Abstract
Background
We conducted a phase 1 trial to determine the maximum tolerated dose (MTD), toxicity profile, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary activity of cabozantinib in children with refractory or relapsed solid tumors.
Methods
Patients received cabozantinib tablets on a continuous dosing schedule in a rolling-six escalating phase 1 trial design. PK and PD studies were performed.
Results
Forty-one patients, median (range) age 13 (4-18) years, received cabozantinib to achieve a weekly cumulative dose equivalent to 30 (n=6), 40 (n=23) or 55 (n=12) mg/m2/day. At 40 mg/m2/d, DLTs were palmar-plantar erythrodysesthesia syndrome, mucositis, and elevated alanine aminotransferase, lipase, and bilirubin. At 55 mg/m2/d, hypertension, reversible posterior leukoencephalopathy syndrome, headache, fatigue, and proteinuria were DLTs. Frequent non-DLTs included diarrhea, hypothyroidism, fatigue, nausea, vomiting, elevated hepatic transaminases, and proteinuria. In subsequent cycles, DLTs occurred at all dose levels. Across all dose levels, the steady state exposure and peak cabozantinib concentrations were similar. Four patients experienced a confirmed partial response: medullary thyroid carcinoma (MTC; n=2), Wilms tumor, and clear cell sarcoma. Stable disease (>6 cycles) was seen in seven patients (MTC (n=2), Ewing sarcoma, synovial sarcoma, alveolar soft part sarcoma, paraganglioma, and ependymoma).
Conclusions
A protocol-defined MTD was not reached; DLTs and dose reductions for toxicity occurred in the first, and subsequent cycles, at all dose levels. Based on the toxicity profile, pharmacokinetics, and responses, the recommended dose of cabozantinib in pediatric patients with refractory solid tumors is 40 mg/m2/day. A phase 2 study of cabozantinib is being conducted.
Keywords: cabozantinib, pediatrics, phase 1
Introduction
Cabozantinib (XL184, COMETRIQ®; CABOMETYX™, Exelixis, San Francisco, CA) is a small molecule inhibitor of multiple tyrosine kinases including MET, VEGFR2, RET, and AXL. Selective inhibition of VEGFR2 may lead to increased invasiveness and metastasis, and preclinical models suggest that inhibition of VEGFR2 together with c-MET may decrease tumor size, decrease invasiveness and metastases1,2. Cabozantinib, 140 mg capsules once daily, was FDA approved in 2012 for patients with progressive metastatic medullary thyroid cancer (MTC) based on improved progression-free survival compared to placebo [11.2 months vs. 4 months; HR (95% CI) 0.28 (0.19, 0.40)]. In 2016, cabozantinib, 60 mg tablet once daily, was approved for adults with advanced renal cell carcinoma following prior antiangiogenic therapy3–5. The most commonly reported adverse drug reactions (≥25%) in adult trials were diarrhea, stomatitis, palmar-plantar erythrodysesthesia (PPE) syndrome, decreased weight, decreased appetite, nausea, vomiting, fatigue, oral pain, hair color changes, dysgeusia, hypertension, abdominal pain, and constipation3,4.
The approved dose of cabozantinib for adults with MTC is 140 mg/d (capsules); however, in the trial that lead to approval, 79% and 41% of patients had dose-reductions to 100 mg/d and 60 mg/d, respectively. Adverse events (AEs) led to discontinuation in 16% of patients4. There is an ongoing trial to fulfill a post-marketing requirement to evaluate two different dosing regimens of cabozantinib in adult patients with MTC, 140 mg capsules and 60 mg tablets (NCT01896479)6,7. Cabozantinib is being evaluated in adults with prostate, genitourinary, colon, non-small cell lung, breast, hepatocellular carcinoma, sarcoma, and AML.
