Abstract
Socially disadvantaged individuals are at greater risk for simultaneously being exposed to adverse social and environmental conditions. Although the mechanisms underlying joint effects remain unclear, one hypothesis is that toxic social and environmental exposures have synergistic effects on inflammatory processes that underlie the development of chronic diseases, including cardiovascular disease, diabetes, depression, and certain types of cancer. In the present review, we examine how exposure to two risk factors that commonly occur with social disadvantage—early life stress and air pollution—affect health. Specifically, we identify neuroimmunologic pathways that could link early life stress, inflammation, air pollution, and poor health, and use this information to propose an integrated, multi-level model that describes how these factors may interact and cause health disparity across individuals based on social disadvantage. This model highlights the importance of interdisciplinary research considering multiple exposures across domains and the potential for synergistic, cross-domain effects on health, and may help identify factors that could potentially be targeted to reduce disease risk and improve lifespan health.
Keywords: early adversity, inflammation, cytokine, inflammatory reactivity, pro-inflammatory phenotype, stress responsivity
1. Introduction
Socially disadvantaged individuals, such as those with low educational attainment or income, or who belong to racial or ethnic groups that have historically experienced discrimination, systematically experience relatively worse health across the lifespan, compared to those in more socially advantageous circumstances (Adler and Stewart, 2010). The determinants of these health disparities include both social and physical environmental factors that interact to influence a broad range of psychological, biological, and behavioral processes that in turn affect health (Braveman and Gottlieb, 2014). Although it is well documented that individuals in socially disadvantaged conditions are more likely to be exposed to harmful social and physical environments compared to those in better social conditions, research on the interaction of these factors is limited.
A harmful exposure common among socially disadvantaged populations is psychosocial stress during childhood (Andersen and Blosnich, 2013; Slopen et al., 2016). Indeed, early life exposure to psychosocial stress has been identified as a determinant of social disparities in health that emerge over the life course (Miller et al., 2011; Shonkoff et al., 2012), and a large body of research now suggests that early life stress increases adulthood risk for cardiovascular disease, stroke, diabetes, autoimmune disease, and certain cancers, in addition to substance abuse and depression (Brown et al., 2013; Carroll et al., 2013; Chapman et al., 2004; Dube et al., 2009; Felitti et al., 1998). Moreover, the effects of severe early life stressors that cause repeated biological stress responses or prolonged biological dysregulation, such as poverty, abuse, neglect, isolation, discrimination, humiliation, or violence, appear to be particularly deleterious for lifespan health (Goodman et al., 2005; Horwitz, 2002; Nurius et al., 2013).
Like early life stress, exposure to air pollution is also patterned by social disadvantage. In the United States, for example, land values decrease substantially near highways and industrial sites, which are major sources of air pollution (Boehmer et al., 2013). Consequently, poor communities tend to be comprised of socially disadvantaged individuals who are commonly exposed to high levels of air pollution. This is notable since exposure to air pollution is also strongly associated with poor lifespan health, causing an estimated seven million premature deaths worldwide each year, primarily due to inflammation-related diseases such as cardiovascular disease, chronic obstructive pulmonary disease, and lung cancer (World Health Organization, 2014). Inflammation is central to the biological machinery that links air pollution with adverse health outcomes (Du et al., 2016; Mostafavi et al., 2015). Consequently, alterations in inflammatory activity that are caused by early life stress may be one key mechanism that potentiates the negative effects that air pollution has on health over the lifespan.
In the present review, we integrate research from several fields—especially psychoneuroimmunology, epidemiology, environmental toxicology, and genomics—to elucidate how early life stress may influence immune system processes to increase vulnerability to air pollution and risk for inflammation-related chronic illness. To accomplish this, we first summarize studies linking early life stress and poor lifespan health. Second, we review research suggesting that psychosocial stress may promote poor health in part by initiating chronic, low-grade inflammation. Third, we examine toxicology studies linking exposure to particle air pollution and inflammation, and connect this work to related research on psychosocial stress and inflammation. Fourth, based on these literatures, we propose an integrated, multi-level model of early life stress, air pollution exposure, and lifespan health. This model suggests that stressors occurring during childhood program a “defensive” immunologic phenotype characterized by increased inflammatory reactivity to air pollution. As a result, individuals exposed to high levels of stress early in life may have more potent inflammatory responses upon exposure to a range of harmful social and physical factors, including air pollution, and thereby be at high risk of inflammation-related diseases and conditions (Gouin et al., 2012; Miller and Chen, 2007; Miller and Chen, 2010; Steptoe et al., 2002b). Finally, we suggest some possible avenues for future research.
