVA with post-hoc simple effects tests. Horizontal bars: mean, vertical bars: +/− s.e.m.
G. Percent time in motion for all groups before and during social stimulus presentation. Horizontal bars: mean, vertical bars: +/− s.e.m.
See also Figure S1 and Video S1.
To determine whether this behavior is explicitly social and driven specifically by conspecifics, we performed identical experiments using an empty tank or a novel object and found no similar transient increase in orienting behavior (p = .367, n = 20 and p = .355, n = 20 respectively, Fig S1A). Interestingly, when exposed to an empty tank stimulus zebrafish exhibit a statistically significant increase in preference for the side of the tank near the divider even in the absence of orienting (p = .001, n = 20, Fig S1B) such that there is an equal preference for both transparent sides, an effect not seen with a novel object stimulus (p = .948, n = 20, Fig S1B). These results suggest that while zebrafish approach the divider in the absence of a social stimulus, orienting behavior requires an interaction between two fish. We conclude that orienting with an angle between 45-90° to the divider is a rigorous measure of social interaction that may reflect parallel swimming and orienting behavior in naturalistic settings [5,6,8,9].
We tested male-female pairs in two wild-type laboratory strains (ABxTU and WIK) and found they respond similarly to a social stimulus, suggesting that social orienting occurs across zebrafish strains (Figure 1F; p = .997, n = 112 and 16 respectively). We also found no differences in percentage of time in motion between strains (Figure 1G; p = .393). Measuring time spent between 45-90° allowed us to identify subtle differences between ABxTU dyads based on sex (Figure S1C), indicating that females are less likely to engage in social orienting than males regardless of the sex of the stimulus fish (p = .014, n = 57 and 55 respectively, Figure S1C). These findings replicate previous descriptions of sex differences in zebrafish social behavior [10,11]. There were no sex differences in the percentage of time spent in motion (Figure S1D; p = .630), suggesting that sex differences are specific to social engagement. In all subsequent experiments, we used ABxTU male-female dyads evenly distributed across experimental groups to equally represent both sexes in our dataset and to reduce potential confounds due to male-male aggression [11].
To determine if the orienting assay is sufficiently sensitive to detect impaired behaviors, we treated zebrafish with apomorphine (apo), a broad dopamine receptor agonist known to impair social interactions in mice [12]. Consistent with mammalian studies, apo-treated fish had impaired social interactions and showed no significant increase in time spent at 45-90° when exposed to control fish (Figure 1F; p = .260, n = 23). We observed suppression in the percentage of time spent in motion for apo-treated fish, but this effect was not statistically significant (Figure 1G, p = .635). Interestingly, control fish paired with drug-treated stimulus fish (ctl > apo) also significantly reduced their orienting behavior and place preference, to the extent that they do not differ from the ‘no stimulus’ period. This effect was sustained over the 5 minute recording period (Figures 1C-D; Figure 1F; Figure S1E; p = .516, n = 23), suggesting that active social engagement of the partner is necessary for a fish to exhibit social orienting behavior. We observed suppression in the percentage of time spent in motion for apo-treated fish, but this effect was not statistically significant (Figure 1G, p = .635).
We probed whether social engagement is reduced because apo-treated fish spend more time distant from the divider. We placed apo-treated fish in a shortened tank that restricted their movement away from the divider, and found that test fish exposed to apo-treated stimulus fish still had suppressed social orienting relative to controls (Figures 2A-D; p = .009, n = 26). We conclude from these results that another socially-engaged fish is the stimulus required for social orienting, and that the presence of another fish that is not socially engaged is insufficient regardless of their proximity.
Fig 2. Behavioral feedback drives social orienting within a zebrafish dyad.
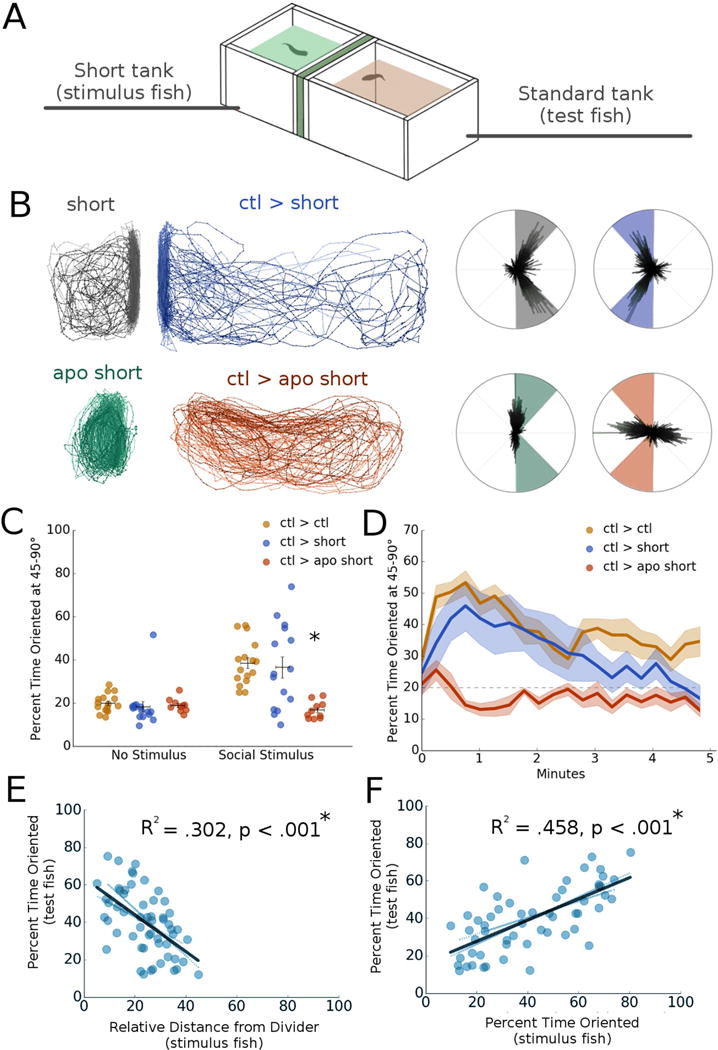
A. Schematic of short tank dyad assay apparatus, where the stimulus fish’s tank is truncated to half size to restrict movement away from the divider.