The primary objectives of this phase I trial were to identify a maximum tolerated dose (MTD) or recommended phase 2 dose (RP2D), characterize the toxicity profile, and describe the pharmacokinetics (PK) of cabozantinib in pediatric patients with refractory or recurrent solid tumors. Additional objectives were to describe preliminary evidence of activity and pharmacodynamics (PD) of cabozantinib in this patient population.
Patients and Methods
Patient Eligibility
Eligible patients were between the ages of 2 and 18 years (y), had a body surface area ≥0.35m2; had a relapsed or refractory solid tumor including CNS tumors (Part A), or MTC (Part B); measurable or evaluable disease; no known curative therapy or therapy proven to prolong survival with an acceptable quality of life; a Lansky (≤16y) or Karnofsky (>16y) performance status ≥ 50%; and were recovered from the acute toxic effects of prior anticancer therapy. In addition, they were able to swallow intact tablets, had adequate organ function defined as: absolute neutrophil count (ANC) ≥1000/mm3, platelet count ≥100,000/mm3, creatinine clearance or radioisotope GFR ≥70 ml/min/1.73 m2 or serum creatinine(Cr) (mg/dL) based on age and gender; urine protein ≤ 30 mg/dL in urinalysis or ≤ 1+ on dipstick; total bilirubin ≤ 1.5 x upper limit of normal (ULN) for age, ALT ≤ 110 U/L, serum albumin ≥ 2.8 g/dL; PT and INR ≤ 1.5x ULN; serum amylase and lipase ≤ 1.5x ULN; blood pressure ≤ 95th percentile for age, height, and gender, and were not receiving medication for treatment of hypertension.
Patients were not eligible if they had a history of congenital prolonged QTc syndrome, QTc >480msec; significant cardiac arrhythmias, stroke, or myocardial infarction within 6 months prior to enrollment; were receiving potent CYP3A4 inducers or inhibitors, systemic treatment anticoagulation, enzyme-inducing anticonvulsants, escalating doses of corticosteroids, or drugs that prolong QTc; or had any active bleeding or major surgical procedure within 28 days of enrollment or minor procedures within 7 days of enrollment.
This multi-institutional trial was conducted at Children’s Oncology Group (COG) Phase I Consortium Institutions and approved by local IRBs. Informed consent, and assent as appropriate, was obtained from patients and their guardians prior to enrollment. The trial is listed on clinicaltrials.gov as NCT017094356.
Treatment Regimen and Dose Escalation
Cabozantinib (20 and 60mg tablets supplied by Exelixis and distributed by the National Cancer Institute’s Division of Cancer Treatment and Diagnosis) was administered orally in a fasted state (2 hours after or 1 hour before food) to achieve a weekly cumulative dose on a continuous dosing schedule in 28-day cycles. Due to limitations of available tablet strengths, dosing was based on a nomogram to achieve a total weekly dose within 10% of the intended dose (Supplemental Table S1). Three dose levels were evaluated: 30, 40, and 55 mg/m2/day (d). The trial was conducted in several parts: in Part A, the starting dose was 30 mg/m2/d with escalation to 40 and 55 mg/m2/d in subsequent cohorts utilizing the rolling-six design8; Part B was continuously open to enrollment for patients with MTC at one dose level below Part A if no openings were available on Part A. Patients were also enrolled in expansion cohorts to gather additional information on toxicity and PK in various ages.
Toxicities were graded according to CTCAE v.4. Patients were monitored with weekly complete blood counts, chemistries including liver function tests, blood pressure, and physical exams during Cycle 1 (C1). Tibial radiographs were obtained at baseline and for patients with open growth plates, radiographs were monitored during protocol therapy for growth plate toxicity by local and central review. Patients with MTC had serum levels of calcitonin and CEA measured at baseline and periodically thereafter.
Subsequent cycles were administered if the patient met eligibility laboratory parameters, did not experience study-drug related adverse events (AEs) warranting discontinuation, and had no evidence of disease progression.
Radiographic disease evaluation was conducted at baseline, at the end of C1, C3, C5, and then every three cycles. Radiographic response was assessed using RECIST v 1.1 by the treating physician-investigator. In addition, objective responses or prolonged stable disease, defined as ≥6 cycles, were centrally reviewed by an independent radiologist.