2. Early life stress and lifespan health
The notion that early life stress can affect health has been around for centuries (Monroe and Slavich, 2016; Slavich, 2016). This idea was further concretized in 1998 when the Adverse Childhood Experiences (ACE) study revealed a strong dose-response relationship between exposure to different forms of childhood adversity and increased risk for several of the leading causes of death among adults in the United States (Felitti et al., 1998). Specifically, individuals experiencing four or more types of childhood adversity were found to have a 1.4- to 1.6-fold increase in physical inactivity and severe obesity compared to individuals experiencing none of these types of adversity, and a 4- to 12-fold increase in risk for smoking, self-rated health complaints, alcoholism, drug abuse, depression, heart disease, and suicide (Anda et al., 2008; Dong et al., 2004; Dube et al., 2009; Felitti et al., 1998). In this context, the terms early life stress or early adversity, have referred to different types of stressors, including violence, poverty, maltreatment, abuse, and neglect, as well as parental substance abuse or mental illness (Carroll et al., 2011; Dube et al., 2001; Hughes et al., 2017; Infurna et al., 2016; Lacey et al., 2014; Lindert et al., 2014; McCrory et al., 2011; Miller and Chen, 2010; Norman et al., 2012; Pollitt et al., 2007; Sripada et al., 2014; Varese et al., 2012; Zatti et al., 2017). It has been estimated that more than 60% of adults in the United States experience at least one these significant life stressors during childhood (Felitti et al., 1998). Such exposures have been found to reduce overall life expectancy by up to 4.5 years and active (i.e., non-functionally impaired) life expectancy by up to 6.6 years (Felitti et al., 1998; Montez and Hayward, 2014). Moreover, studies have shown that many of these stressors are more common among socially disadvantaged populations, suggesting that these exposures may contribute to structuring social disparities in health (Slopen et al., 2016).
Since the publication of the ACE study and others, the list of adverse health outcomes linked with childhood adversity has grown considerably (Table 1). Indeed, early adversity has been linked with increased risk for a wide variety of mental and physical health problems in addition to those identified above, including asthma, rheumatoid arthritis, cardiovascular disease, type 2 diabetes, certain types of cancer, and accelerated cognitive decline (Brown et al., 2010; Danese and McEwen, 2012; Fagundes et al., 2013; Huang et al., 2015; Palmier-Claus et al., 2018; Wolitzky-Taylor et al., 2017). As a result, early life stress exposure is now recognized as a critical public health concern across the world (Shonkoff, 2010).
Table 1.