B. Representative traces and polar histograms of control fish (ctl > short) and fish exposed to a sub-optimal social stimulus (ctl > apo short). Test fish exposed to a sub-optimal stimulus had significantly suppressed orienting behavior relative to controls (*p = .009, n = 26).
C. Average percent time oriented at 45-90° for shor t tank experiments before (no stimulus) and after (social stimulus) social stimulus presentation. *p < .05, repeated measures mixed model ANOVA with post-hoc simple effects tests. Horizontal bars: mean, vertical bars: +/− s.e.m.
D. Percent time oriented over 5 minute period for short tank experiments.
E. Correlation plot between the test fish’s percent time oriented and the stimulus fish’s relative distance from the divider (R2 = .302, *p < .001, n = 112). *p < .05, linear regression.
F. Correlation plot between the test fish’s percent time oriented and the stimulus fish’s percent time oriented (R2 = .458, p < .001, n = 112) *p < .05, linear regression.
See also Figure S2.
We further examined the role of behavior versus distance of the stimulus fish from the divider in driving social orienting. Orientation of the test fish and distance of the stimulus fish are highly correlated (R2 = .302, p < .001), as is the orienting behavior of the stimulus fish (R2 = .458, p < .001; Figures 2E-F). However, multiple linear regression reveals that when both the orientation and distance of the stimulus fish are taken into account as predictive variables, only orienting behavior significantly accounts for variability in the test fish (p < .001 and p = .178 respectively, n = 112), suggesting that proximity of the stimulus fish exerts less influence than its orientation. There are no such relationships between the test and stimulus fish in the pre-stimulus period when they are not yet visible to one another (Figures S2A-B). We conclude that orienting behavior, and not proximity, of the stimulus fish is what drives orienting behavior in the test fish.
We examined whether visual cueing between fish might account for the simultaneous orienting behavior of both stimulus and test fish. Time-lag cross correlation reveals that test fish mirror the stimulus fish by matching their angle, with a lag of about 1 second (Figure S2C). To determine if this correlation was a spurious relationship and would occur regardless of the behavior of the stimulus fish, we performed a permutation analysis by shuffling data randomly such that results from each test fish were matched with results from a stimulus fish from a different dyad. We found that the correlation was lost and therefore directly reflects dynamic interactions between individuals orienting to one another (Figure S2C). These results suggest that zebrafish copy each other’s motions, consistent with findings reported at earlier developmental stages [9]. In summary, our analysis of orienting behavior revealed that under normal conditions social interactions are reciprocated between fish, and that this effect is primarily driven by orienting behavior rather than absolute distance from the divider.
Neuroanatomical correlates of social behavior
The telencephalon, a region of the teleost brain proposed to be evolutionarily and functionally homologous to mammalian subcortical structures that regulate memory, emotion, and social behavior [13, 14], has been implicated in social behaviors in fish [15, 16, 17]. We investigated the role of the telencephalon in social orienting by manually lesioning it via insertion of a fluorescent dye-coated needle through the right nostril [18]. The needle pointed toward the brain midline, and was angled up or down to cause dorsal or ventral injury respectively (Figures 3A-B). Lesioned zebrafish were allowed to recover for one hour and then tested in our dyad assay against unlesioned controls. Injuries to the ventral telencephalon (vTel) significantly disrupted orienting behavior. In contrast, the behavior of dorsally lesioned animals was not significantly different from ABxTU controls (n = 16) from a separate experiment (Figures 3D-F; ventral: p < .001, n = 7; dorsal: p = .076, n = 9). To rule out the possibility that lesioned animals had locomotor deficits, we measured the percentage of time spent in motion and found that this parameter did not differ from controls (Figure 3G). We dissected a subset of brains (n = 7) and located the lesions using confocal microscopy (Figure 3B) [19]. We confirmed that severe social orienting deficits are most associated with injury to the ventral forebrain (Figure 3C). These deficits are accompanied by a disruption in the correlation between distance from the divider and social orienting (Figure 3H; R2 = .188, p = .243).