Definition of Dose Limiting Toxicity
Hematologic dose-limiting toxicity was defined as Grade 4 decrease in ANC or platelet count, any myelosuppression that caused a delay of >14 days between treatment cycles and, for patients with CNS tumors, more than one platelet transfusion administered for a platelet count ≤50,000/mcL during a cycle.
Non-hematologic DLT was defined as any Grade 3 or 4 non-hematological toxicity with the exception of Grade 4 fever and the following Grade 3 toxicities: nausea and vomiting of < 3 days duration, diarrhea ≤ 3 days duration, liver enzyme elevation that returned to eligibility or baseline levels within 7 days of drug interruption and did not recur upon re-challenge, electrolyte abnormality responsive to supplementation, asymptomatic amylase or lipase elevation that resolved to ≤ Grade 1 within 7 days of drug interruption and did not recur upon re-challenge, fever, infection < 5 days duration, and proteinuria unless it was confirmed with a second measurement within 72 hours. In patients who began antihypertensive therapy, a blood pressure > 10 mmHg but ≤ 25 mmHg above the 95th percentile for age, height, and gender for > 14 days was a DLT. In addition, any Grade 2 non-hematological toxicity that persisted for ≥ 7 days and was considered sufficiently medically significant or intolerable by patients that it required treatment interruption, and any toxicity requiring interruption of study drug during a cycle of therapy for ≥ 7 days or which recurs upon drug challenge was considered a DLT.
Definition of Maximum Tolerated Dose (MTD) and Dose Modifications for Toxicity
The MTD, determined from toxicity during C1, was defined as the dose level immediately below the level in which two or more in a cohort of six patients experienced a DLT. Two dose reductions were permitted following recovery from a DLT.
Pharmacokinetics
Plasma pharmacokinetic sampling was performed at the beginning of C1 and at steady state. Samples were obtained on all patients prior to and 4 hours after the dose on Cycle 1 Day 1 (C1D1), prior to and 2, 4, 8, and 24 hours after the dose on C1D21, and prior to the dose on C3D1. Plasma concentrations of cabozantinib were measured using a validated, previously described, LC-MS/MS method9. Pharmacokinetic parameters were calculated by standard non-compartmental analysis using Phoenix WINNONLIN 6.3 (Certara, L.P., St. Louis MO).
Pharmacodynamics
Blood samples for biomarker analysis for known targets of cabozantinib and markers of hypoxia, inflammation, and angiogenesis were collected at baseline and prior to cabozantinib dosing on C1D1, C1D21 and C1D28. Plasma (EDTA) samples were shipped on dry ice to AssayGate (Ijamsville, MD) for multiplex immunoassay using standard protocols for quantitation of soluble forms of c-MET, VEGFR2, HGF, AXL, CA9, EPO, OPN, PIGF, IL-6, and TIMP-1. Analyte concentrations in samples were determined by either four or five-parameter logistic regression algorithm with analysis of the median fluorescence intensity or optical density readings of each eight-point standard curve.
Results
Patient characteristics
Forty-one patients were enrolled; all were eligible and 36 were evaluable for the primary endpoints. The five inevaluable patients either did not receive at least 85% percent of protocol-prescribed drug during C1 as a result of early disease progression (n=4) or withdrew consent for non-toxicity reasons (n=1). Patient demographics and disease characteristics are presented in Table 1. The median (range) number of cycles administered was 3 (1-25). At the time of data cut-off, 4 patients continued on protocol therapy, two patients in C13 (ependymoma and Ewing sarcoma), and two patients with MTC, one in C20, and one in C25.
Table 1.