Associations between early life stress and health problems in adulthood
| Citation | Cited association | Type of study | Population | Sample size | Key metrics |
|---|---|---|---|---|---|
| Anda et al. (2008) | Association between childhood adversity and Chronic Obstructive Pulmonary Disease (COPD) | Quasi-prospective | Adults | 15,472 | Adverse Childhood Experiences (ACE; retrospective at baseline), COPD self-report, COPD incidence at follow-up, medication use at follow-up |
| Brown et al. (2010) | Association between childhood adversity lung cancer | Quasi-prospective | Adults | 17,337 | ACE (retrospective at baseline), hospital discharge at follow-up, mortality records at follow-up, adult smoking as mediator |
| Dong et al. (2004) | Association between childhood adversity and ischemic heart disease (IHD) | Retrospective | Adults | 17,337 | ACE, self-report of IHD, IHD risk factors (e.g., physical activity) as mediators |
| Dube et al. (2001) | Association between childhood adversity and suicide attempt | Retrospective | Adults | 17,337 | ACE, self-report suicide attempt, illicit drug use, depression, and alcoholism as mediators |
| Dube et al. (2009) | Association between childhood adversity and autoimmune diseases (AD) | Retrospective | Adults | 15,357 | ACE, hospitalization for 21 types of AD after baseline measurement of ACE, AD immunopathology grouping |
| Felitti et al. (1998) | Association between childhood adversity, health risk behaviors, obesity, and depression | Retrospective | Adults | 9,508 | ACE, health risk behaviors and disease from self-report and health screening |
| Miller and Chen (2010) | Association between early life harsh family climate and pro-inflammatory phenotype in adolescence | Prospective | Female adolescents | 135 | Harsh family climate, cytokine response to in-vitro bacterial challenge, sensitivity of glucocorticoid receptor |
| Montez and Hayward (2014) | Association between childhood adversity and active life expectancy | Retrospective | Adults | 30,671 | Childhood health and SES, mortality, activities of daily living (ADL), educational attainment as mediator |
| Norman et al. (2012) | Association between childhood adversity and physical and mental health problems | Meta-analysis | Mixed | >500,000 | Physical abuse, sexual abuse, emotional and psychological abuse, neglect, depressive disorder, anxiety, alcohol abuse |
| Sripada et al. (2014) | Association between childhood poverty and stress reactivity in adulthood | Case-control | Adults | 52 | Childhood poverty, Trier Social Stress Test, salivary cortisol |
| Hughes et al. (2017) | Association between childhood adversity an poor health in adulthood | Meta-analysis | Mixed | 253,719 | ACE, cancer, heart disease, mental illness, drug use, and interpersonal and self-directed violence |
| Varese et al. (2012) | Association between childhood adversity and psychosis in adulthood | Meta-analysis | Mixed | 81,253 | Childhood sexual abuse, physical abuse, emotional/psychological abuse, neglect, parental death, bullying, psychotic disorder, schizophrenia, schizoaffective disorder |
| Huang et al. (2015) | Association between childhood adversity and type 2 diabetes in adulthood | Meta-analysis | Mixed | 87,251 | ACE, type 2 diabetes |
| Infurna et al. (2016) | Association between childhood abuse and neglect and depression | Meta-analysis | Adults | 4,372 | Childhood experience of care and abuse (CECA), clinically assessed depression |
| Lindert et al. (2014) | Association between childhood sexual and physical abuse and depression | Meta-analysis | Mixed | 115,579 | Sexual and physical abuse prior to 16 years of age, depression and anxiety after 16 years of age |
| Palmier-Claus et al. (2018) | Association between childhood adversity and bipolar affective disorder | Meta-analysis | Mixed | >1,000,000 | Child abuse, neglect, bullying, loss of parent, trauma, bipolar disorder |
| Zatti et al. (2017) | Association between childhood trauma and suicide attempt | Meta-analysis | Children → Adults | 6,612 | Childhood sexual, physical, and emotional abuse, neglect, and suicide attempt |
3. Inflammatory mechanisms linking early life stress and health
Recent studies in health psychology and psychoneuroimmunology have focused on elucidating biological mechanisms that may explain how early life stress affects mental and physical health in later life (Shields and Slavich, 2017; Slavich, in press). One of the most recent and potentially important discoveries in this context has involved the recognition that inflammation may be a key process linking stress and health (Table 2) (Miller et al., 2011; Slavich, 2015). At least two major lines of research support this possibility. First, high levels of chronic peripheral inflammation, indexed by circulatory inflammatory markers, have been associated with experiences of early life stress in both cross-sectional (Carroll et al., 2011; Dixon et al., 2009; Gouin et al., 2012; Grosse et al., 2016; Hartwell et al., 2013; Lacey et al., 2013; Lehto et al., 2012; Lin et al., 2016; Moreira et al., 2018; Munjiza et al., 2018; Packard et al., 2011) and longitudinal studies (Baldwin et al., 2018; Boch and Ford, 2015; Chen and Lacey, 2018; Danese et al., 2011; 2009; 2008; 2007; Lacey et al., 2014; Slopen et al., 2013). For example, Slopen et al. (2013) found that cumulative adversity from birth to 8 years of age, as well as acute adverse events experienced between 6 and 8 years of age were associated with higher circulatory levels of interleukin-6 and C-reactive protein (CRP) at age 10, and CRP at age 15. Researchers have also found associations between experiences of bullying in childhood (9–16 years) and elevated CRP levels in young adulthood (19–21 years) (Copeland et al., 2014), as well as exposure to victimization during childhood (birth-12 years) and elevated levels of CRP at age 18 years (Baldwin et al., 2018).