Fig 3. Ventral telencephalic lesions disrupt social orienting.
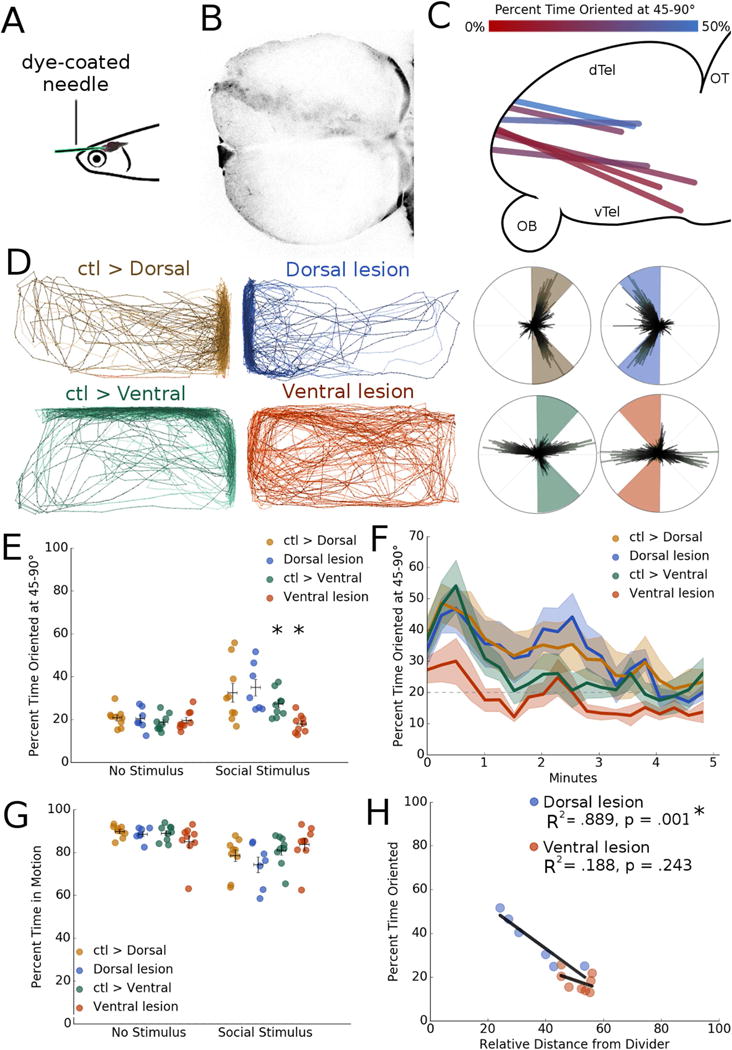
A. Schematic of lesion technique, where a dye-coated needle is inserted into the nostril of an anesthetized zebrafish to injure the forebrain.
B. Representative image of lesion track through forebrain (dorsal view).
C. Lesion tracks localized from a subset of zebrafish, color-coded to indicate severity of social deficit by location (sagittal view).
D. Representative traces of lesioned zebrafish and control stimulus fish. Dorsally lesioned zebrafish exhibit no social impairments relative to controls (p = .974, n = 9), however ventral injuries result in a severe reduction in both distance from the divider and orienting behavior (*p = .007, n =7).
E. Average percent time oriented at 45-90° for lesi on experiments before (no stimulus) and after (social stimulus) social stimulus presentation. *p < .05, repeated measures mixed model ANOVA with post-hoc simple effects tests. Horizontal bars: mean, vertical bars: +/− s.e.m.
F. Percent time oriented over 5 minute period for lesion experiments.
G. Percent time in motion for all lesion groups before and after social stimulus presentation. Horizontal bars: mean, vertical bars: +/− s.e.m.
H. Correlation between orienting behavior and relative distance from divider in dorsally and ventrally lesioned zebrafish. Dorsally lesioned fish retain a significant correlation between orienting and distance from the divider (R2 = .889, *p < .001), but ventrally lesioned fish lose this relationship and more closely resemble the no stimulus period (R2 = .188, p = .243). *p < .05, linear regression.
We further validated our finding that the vTel is important for teleost social behavior by using the GAL4/UAS system to chemo-genetically ablate different neuronal populations in the forebrain. We drove the expression of nitroreductase, a bacterial enzyme that is inert until exposed to its substrate metronidazole (MTZ), at which point it generates toxic metabolites. This paradigm provides a method for temporally-controlled ablation of discrete cell populations. We selected three transgenic lines with distinct expression patterns in the forebrain and elsewhere (Figure 4A-B), y321 [ventromedial telencephalon and hindbrain, Et(cfos:kGal4ff)y321;UAS:nfsB-mCherry], y299 [dorsal and anterior telencephalon, olfactory bulb, optic tectum, and hindbrain, Et(cfos:kGal4ff)y299;UAS:nfsB-mCherry], and dlx (ventrolateral telencephalon, optic tectum, ventral diencephalon and cerebellum, dlx5a/6a:kalTA4;UAS:nfsB-mCherry) [20]. We exposed each line to 10 mM MTZ by 24 hour bath application and subsequently tested behavior after washout. We confirmed the efficacy of this protocol by imaging cleared brains [19] (Figure S3A).