Patient Demographics and Disease Characteristics
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median (Range) | 13 (4 – 18) |
| Sex | |
| Male | 21 (51) |
| Female | 20 (49) |
| Race | |
| White | 26 (63) |
| Asian | 3 (7) |
| American Indian or Alaska Native | 0 (0) |
| Black or African American | 7 (17) |
| Unknown | 5 (12) |
| Ethnicity | |
| Non-Hispanic | 38 (93) |
| Hispanic | 3 (7) |
| Unknown | 0 (0) |
| Prior Chemotherapy Regimens | 31 (76) |
| Median number of regimens (Range) | 3 (1 – 9) |
| Prior Radiation | 28 (68) |
| Tumor Type | |
| Sarcoma | 12 (29) |
| Alveolar soft part sarcoma | 2 |
| Clear cell sarcoma | 1 |
| Embryonal rhabdomyosarcoma | 2 |
| Ewing sarcoma | 4 |
| Osteosarcoma | 2 |
| Synovial sarcoma | 1 |
| CNS | 9 (22) |
| Ependymoma | 2 |
| Glioma | 6 |
| Medulloblastoma | 1 |
| Medullary thyroid carcinoma | 5 (12) |
| Renal cell carcinoma | 3 (7) |
| Epithelial-myoepithelial carcinoma | 2 (5) |
| Hepatoblastoma | 2 (5) |
| Wilms tumor | 2 (5) |
| Neuroblastic tumors | |
| Neuroblastoma | 2 (5) |
| Ganglioneuroblastoma | 1 (2) |
| Hepatocellular carcinoma | 2 (5) |
| Paraganglioma | 1 (2) |
Toxicity and MTD
Six patients were enrolled at 30 mg/m2/d. No C1 DLTs were observed.
Twenty-three patients were enrolled at 40 mg/m2/d (nine in Part A, four in Part B, and 10 in the PK cohort). Nineteen patients were evaluable for toxicity; five patients (26%) had a DLT during C1 (PPE; mucositis and PPE; and increased ALT, lipase, and bilirubin).
Twelve patients were enrolled at 55 mg/m2/d (six in Part A and six in the PK expansion cohort). Eleven were evaluable for toxicity; three patients (27%) had a C1 DLT (headache and fatigue, reversible posterior leukoencephalopathy syndrome (RPLS) and hypertension in one patient, proteinuria and hypertension in one patient).
Table 2 summarizes DLTs in the first and later cycles, dose modifications for DLTs, and the average daily dose received by patients in each dose level. Across dose-levels, nine patients accounted for 13 dose-reductions for toxicity after C1; the majority of dose reductions (10/13; 77%) occurred in C2 or C3. Reasons for dose-reductions included increased ALT, arthralgia, fatigue, hypertension, increased lipase, oral mucositis, nausea, decreased neutrophil count, PPE, proteinuria, thromboembolic event, vomiting, and weight loss. Overall, 3 patients discontinued therapy due to DLTs. One patient who discontinued cabozantinib (55 mg/m2/d) due to dose limiting RPLS and hypertension during cycle 1, experienced toxicity possibly related to cabozantinib during the 30 day follow up period. The patient developed Grade 4 adult respiratory distress syndrome 4 days after the last dose of cabozantinib, and Grade 5 multi-organ failure 17 days after discontinuation of cabozantinib.
Table 2.
Incidence of Dose-Limiting Toxicities and Dose Modifications
| Dose level | DLT during Cycle 1 | DLT after Cycle 1 | Dose reduction for DLT | Discontinuation due to DLT | Average daily dose a |
|---|---|---|---|---|---|
| (mg/m2/d) | Patients with DLT/Evaluable patients(%) | Number of DLT events/Evaluable patients (%) | Patients having dose reduction/Evaluable patients (%) | Patients discontinuing/Evaluable Patients (%) | mg/m2/d (SD) |
| 30 | 0/6 (0) | 2/5 (40) | 0/6 (0) | 1/6 (17) | 30.8 (3.6) |
|
| |||||
| 40 | 5/19 (26) | 8/16 (50) | 5/19b (26) | 0/19 (0) | 31.5 (10.3) |
|
| |||||
| 55 | 3/11 (27) | 5/9 (56) | 4/11c (36) | 2/11 (18) | 46.0 (11.1) |
Abbreviations in table: DLT=dose limiting toxicity; SD=standard deviation
Average daily dose for each patient over all cycles was calculated as total dose in mg received per cycle divided by body surface area and cycle length.