Table 2.
Associations between early life stress and inflammation
| Citation | Cited association | Type of study | Population | Sample size | Key metrics |
|---|---|---|---|---|---|
| Balwin et al. (2018) | Association between victimization in childhood and inflammation (CRP) in young adulthood | Prospective | Children and Young Adulthood | 2,232 | Childhood victimization, C-reactive protein (CRP), latent genetic risk score, socioeconomic status (SES), waist-hip ratio at age 18, body temperature |
| Baumesiter et al. (2016) | Association between childhood trauma and adult inflammation | Meta-analysis | Adults | varied; 16,870–881 | Adverse Childhood Experiences (ACE) Questionnaire; Childhood experiences of care and abuse; Childhood Trauma Questionnaire (CTQ) and CRP, tumor necrosis factor alpha (TNF-α), interleukin (IL)-6 |
| Chen et al. (2018) | Graded association between adverse childhood experiences and inflammation (CRP, fibrinogen) in adulthood | Prospective | Children | 7,464 | ACE (0–16 years) and CRP, fibrinogen, Von Willebrand factor (vWF) at age 44–45 years, SES |
| Danese et al. (2007) | Association between childhood maltreatment and adult inflammation | Prospective | Adults (32 years old) | 1,037 | Childhood maltreatment between 3–11 years, CRP, depression, perceived stress, cardiovascular risk, smoking, physical activity |
| Danese et al. (2008) | Association between childhood maltreatment and the co-occurrence of depression and inflammation (CRP) in adulthood | Prospective | Adults | 1,015 | Childhood maltreatment, CRP, fibrinogen, white blood cell count, depression, childhood SES, adulthood SES, smoking, cardiovascular risk |
| Danese et al. (2009) | Association between adverse psychological childhood experiences and elevated levels of CRP | Prospective | Adults | 1,037 | Childhood maltreatment, childhood social isolation, adverse childhood experiences, childhood SES, CRP, depression, overweight, high blood pressure, total cholesterol, low high-density lipoprotein cholesterol, high glycated hemoglobin, and low maximum oxygen consumption levels adjusted for body weight |
| Danese et al. (2011) | Association between physical maltreatment and CRP in 12 year-old children | Prospective | Children | 174 | Physical maltreatment, CRP, depression, temperature, waist-hip ratio |
| Dixon et al. (2009) | Association of stress and TNF-α levels in children | Cross-sectional | Children | 112 | Stressful life events, TNF-α, body mass index (BMI), sex, family history of type 2 diabetes |
| Gouin et al. (2012) | Association between childhood abuse and inflammatory response, indexed by IL-6, to daily stressors | Cross-sectional | Older adults | 130 | CTQ, IL-6, TNF-α, and CRP assays, Center for Epidemiological Scale-Depression, Beck Anxiety Inventory, Pittsburgh Sleep Quality Index, Community Healthy Activities Model Program for Seniors (CHAMPS) Questionnaires, Older Adult Resources and Services |
| Grosse et al. (2016) | Association between childhood trauma and elevated cytokine (IL-6, TNF-α) levels in adults with major depression | Cross-sectional | Adults | 394 | CTQ, Threatening Experiences Questionnaire, IL-6, TNF-α, major depression, perceived stress |
| Hartwell et al. (2013) | Association between childhood traumatic experiences and elevated cytokine (IL-1β, IL-6, TNF-α) levels | Cross-sectional | Adults | 38 | Early Trauma Inventory (ETI), IL-1β, IL-6, TNF-α, CRP |
| Lacey et al. (2013) | Association between parental separation during childhood and increased inflammation (CRP) in adulthood | Cross-sectional | Adults | 7,462 | Parental separation measured at 16 years, CRP and BMI levels measured at 44 years |
| Lehto et al. (2012) | Association between lower levels of anti-inflammatory marker adiponectin and history of childhood maltreatment in adults | Cross-sectional | Adults | 147 | Experiences of childhood maltreatment, adiponectin, resisting, depression, BMI |