Fig 4. Chemo-genetic ablation of cholinergic neurons in the ventral telencephalon disrupts social orienting.
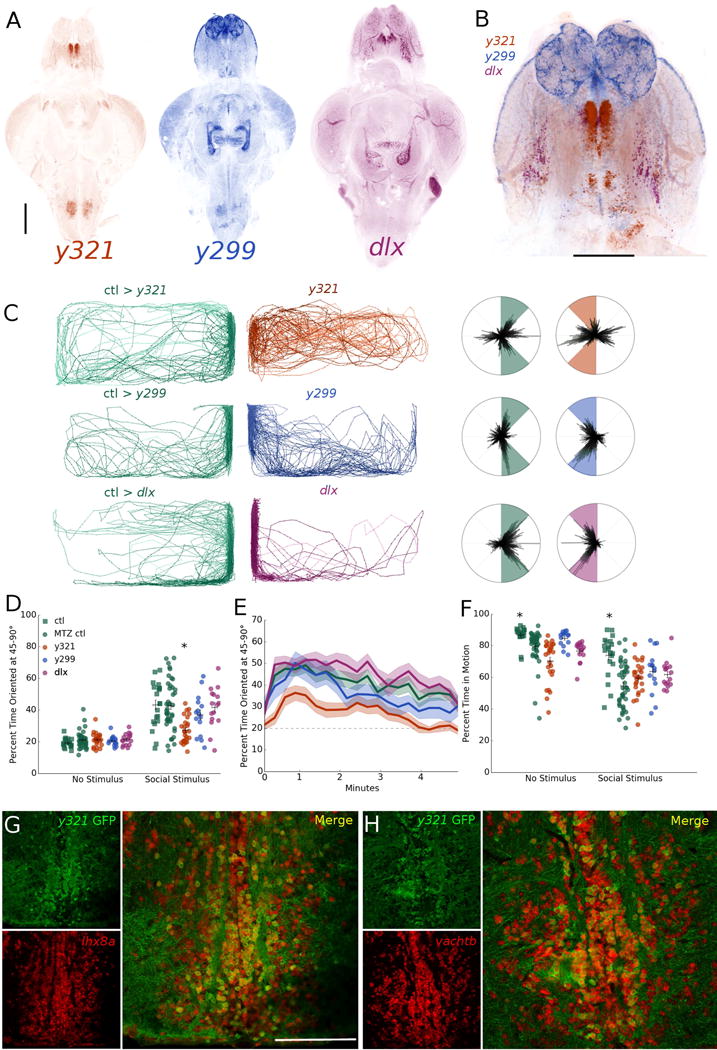
A. Whole-brain z projections of transgenic expression in y321, y299, and dlx gal4 lines. Scale bar: 200 μm.
B. Z-projection overlay of registered brains showing expression overlap and differences in the telencephalon. Scale bar: 200 μm.
C. Representative traces and polar histograms of y321, y299, and dlx lines following nitroreductase ablation of transgene-expressing cell populations.
D. Average percent time oriented at 45-90° for chem o-genetic ablation experiments before and after social stimulus presentation. *p < .05, repeated measures mixed model ANOVA with post-hoc simple effects tests. Horizontal bars: mean, vertical bars: +/− s.e.m.
E. Percent time oriented over 5 minute period for chemo-genetic ablation experiments.
F. Percent time in motion for all ablation groups before (no stimulus) and after (social stimulus) social stimulus presentation. *p < .05, repeated measures mixed model ANOVA with post-hoc simple effects tests. Horizontal bars: mean, vertical bars: +/− s.e.m.
G. In situ hybridization images of y321:gal4;UAS:GFP neurons labeled for lhx8a transcripts. Scale bar: 100 μm.
H. In situ hybridization images of y321:gal4;UAS:GFP neurons labeled for vachtb transcripts. See also Figure S3.
We found significant reductions in orienting behavior in y321 ablated zebrafish relative to drug-treated controls (Figures 4C-E, S3B; p = .006, n = 26), but not in y299 or dlx ablated animals (p = .995, n = 16 and p = .390, n = 16 respectively). Wild-type siblings exposed to MTZ did not differ from untreated transgenic or wild-type controls in any group (Figure S3B), ruling out drug effects. MTZ treatment significantly reduced percentage of time in motion relative to untreated controls in all conditions (Figure 4F; p < .001), however no ablated animals differed from drug-treated control siblings during the stimulus period as determined by a post-hoc Tukey’s test (y321: p = .512, y299: p = .132, dlx: p = .299). No changes in visually-mediated bias toward the transparent side of the tank during the no stimulus period were detected in any ablated or drug-treated animals (Figure S3C), indicating the behavioral deficits in y321 ablated zebrafish are specific to social orienting. We ruled out the possibility that the reduction in orienting is driven by the reduction in preference for the side of the tank adjacent to the divider in y321 ablated fish by analyzing only the percent time oriented when the animals occupy the 50% of the tank nearest the divider, and found y321 ablated fish still differed significantly from controls (p = .004). Similarly, the correlation between distance from the divider and orienting is disrupted in y321 ablated fish such that there is no significant relationship between the two during the social stimulus period (Figure S3D; R2 = .131, p = .069).