Three patients had one dose reduction, two patients had two dose reductions
Two patients had one dose reduction, two patients had two dose reductions
Hematologic and non-hematologic adverse events as well as laboratory abnormalities that were assessed by the investigator to be at least possibly attributed to cabozantinib are summarized in Supplemental Table S2. Hypertension, gastrointestinal disorders (diarrhea, nausea and vomiting), constitutional (fatigue, arthralgia, weight loss), dermatological (rash and hypopigmentation of hair), and laboratory abnormalities (increased hepatic transaminases, hypothyroidism, proteinuria) were prevalent. All of these toxicities were grade 1 or 2 except for cases of grade 3 hypertension (n=3), PPE (n=3), diarrhea (n=2), increased ALT (n=2), proteinuria (n=1), fatigue (n=1), and weight loss (n=1). Overall, the toxicity profile was similar to adults.
Twenty-six patients underwent growth plate evaluations. Growth plate widening on therapy (Figure 1) was identified in 5 of 23 patients who had open physes at baseline. Of the five patients, three patients were treated at 40 mg/m2/d and two patients at 55 mg/m2/d; diagnoses were anaplastic ependymoma (n=2), Ewings sarcoma (n=1), clear cell sarcoma (n=1), hepatocellular carcinoma (n=1). Despite the presence of physeal widening, longitudinal growth occurred in all 5 patients, even in those treated for greater than 12 months.
Figure 1. Growth Plate Toxicity.
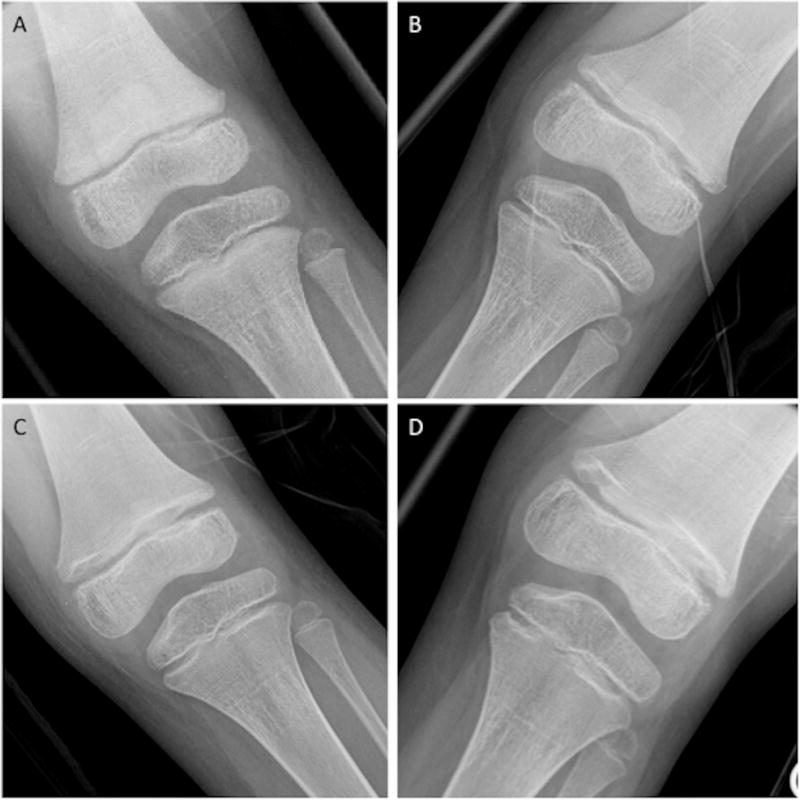
Progressive growth plate widening on therapy. Tibial radiographs obtained prior to Cycle 1 (A), Cycle 9 (B), Cycle 16 (C), and Cycle 20 (D) show abnormal widening and irregularity of the distal femoral and proximal tibial physes. The physeal widening increases from the normal baseline seen prior to Cycle 1 (A) to become progressively more widened during the course of treatment.