| Lin et al. (2016) | Association between childhood adversity and CRP | Cross-sectional | Adults | 11,198 | Childhood adversity, CRP, adulthood trauma, education, smoking, BMI, alcohol use, physical activity |
| Miller and Chen (2010) | Association between early life harsh family climate and pro-inflammatory phenotype in adolescence | Prospective | Female adolescents | 135 | Harsh family climate, cytokine response to in-vitro bacterial challenge, sensitivity of glucocorticoid receptor |
| Miller and Cole (2012) | Clustering of depression and inflammation (CRP, IL-6) in female adolescents exposed to childhood adversity | Prospective | Female adolescents (15–19 years old) | 147 | Depressive episodes Structured Clinical Interview for DSM-IV-TR (SCID) Axis I Disorders—Non-Patient Edition, childhood adversity index, CRP and IL-6 |
| Munjiza et al. (2018) | Association between childhood abuse and IL-6 among depressed adults | Cross-sectional | Adults | 64 | CTQ, IL-6, Beck Depression Inventory (BDI), Hamilton Depression Rating Scale (HDRS), and Hamilton Anxiety Rating Scale (HARS) |
| Pedrotti Moreira et al. (2018) | Association between childhood trauma and higher levels of IL-6 and IL-10 among young adults with major depression | Cross-sectional | Young Adults | 166 | CTQ, IL-6, IL-10, TNF-α, |
| Slopen et al. (2013) | Association between adverse events in childhood (ages 6–8 years) and CRP and IL-6 levels at age 10 years | Prospective | Children | 4,647 | Adverse life events, CRP, IL-6, BMI, depression |
Moreover, elevated levels of inflammation appear to persist into adulthood, even among disease-free adults with a history of disadvantage or maltreatment during childhood (Juonala et al., 2006). In one longitudinal study, for example, Danese et al. (2007) followed individuals from birth until age 32 and found that risk of clinically relevant CRP levels in adulthood was higher among individuals that experienced maltreatment as children, and that this association was independent of the co-occurrence of other early life risks, stress in adulthood, adult health status, and adult health behaviors. Similarly, a longitudinal birth-cohort study of 7,462 participants recently found that social isolation during ages 7–11 predicted higher levels of CRP at 44 years of age (Lacey et al., 2014). Moreover, this association was robust while controlling for obesity and cigarette smoking at 42 years of age, as well as social disadvantage at 42 years of age, educational attainment at 23 years of age, and stress in adulthood (23–42 years).
Second, early life stress has been associated with the types of health problems that are suspected of having an underlying inflammation-related etiology. Indeed, several psychiatric disorders including substance abuse, post-traumatic stress disorder, depression, and schizophrenia have been linked to both early adversity exposure and inflammation, as have many somatic conditions, such as metabolic, cardiovascular, neoplastic, and rheumatic diseases (Anda et al., 2008; 2006; Batten et al., 2004; Brown et al., 2013; Chapman et al., 2004; Dong et al., 2004; 2003; Dube et al., 2009; Felitti et al., 1998; Halonen et al., 2015; Kivimaki et al., 2006; Korkeila et al., 2010; Mantovani et al., 2008; Miller et al., 2011; Nanni et al., 2012; Packard et al., 2011; Scrivo et al., 2011; Slavich and Irwin, 2014; Wolitzky-Taylor et al., 2017). For instance, higher levels of inflammatory activity have been associated with cardiovascular disease, type 2 diabetes, Alzheimer’s disease, depression, rheumatoid arthritis, and some cancers, as indexed by the inflammatory markers CRP and fibrinogen (an inflammatory protein), as well as pro-inflammatory cytokines that drive the acute inflammatory response—namely, interleukin-1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) (Fagundes and Way, 2014; Miller and Cole, 2012; Slavich and Auerbach, 2018).