To further test how these cells might contribute to social orienting and how y321 ablated animals respond to a non-social stimulus, we paired y321 ablated zebrafish with apomorphine-treated stimulus fish. Ablated y321 fish showed a nonsignificant reduction in orienting when presented with a suboptimal social stimulus, demonstrating the y321 line may not capture the entire population of vTel cells downstream of visual, behavioral input (Figure S3E-F). Although it is not possible in the current experiment to completely rule out the contribution of hindbrain neurons, we confirmed that there is a high degree of spatial overlap between the hindbrain cells expressing in the y321 and y299 lines (Figure S3G), suggesting that vTel neurons likely play a more significant role.
To characterize the molecular identity of these vTel neurons, we performed immunohistochemistry and in situ hybridization on coronal sections of adult y321:GAL4;UAS:GFP zebrafish forebrains. The y321 enhancer trap insertion is located close to the lhx8a locus [20], which encodes a transcription factor associated with cholinergic neuron fate in the mouse forebrain [3]. In situ hybridization with probes to the gene encoding this transcription factor confirmed the transgenic population represents a subset of neurons expressing lhx8a (Figure 4G). We found that the overwhelming majority (97.6% +/− 2.58 on average, 625/640 total neurons across 4 brains) of GFP expressing cells were cholinergic (Figure 4H). Based on their anatomical location and gene expression patterns, these cells may be homologous to a population of lhx8-expressing, cholinergic basal forebrain neurons that is also found in mammals [3].
Conclusions
Our data show that social engagement in zebrafish requires a behavioral visual stimulus provided by another socially-engaged fish. We demonstrated that both pharmacological manipulation of dopaminergic systems and ablation of a portion of the ventral telencephalon produce predictable deficits in social behavior. Our results also provide evidence that an as yet uncharacterized population of cholinergic neurons in the ventral telencephalon are critical for social interactions in zebrafish. The ventral telencephalic region corresponds to mammalian forebrain regions, such as the lateral septum, that have been implicated in social behavior [21,22], suggesting an evolutionarily conserved population of cells may drive social orienting in zebrafish and mammals. Given that the inputs to these cells are undescribed, these findings are promising for future studies into the visual circuitry required to drive social behavior.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Philip Washbourne (pwash@uoregon.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Zebrafish (ABxTU and WIK) were maintained according to standard protocols [24] at 28°C with a 14/10 light/dark cycle. All procedures were performed according to a protocol approved by the University of Oregon Animal Care and Use Committee (#15-33). Tg(dlx5a/6a:kalTA4) fish were generated by inserting the optimized Gal4 element kalTA4 [25] into a plasmid containing the dlx5a/6a intergenic regulatory elements ([26]; generous gift of Marc Ekker). The DNA construct was injected into 1-cell stage Tg(UAS:GFP) embryos. Transgenic fish recapitulate the expression pattern of the line ot1 (Tg[1.4dlx5a-dlx6a:GFP]).
Unless otherwise specified, all experiments consisted of male and female pairs of zebrafish from the ABxTU background evenly distributed across conditions. Animals were between 2-12 months for all experiments and test and stimulus fish were age-matched. Transgenic animals were screened for fluorescent protein expression at 2-5 days post fertilization.
METHOD DETAILS
Social Behavior Assay
Two custom acrylic tanks composed of ¼″ panels measuring 3.5″ (width) × 7″ (length) × 2.5″ (depth) separated by a divider (a panel of opaque electrochromic film encased in ⅛″ thick acrylic sheets) were used. One long (7″) side of the tank was opaque white acrylic, while all other sides were transparent panels, allowing for an additional measure of visually-mediated bias to the transparent side of the tank within the same experiment. The behavior apparatus was illuminated from above using a daylight white LED panel measuring 18” × 24″ (Environmental Lights, UTLP-18-24), and contrast enhanced using a panel of light-diffusing plastic as the tank lid. The behavior apparatus was located in a room heated to 28°C. Tanks were placed on a 1″ thick sheet of clear acrylic and imaged from below with a Logitech HD Webcam C310 at 640×480 resolution. Zebrafish were placed individually into tanks with clean fish water at a depth of 2″ and recorded for 5 minutes at 10fps (no stimulus stage). The electrochromic film was then switched on to become transparent and allow the individuals to view one another. In the case of non-social stimulus experiments, the adjacent tank was either empty or contained a novel object placed near the divider (a plastic yellow object approximately the same size as an adult zebrafish). Recordings were performed for an additional 5 minutes (social or control stimulus stage). Both average relative distance from the divider (0-100%) and percentage of time oriented between 45-90° were used as dependent measures. Percentage of time spent in motion (as defined by moving a minimum of ⅓ the fish’s body length from one frame to another) and x,y location in the tank was also computed for each frame.