Pharmacokinetics
Steady-state pharmacokinetics (day 22) were evaluated in 32 patients. Estimates of the cabozantinib pharmacokinetic parameters are summarized in Table 3. The median time of the peak concentration was 2 hours after drug administration. Mean peak concentrations were greater than 1693 ng/ml, and similar across the dose levels studied. Cabozantinib AUC was similar across dose levels. The steady-state oral clearance (Cl/F) was calculated over the 24-hour dosing interval, and BSA-normalized oral clearance (mean ± SD) was 1.68 ± 0.76 L/h/m2 for all patients and appeared to be similar across the dose levels. BSA-normalized Cl/F was similar in females and males (1.54 ± 0.55 L/h/m2 versus 1.72 ± 1.02 L/h/m2). BSA- normalized values tended to be higher in children less than 12 years of age compared with older children (1.89 ± 0.83 L/h/m2 versus 1.53 ± 0.69 L/h/m2); however, this was not significantly different (p=0.31, Wilcoxon rank sum test). The elimination half-life could not be calculated for most patients. Therefore, the day 21:day 1 accumulation ratio was calculated as 4.3 ± 3.9, and used to estimate the cabozantinib half-life of 66.2 ± 64.7 hours.
Table 3.
Plasma Pharmacokinetic Parameters
| Dose Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 mg/m2/d | 40 mg/m2/d | 55 mg/m2/d | |||||||
| N | Mean | StdDev | N | Mean | StdDev | N | Mean | StdDev | |
| Cmax, ng/ml | 6 | 2025 | 658 | 16 | 1693 | 692 | 10 | 2389 | 802 |
| Tmax, h | 6 | 5.24 | 8.93 | 16 | 7.38 | 8.46 | 10 | 4.7 | 6.57 |
| AUC0-24h, h·ng/ml | 6 | 32217 | 8022 | 16 | 30010 | 10985 | 10 | 38101 | 12785 |
| Cl/f, L/h | 6 | 1.62 | 0.43 | 16 | 2.21 | 0.86 | 10 | 2.31 | 1.05 |
| Cl/f/m2, L/h/m2 | 6 | 1.14 | 0.36 | 16 | 1.81 | 0.87 | 10 | 1.79 | 0.64 |
| Accumulation Ratio C4hr,day21/C4hr,day1 | 6 | 5.25 | 2.17 | 16 | 4.72 | 5.06 | 9 | 2.82 | 1.23 |
| Accumulation Adjusted t1/2, h | 6 | 78.7 | 36.3 | 14 | 79.1 | 86.3 | 9 | 37.8 | 20.8 |
Pharmacodynamics
Change from baseline in plasma pharmacodynamic markers is presented in Table 4. AXL, CA9, and PIGF increased on study D21 and D28 versus baseline. OPN, TIMP-1, and VEGFR2 decreased on D21 and D28 versus baseline. The Wilcoxon signed rank test was used to test the null hypothesis that there was no change in biomarkers at D21 and D28 versus baseline (p-values were adjusted for multiple hypothesis testing using the Bonferroni correction). There were significant changes in AXL, CA9, OPN, PIGF, TIMP-1, and VEGFR2 (p<0.05) at both D21 and D28 versus baseline. There was no significant change in cMET, EPO, HGF, or IL-6. There was no significant biomarker change among cabozantinib dose levels or among patients who achieved response (data not shown).
Table 4.