Although numerous factors likely moderate the effects of early life stress on inflammation and health, gene-environment interactions likely play an important role in moderating the effects of early life stress on inflammation discussed above. In one case-control study of 262 cases of depression and 288 controls, childhood maltreatment interacted with single nucleotide polymorphisms in genes that regulate inflammation—namely, IL-6 [rs1818879] and CRP [rs3093077]—to predict increased risk of depression (Cohen-Woods et al., 2018). Indeed, genes involved in inflammation are well-known to influence the effects that life stress has on health (for a review, see Slavich and Cole, 2013).
4. Epigenetic embedding of a pro-inflammatory phenotype by early life stress
How might early life stress exposure initiate chronic, low-grade inflammation that persists into adulthood? One possibility is that early life stress becomes embedded in neural systems that regulate peripheral stress physiology (Nusslock and Miller, 2016; Slavich and Irwin, 2014) and in genes that coordinate the systemic inflammatory response (Slavich and Cole, 2013). Additionally, the bi-directional interaction between neural and immune systems may become more interconnected and self-promoting over time, allowing elevations in inflammatory activity to persist even after the initial provoking stimulus has passed (Nusslock and Miller, 2016; Slavich and Irwin, 2014).
Consistent with this possibility, growing evidence suggests that individuals reared in adverse childhood environments might tend to exhibit amplified (phenotypic-like) inflammatory responses to varied challenges including those that are both pathogen-related (e.g., bacteria) and threat-related (psychosocial stress). These pro-inflammatory patterns are evident after many types of challenges, and are marked by elevated circulating levels of the key pro-inflammatory cytokines IL-6 and TNF-α (Danese and McEwen, 2012; Gouin et al., 2012; Heim et al., 2002; Heleniak et al., 2016; Hornung and Heim, 2014; Levine et al., 2015; McLaughlin, 2016; Miller and Chen, 2010; Nusslock and Miller, 2016; Schwaiger et al., 2016; Slopen et al., 2013; Steptoe et al., 2002b; 2002a; Winzeler et al., 2017). For instance, adults exposed to early life stress have been found to exhibit greater IL-6 responses, and weaker anti-inflammatory cortisol responses, to real or simulated acute social stressors. Findings from simulated laboratory-based social stressors, such as the Trier Social Stress Test that induces an experience of psychosocial stress involving social evaluation and threat, are particularly interesting as they provide experimental evidence that social stress plays a causal role in activating inflammatory processes (Carpenter et al., 2007; 2010; 2009; Elzinga et al., 2008; Gouin et al., 2012). Similarly, adults reared in adverse childhood environments tend to exhibit exaggerated reactivity to standardized in vitro immunologic challenges, as indexed by greater TNF-α levels responses to ex vivo lipopolysaccharide (LPS) exposure (Baumeister et al., 2016).
In addition to these findings, lower family socioeconomic status in childhood has been associated with increased cellular inflammatory reactivity to ex vivo LPS exposure, as indexed by the cytokines IL-6 and TNF-α (Azad et al., 2012; Miller et al., 2009; Schreier et al., 2014; Wright et al., 2010). Likewise, a recent longitudinal study of female adolescents found that being raised in a harsh family environment was associated with a pronounced cytokine response to ex vivo LPS stimulation, as well as with declining glucocorticoid sensitivity, over time (i.e., cortisol became progressively less effective at suppressing LPS-evoked cytokine production; (Miller and Chen, 2010). Schreier et al. (2014) also observed that monocytes derived from adolescents from low socioeconomic status (SES) backgrounds exhibited a relative insensitivity to glucocorticoids. This altered sensitivity to glucocorticoids is potentially harmful for health given that glucocorticoids normally down-regulate inflammation and promote recruitment and survival of anti-inflammatory monocytes that aid in resolving tissue injury. Finally, there is evidence that experiencing even one major life stressor involving social threat during adolescence can lead to greater upregulation of pro-inflammatory molecular signaling pathways (Murphy et al., 2013) and downregulation of anti-inflammatory pathways (Murphy et al., 2015).