Electronics Control
Data acquisition and electronics control were achieved with a combination of Python 2.7 and an Arduino Uno R3 microcontroller. A Python script captured individual frames from a Logitech HD Webcam C310 camera for all behavior experiments.
Electrochromic film (Justin Cary, CaryShop) was switched on by a Python script communicating via USB with an Arduino Uno R3 microcontroller, using a DC 12V relay module (SainSmart) and a 12-60V inverter.
Apomorphine Treatment
ABxTU zebrafish (ages 2-12 months) were incubated in a 20 μM solution of apomorphine (R-(−)-Apomorphine hydrochloride hemihydrate, CAS 41372-20-7, Sigma) prepared in fish water for ten minutes. They were then netted to a rinse solution of clean fish water, allowed to recover for approximately one minute, and transferred again into fresh fish water and tested in our social behavior assay.
Manual lesions
Adult ABxTU zebrafish (6-12 months) were anesthetized in 4 mg/mL MS-222, then placed into a slit cut in a sponge soaked in MS-222. A 27½ G needle coated in DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Invitrogen) was inserted 1 mm into the right nostril and pointed toward the brain midline. The needle was angled upward or downward to cause dorsal or ventral injury respectively. Following injury, zebrafish were returned to fresh fish water and allowed to recover for 1 hour before behavioral analysis. Animals were monitored for signs of discomfort or distress as per our IACUC protocol. A subset of zebrafish were euthanized by hypothermal shock and processed using our modified CUBIC protocol [20].
Chemo-genetic ablation
Transgenic zebrafish (ages 2-3 months) expressing UAS:nfsB-mCherry were incubated in 10mM metronidazole (MTZ) prepared in fish water in groups of 20 overnight in the dark. They were then transferred to fresh fish water and allowed to recover for 1 hour before behavioral testing. Zebrafish with the genotypes y321:GAL4;UAS:nfsB-mCherry, y299:GAL4;UAS:nfsB-mCherry and dlx5/6:KaltA;UAS:nfsB-mCherry maintained in an ABxTU background were chemo-genetically ablated. For all experiments, three control groups consisting of clutchmates were included to control for effects of genotype and drug exposure: wild-type MTZ-, wild-type MTZ+, and transgenic MTZ-. No chemogenetically ablated animals exhibited signs of discomfort or distress as described by our IACUC protocol.
Brain Clearing & Imaging
We utilized a modified (one-step) CUBIC protocol [20] to clear transgenic and lesioned brains for imaging. Zebrafish were anesthetized and euthanized in ice water, immediately decapitated, and their heads fixed in 4% paraformaldehyde (PFA) in 1X phosphate buffered saline (PBS) at room temperature. Brains were then dissected and fixed overnight in 4% PFA at room temperature. After rinsing in 1X PBS, brains were transferred to CUBIC 1 solution (25 wt% of 80 wt% Quadrol, 25 wt% urea, 15 wt% Triton X-100 in dH2O) [20] and kept in a 37°C water bath for 2-3 days until transparent. Brains were removed from CUBIC 1 reagent and immediately mounted in either 1.5% low-melt agarose (lesions) or ProLong Gold antifade mounting medium (Invitrogen, transgenics).
Cleared brains were imaged by fluorescence confocal microscopy with a Leica DMI8-CS and a 10x objective by tiling multiple z stacks using Leica LAS X 2.0.0.14332.2 software. Overlays of multiple GAL4 lines were generated via nonrigid registration using the Computational Morphometry Toolkit (CMTK) [21,27].
In-situ hybridization
Adult y321:GAL4;UAS:GFP zebrafish (age 2-12 months) were anesthetized and euthanized in ice water, then decapitated and placed into 4% PFA for 1-1.5 hours before brains were dissected and fixed overnight in 4% PFA at room temperature. After fixation, brains were rinsed 3x in PBS and dehydrated in 20% sucrose in PBS for 24 hours, followed by cryosection after mounting in agarose.
RNA in situ hybridization on 16 μm brain sections was carried out according to the protocol by J. Talbot [28] using digoxigenin labeled probes for either lhx8 or vachtb. Sections were incubated overnight at 65°C with 200ng of probe per section. The following day, sections were washed in a graded concentration series of 5X saline sodium citrate (SCC) with 50% formamide/2X SSC ending in an incubation step with anti-digoxygenin Fab fragments (Roche) overnight at 4°C. Sections were washed 8x in 1M Tris HCl pH 7.5, 5M NaCl and 0.25% Tween 20 (TNT) and incubated for 5 minutes in the dark with amplification diluent, then incubated for 1 hour at room temperature in the dark with cyanine 3 (Cy3) for subsequent visualization. Endogenous peroxidase activity was quenched by incubation in 2% hydrogen peroxide in TNT for 1 hour. Sections were first incubated in primary antibody (chicken anti-GFP, 1:500, Aves Laboratories) and then secondary antibody (goat anti-chicken IgY-488, 1:500, Molecular Probes) overnight in 0.25% PBSTx. Sections were imaged on a Leica DMI8-CS confocal fluorescence microscope using a 40x objective.