Pharmacodynamic Markers Change from Baseline
| Biomarker | Study day | Median change | p-valueˆ |
|---|---|---|---|
| AXL* | 21 | 2383.16 1823.11 32.72 |
0.011 0.004 <0.001 |
|
28 21 | |||
| CA9* | |||
| 28 | 35.52 | <0.001 | |
| PIGF* | 21 | 40.59 | <0.001 |
| 28 | 41.41 | <0.001 | |
| OPN* | 21 | −18.41 | 0.008 |
| 28 | −21.02 | 0.018 | |
| TIMP-1* | 21 | −42.13 | <0.001 |
| 28 | −28.13 | <0.001 | |
| VEGFR2* | 21 | −1581.01 | <0.001 |
| 28 | −1992.72 | <0.001 | |
| cMET | 21 | 15.61 | 0.173 |
| 28 | 14.33 | 0.111 | |
| EPO | 21 | 2.77 | 0.524 |
| 28 | 6.46 | 0.119 | |
| HGF | 21 | −144.25 | 0.077 |
| 28 | −123.01 | 0.650 | |
| IL-6 | 21 | 0.00 | 1 |
| 28 | 0.00 | 1 |
Significant change from baseline
P-values testing the null hypothesis that there is a change in the biomarker level versus baseline using Wilcoxon signed rank test and adjusted for multiple comparisons using the Bonferroni correction.
Response
There were no complete responses. Four patients had a partial response (PR), and seven patients had prolonged stable disease (SD). PRs were achieved in two patients with MTC at the 40 mg/m2/d dose level (both continued on study at the time of data cut-off, C20 and C25), one patient with Wilms tumor at 55 mg/m2/d received 24 cycles before progressive disease (PD), and one with clear cell sarcoma at 55 mg/m2/d received seven cycles prior to PD. Of the seven patients with prolonged SD, two continue cabozantinib (40 mg/m2/d) at cycle 13 (Ewing sarcoma and ependymoma); three patients receiving 40 mg/m2/d discontinued therapy for PD after 8-10 cycles (MTC (n=2), alveolar soft part sarcoma). One patient with synovial sarcoma discontinued protocol therapy due to toxicity; this patient was receiving cabozantinib 30 mg/m2/d and experienced dose limiting weight loss in cycle 10. One patient with paraganglioma discontinued cabozantinib (55 mg/m2/d) after C11 when the family withdrew consent for non-toxicity reasons.
Calcitonin and CEA
Five patients with advanced MTC were treated on the trial. All had multiple endocrine neoplasia type 2B (MEN2B), a genetic cancer predisposition syndrome caused by germline activating mutations in the RET proto-oncogene. All had a point mutation in exon 16 (codon 918). A decrease in calcitonin from baseline to the end of C1, ranging from 24 to 87%, was observed. Changes in CEA were more variable with three patients having a decrease and two patients, who remained on trial for 20 and 2 cycles, with an increase from baseline to post C1.
Discussion
No protocol-defined MTD was reached in this dose-escalation phase 1 study. No patients in the 30 mg/m2/d cohort, 5 of 19 (26%) patients in the 40 mg/m2/d cohort, and 3 of 11 (27%) patients in the 55 mg/m2/d cohort, had first cycle DLTs. Due to the long half-life of cabozantinib estimated in pediatric patients (66 h), steady-state drug concentrations are not achieved until the beginning of the third week of therapy. Thus, evaluation of toxicity for dose-determination during the first 28 day cycle is likely inadequate to evaluate the long-term tolerability of the drug and consideration should be given to toxicities in later cycles when determining the MTD or recommended dose. Delayed emergence of cabozantinib toxicity was observed in adults with MTC; the majority of patients required a dose reduction in the pivotal trial.
Patients in this trial received a median of 3 cycles (range 1-25). DLTs after C1 occurred at all dose levels. To further evaluate the toxicity profile across dose levels, the average daily dose actually received was calculated for each patient and then averaged for each dose level (Table 2). This average daily dose was the sum of all course doses divided by the cycle length. This calculation accounted for dose reductions for DLTs and dose delays for resolution of dose-limiting and non-dose limiting toxicity. For 30, 40, and 55 mg/m2/d cohorts, the average daily dose actually received was 30.8 ±3.6, 31.5 ±10.3, and 46.0 ±11.1 mg/m2/d, respectively. The variation in daily dosing was due, in part, to the lack of a pediatric oral formulation, and complicated nomogram (see Supplemental Table S1). In addition, these factors limited the ability for precise dose reductions.