Although the specific mechanisms by which early life stress becomes biologically embedded are not yet fully understood, at least one pathway likely involves early life stress-related programming of a pro-inflammatory phenotype via changes in the epigenetic regulation of gene expression (Table 3) (Khulan et al., 2014; Levine et al., 2015; Szyf and Bick, 2012)}. Consistent with this possibility, initial research has demonstrated associations between early life stress and genome-wide methylation in adulthood, which was in turn related to increased production of IL-6 following an immunologic challenge (Janusek et al., 2017; Lam et al., 2012). In human studies, early life stress has been associated with modifications of hypothalamic–pituitary–adrenal (HPA) axis- and neuroplasticity-related methylation patterns (Labonté et al., 2012; McGowan et al., 2009; Mehta et al., 2013). Specifically, early life stress was associated with increases in methylation of the glucocorticoid receptor gene (NR3C1; a key regulator of inflammatory activity) (McGowan et al., 2009; Romens et al., 2014; Tyrka et al., 2012; van der Knaap et al., 2014) as well as demethylation of the FKBP5 gene, which increases the expression of the FK506 protein, an important regulator of the stress hormone (Klengel et al., 2013). Increased methylation of the glucocorticoid receptor gene reduces its function and disturbs the feedback of the HPA axis, while binding of FKBP5 protein inhibits the glucocorticoid receptor (Tyrka et al., 2012). These epigenetic changes, which suppress the expression and function of the glucocorticoid receptor, can result in increased inflammatory activity.
Table 3.
Evidence of epigenetic embedding of early life stress
| Citation | Cited association | Type of study | Population | Sample size | Key metrics | ||
|---|---|---|---|---|---|---|---|
| Janusek et al. (2016) | Association between childhood adversity and DNA methylation of the interleukin (IL)-6 promoter | Experimental | Adults | 34 | Childhood Trauma Questionnaire (CTQ), household dysfunction, DNA methylation of IL-6 promoter, salivary IL-6 and cortisol, exposure to community violence, social support, anxiety, socioeconomic status (SES), health behaviors | ||
| Khulan et al. (2014) | Association between current mental state of men exposed to early-life stress (ELS) and DNA methylation | Case-control | Adult males | 166 | ELS in the form of separation from their parents during childhood, age at separation, length of separation, DNA methylation, current SES, education level, history of mental health disorders, glucose, insulin, IL6, TNF-α and CRP, depression | ||
| Labonte et al. (2012) | Association between childhood adversity and epigenetic alterations of genes in hippocampal neurons in suicide completers | Case-control | Adult males | 41 | Severe early-life abuse assessed via interviews, methylated DNA, gene expression | ||
| Levine et al. (2015) | Association between childhood stress and increased inflammatory gene expression later in life | Cross-sectional | Adults | 114 | Childhood trauma, gene expression levels for PTGS2, IL-1β, and IL-8, childhood SES, childhood health, adult traumas, adulthood SES | ||
| Mehta et al. (2013) | Gene expression was associated with methylation differences in peripheral blood cells and childhood abuse among individuals with posttraumatic stress disorder (PTSD) | Case-control | Adults | 169 | CTQ, trauma events inventory, gene expression, DNA methylation, PTSD via symptomatic scale and clinician administered scale | ||
| Perroud et al. (2011) | Childhood sexual abuse, its severity and the number of type of maltreatments positively correlated with methylation of the glucocorticoid receptor gene (NR3C1) among individuals with borderline personality disorder (BPD) | Cross-sectional | Adults | 101 | CTQ, NR3C1 methylation, Screening Interview for Axis II Disorders BPD part, depression | ||
| Schwaiger et al. (2016) | Stress-responsive transcripts were enriched for genes involved in cytokine activity among individuals with history of childhood adversity | Experimental | Adults | 60 | CTQ, Early Trauma Inventory (ETI), genome-wide mRNA expression, Structured Clinical Interview for DSM Disorders (SKID I & II), Resilience Scale (RS-25), Brief Symptom Inventory, Trier Social Stress Test | ||
| Tyrka et al. (2012) | Parental loss, childhood maltreatment, and parental care, was associated with increased methylation of NR3C1 | Cross-sectional | Adults | 99 | Parental Bonding Index, childhood parental Loss, CTQ, methylation of NR3C1, Dex/CRH Test, plasma cortisol, Structured Clinical Interview for DSM-IV, Inventory for Depressive Symptoms, State-Trait Anxiety Inventory, Perceived Stress Scale | ||