QUANTIFICATION AND STATISTICAL ANALYSIS
Video Analysis
Behavioral data were analyzed using bespoke software written in Python 2.7 (DaniOPEN, https://github.com/stednitzs/daniopen). This software tracks the center of mass of fish and calculates their orientation for each frame, generating text files of these data for further analysis. Where necessary, images were pre-processed using ImageJ (NIH) [29] to subtract the background and enhance detection accuracy.
Cell quantification
Co-expression of GFP and lhx8 or vachtb was quantified using ImageJ (NIH) [29]. GFP expressing cells were manually identified and the number of GFP+ neurons that also expressed lhx8 or vachtb were counted.
Statistics
For all experiments, data were screened for normality using descriptive statistics of skewness and kurtosis. A p value < .05 was considered significant. Outliers were not removed from any experimental groups. Control zebrafish that exhibited excessive freezing behavior (as determined by spending less than 10% of the duration of the experiment in motion) were removed prior to analysis (6 out of 430 fish across all experiments, 2 female ABxTU dyads and 1 male ABxTU dyad). No lesioned, ablated, or drug-treated animals were excluded from our analyses.
For social behavior experiments, analysis was performed in IBM SPSS Statistics 24 via repeated measures mixed-model ANOVA, comparing the no stimulus and post-stimulus period by group. In the event of a significant time by group interaction effect, main effects between groups were analyzed by post-hoc simple effects tests using the Bonferroni correction for multiple comparisons. Linear regression analyses were also performed in SPSS 24. Cross-correlations were computed in Python using a time lag window of +/− 5 seconds (50 frames).
DATA AND SOFTWARE AVAILABILITY
Behavioral data were analyzed using bespoke software (DaniOPEN) written in Python 2.7, available from github (https://github.com/stednitzs/daniopen).
Behavioral data are archived via Mendeley (http://dx.doi.org/10.17632/d79p9sttkn.1)
Supplementary Material
Video S1. Example experimental video of two ABxTU zebrafish. Related to Figure 1 consisting of female (left) and male (right) dyad. The divider separating the two tanks is overlaid in black in the opaque divider (no stimulus) condition, and switches to white in the transparent divider (social stimulus) condition.
Acknowledgments
We thank Marc Ekker (University of Ottawa), Reinhard Köster (TU Braunschweig) and Harold Burgess (National Institutes of Health) for providing reagents. We also thank Don Pate (University of Oregon) for assistance with electronics engineering and Adam Christensen and the UO Zebrafish Facility for fish husbandry. This work was supported by National Institutes of Health Grant R33MH104188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization: S.J.S. & P.W.; Methodology: S.J.S., E.M.M. & D.N.; Investigation: S.J.S., E.M.M. & D.N.; Software and Formal Analysis: S.J.S.; Resources: A.T.; Writing – Original Draft: S.J.S.; Writing – Review & Editing: S.J.S, A.T., J.S.E. & P.W.; Funding Acquisition: J.S.E. & P.W.; Supervision: P.W.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- 1.Fakhoury M. Autism spectrum disorders: A review of clinical features, theories, and diagnosis. Int J Dev Neurosci. 2015;43:70–7. doi: 10.1016/j.ijdevneu.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Abril-de-Abreu R, Cruz J, Oliveira RF. Social eavesdropping in zebrafish: Tuning of attention to social interactions. Scientific Reports. 2015;5 doi: 10.1038/srep12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Marion O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci USA. 2003;100(15):9005–10. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke A, File SE. Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviors. Pharmacol Biochem Behav. 1982;17(4):623–8. doi: 10.1016/0091-3057(82)90334-3. [DOI] [PubMed] [Google Scholar]
- 5.Suriyampola PS, Shelton DS, Shukla R, Roy T, Bhat A, Martins EP. Zebrafish social behavior in the wild. Zebrafish. 2016;13(1):1–8. doi: 10.1089/zeb.2015.1159. [DOI] [PubMed] [Google Scholar]
- 6.Hinz RC, de Polavieja GG. Ontogeny of collective behavior reveals a simple attraction rule. Proc Natl Acad Sci U S A. 2017;114:2295–2300. doi: 10.1073/pnas.1616926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes Y, Rampersad M, Jia J, Gerlai R. The effect of the number and size of animated conspecific images on shoaling responses of zebrafish. Pharmacol Biochem Behav. 2015;139 doi: 10.1016/j.pbb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Laan A, Gil de Sagredo R, de Polavieja GG. Signatures of optimal control in pairs of schooling zebrafish. Proc Biol Sci. 2017;12(284):1852. doi: 10.1098/rspb.2017.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreosti E, Goncalo L, Kampff AR, Wilson SW. Development of social behavior in young zebrafish. Front Neural Circuits. 2015;9:39. doi: 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Way GP, Kiesel AL, Ruhl N, Snekser JL, McRobert SP. Sex differences in a shoaling-boldness behavioral syndrome, but no link with aggression. Behavioural Processes. 2015;113:7–12. doi: 10.1016/j.beproc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Ruhl N, McRobert SP, Currie WJ. Shoaling preferences and the effects of sex ratio on spawning and aggression in small laboratory populations of zebrafish (Danio rerio) Lab Anim. 2009;38(8):264–9. doi: 10.1038/laban0809-264. [DOI] [PubMed] [Google Scholar]