Cabozantinib capsules (140 mg) are FDA approved in adults with MTC. In adults with renal cell carcinoma, cabozantinib tablets (60 mg) are FDA approved. These formulations are not bioequivalent. The tablet formulation was used in this pediatric study and the apparent clearance (1.1-1.8 L/h/m2) in children was variable but similar to reported clearance of this formulation in healthy adults (1.3-2 L/h/m2)10.
The toxicity profile of cabozantinib in pediatric patients was similar to that seen in adults5. Common toxicities included increased ALT, diarrhea, fatigue, proteinuria, hypothyroidism, hypertension, weight loss, PPE, anorexia, and nausea. These toxicities were generally low grade and manageable with supportive care, dose interruption or reduction.
Evidence of clinical activity of cabozantinib was seen in MTC and several sarcoma subtypes. Of the eleven patients with clinical benefit (PR or prolonged SD) ten received cabozantinib at the 40 or 55 mg/m2/d dose level. There was no significant change in the evaluated biomarkers in these patients.
Given the toxicity profile (including DLT rate and actual dose administered), similar drug exposure and PD profile, and response data across the dose levels, the dose of cabozantinib recommended for further evaluation in pediatric patients with solid tumors is 40 mg/m2/d (tablets) orally. This is equivalent to an adult dose of 72 mg/d, and is similar to the 60 mg/d (tablets) dose approved for renal cell carcinoma and the dose being evaluated in several ongoing trials in other cancers. A Children’s Oncology Group phase 2 trial (ADVL1622) has opened to accrual to further evaluate the activity of cabozantinib in pediatric patients (NCT02867592).
Supplementary Material
Supplemental Table S1: Cabozantinib dosing nomogram
Supplemental Table S2: Hematologic and Non-Hematologic toxicities related to protocol therapy observed in evaluable patients
Acknowledgments
Additional contributions received from Charlotte H. Ahern (formerly from Baylor College of Medicine), Paul Jarosinski from the NCI Clinical Center, Wendy Martinez, Catalina Martinez and Thalia Beeles from the Children’s Oncology Group Operations Center.
Abbreviations
- CNS
Central nervous system
- Mg
Milligram
- d
Day
- AML
Acute myeloid leukemia
Footnotes
Conflict of Interest Statement: All authors declare that they have no COI to disclose.
References
- 1.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Molecular cancer therapeutics. 2011;10(12):2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 3.Cometriq USPI. Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203756s002lbl.pdf.
- 4.Cabometyx USPI. Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208692s000lbl.pdf.
- 5.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabozantinib in Treating Younger Patients With Recurrent or Refractory Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT01709435.
- 7.Nguyen L, Holland J, Ramies D, et al. Effect of Renal and Hepatic Impairment on the Pharmacokinetics of Cabozantinib. J Clin Pharmacol. 2016;56(9):1130–1140. doi: 10.1002/jcph.714. [DOI] [PubMed] [Google Scholar]
- 8.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(2):190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 9.Lacy S, Hsu B, Miles D, Aftab D, Wang R, Nguyen L. Metabolism and Disposition of Cabozantinib in Healthy Male Volunteers and Pharmacologic Characterization of Its Major Metabolites. Drug Metab Dispos. 2015;43(8):1190–1207. doi: 10.1124/dmd.115.063610. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen L, Benrimoh N, Xie Y, Offman E, Lacy S. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anticancer Drugs. 2016;27(7):669–678. doi: 10.1097/CAD.0000000000000366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: Cabozantinib dosing nomogram
Supplemental Table S2: Hematologic and Non-Hematologic toxicities related to protocol therapy observed in evaluable patients