- 12.Awakawa O, Ikeda T. Apomorphine effect on single and paired rat open-field behavior. Physiol Behav. 1991;50(1):189–94. doi: 10.1016/0031-9384(91)90520-x. [DOI] [PubMed] [Google Scholar]
- 13.Mueller T, Dong Z, Berberoglu MA, Guo S. The dorsal pallium in zebrafish, Danio rerio (Cyrpinidae, Teleostei) Brain Res. 2011;1381:95–105. doi: 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieuwenhuys R. The development and general morphology of the telencephalon of actinopterygian fishes: synopsis, documentation and commentary. Brain Struct Funct. 2011;215(3–4) doi: 10.1007/s00429-010-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teles MC, Cardoso SD, Oliveira RF. Social plasticity relies on different neuroplasticity mechanisms across the brain social decision-making network in zebrafish. Front Behav Neuro. 2016;10(16) doi: 10.3389/fnbeh.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinozuka K, Watanabe S. Effects of telencephalic ablation on shoaling behavior in goldfish. Physiol Behav. 2004;81(1):141–8. doi: 10.1016/j.physbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt R, Beil T, Strahle U, Rastegar S. Stab wound injury of the zebrafish adult telencephalon: a method to investigate vertebrate brain neurogenesis and regeneration. J Vis Exp. 2014;90:51753. doi: 10.3791/51753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nature Protocols. 2015;10:1709–1727. doi: 10.1038/nprot.2015.085. [DOI] [PubMed] [Google Scholar]
- 20.Marquart GD, Tabor KM, Horstick EJ, Brown M, Burgess HA. High precision registration between zebrafish brain atlases using symmetric diffeomorphic normalization. GigaScience. 2017;6(8):1–15. doi: 10.1093/gigascience/gix056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke A, File SE. Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviors. Pharmacol Biochem Behav. 1982;17(4):623–8. doi: 10.1016/0091-3057(82)90334-3. [DOI] [PubMed] [Google Scholar]
- 22.Shin S, Pribiag H, Lilascharoen V, Knowland D, Wang X, Lim BK. Drd3 signaling in the lateral septum mediates early life stress-induced social dysfunction. Neuron. 2018;97(1) doi: 10.1016/j.neuron.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerfield M. The zebrafish book A guide for the laboratory use of zebrafish (Danio rerio) 4th. Eugene: Univ of Oregon Press; 2000. [Google Scholar]
- 24.Rohlfing T, Maurer CR. Nonrigid image registration in shared-memory multiprocessor ernvironments with application to brains, breasts, and bees. IEEE Trans Inf Technol Biomed. 2003;7(1):16–25. doi: 10.1109/titb.2003.808506. [DOI] [PubMed] [Google Scholar]
- 25.Distel M, Wullimann MF, Köster RW. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci USA. 2009;106(32):13365–70. doi: 10.1073/pnas.0903060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu M, Xi Y, Pollack J, Debiais-Thibaud M, Macdonald RB, Ekker M. Activity of dlx5a/dlx6a regulatory elements during zebrafish GABAergic neuron development. Int J Dev Neurosci. 2011;29(7):681–91. doi: 10.1016/j.ijdevneu.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Talbot JC, Nichols JT, Yan Y, Leonard IF, BreMiller RA, Amacher SL, Postlethwait JH, Kimmel CB. Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial mesenchymal interaction. Dev Biol. 2016;415(1):136–148. doi: 10.1016/j.ydbio.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7) doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granato M, Pascal H, Rauch G. A polymorphic zebrafish line for genetic mapping using SSLPs on high-percentage agarose gels. Tech Tips Online. 1997:T01208. [Google Scholar]
- 30.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mechanisms of Development. 2007;124(3) doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Developmental Biology. 2004;274(1):139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Hong E, Santhakumar K, Akitake CA, Ahn SJ, Thiss C, Thiss B, Wyart C, Mangin J, Halpern ME. Cholinergic left-right asymmetry in the habenulo-interpeduncular pathway. Proc Natl Acad Sci USA. 2013;110(52) doi: 10.1073/pnas.1319566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Example experimental video of two ABxTU zebrafish. Related to Figure 1 consisting of female (left) and male (right) dyad. The divider separating the two tanks is overlaid in black in the opaque divider (no stimulus) condition, and switches to white in the transparent divider (social stimulus) condition.