| van der Knaap et al. (2014) | NR3C1 methylation rates were higher after exposure to stressful life events (SLEs) during childhood and after exposure to traumatic youth experiences | Prospective | Adolescents | 468 | Perinatal stress, SLEs and traumatic youth experiences, methylation of NR3C1 | ||
| McGowan et al. (2009) | Methylation and gene expression of NR3C1 decreased in suicide victims with a history of childhood abuse compared with controls (victims of sudden, accidental death with no history of abuse) | Case-control | Adults | 36 | Childhood Experience of Care and Abuse Interview, NR3C1 methylation and gene expression, Structured Clinical Interview for DSM Disorders (III-R) | ||
| Klengel et al. (2013) | Decreased methylation of the glucocorticoid receptor regulator (FKBP5) was observed in individuals exposed to child abuse and carriers of a risk allele | Case-control | Adults | 152 | CTQ, methylation of FKBP5, genotyping, clinician-administered PTSD scale | ||
| Romens et al. (2015) | Physical maltreatment in children was associated greater methylation of NR3C1 | Cross-sectional | Children (11–14 years) | 56 | Physical maltreatment, NR3C1 methylation, child SES, parent martial status, parent SES (employment status, educational attainment, and occupational prestige) | ||
The persistence of a phenotype into adulthood is also plausible as increased inflammatory activity can sustain and further exacerbate the impaired function of the glucocorticoid receptor (Miller et al., 2009; Pace et al., 2007; Zunszain et al., 2011). Because the glucocorticoid receptor is central to the regulation of TNF-α signaling, the connections identified between early life stress and pro-inflammatory responses indexed by TNF-α serve as further evidence of an underlying epigenetic mechanism (Van Bogaert et al., 2010). However, support for an epigenetic explanation of the biological embedding of early life stress is still in a preliminary state. Recently, for example, Marzi et al. (2018) conducted epigenome-wide analyses in peripheral blood from participants of the Environmental Risk (E-Risk) Longitudinal Study (Moffitt, 2002) and found no support for the hypothesis of robust changes in DNA methylation in young (18-year olds) individuals with history of victimization (Marzi et al., 2018).
Early life stress might also activate other epigenetic pathways to promote pro-inflammatory states, such as histone modification. For instance, in one animal model study with rats, the gene (GR/NR3C1) that encodes the glucocorticoid receptor exhibited differences in DNA methylation and histone acetylation (involved in chromatin remodeling) in the hippocampus of offspring of high versus low licking and grooming mothers (Weaver et al., 2004). These differences in DNA methylation and histone acetylation emerged very quickly over the first week of life and persisted into adulthood. Furthermore, these differences were reversed with cross-fostering. A causal relationship between epigenomic state, GR expression, and the maternal effect on stress responses in the offspring was supported by the removal of group differences in histone acetylation, DNA methylation, GR expression and HPA responses via central infusion of a histone deacetylase inhibitor (Weaver et al., 2004). In animals, maternal separation also affected gene regulation of both writer (histone acetyltransferases and histone methyltransferases) and eraser (histone deacetylases and histone lysine demethylases) classes of histone modifiers within the medial prefrontal cortex (mPFC), a key limbic brain region that regulates stress responses and mood-related behavior (Pusalkar et al., 2015)1.
Generally speaking, this shift toward a more pro-inflammatory phenotype has biological advantages, especially under conditions of acute threat of physical harm when inflammation is helpful for promoting wound healing and recovery. If persistently stimulated by inflammation-inducing factors such as pathogens, chronic psychosocial stress, or air pollution, however, the pro-inflammatory phenotype may become more self-promoting and prolonged, which could in turn promote low-grade, chronic inflammation that increases an individuals’ risk for disease (Slavich and Irwin, 2014). These effects are thus relevant for understanding how early life stress leads to poor health in general. Because exposure to stressors that trigger inflammation is socially patterned, however, these findings are also important for understanding how disparities in immunologic-related health outcomes may develop and persist over time (Miller et al., 2011).
5. Air pollution and health
The World Health Organization (WHO) presently considers air pollution the biggest environmental threat to health, with one of every nine deaths around the world attributed to air-pollution related conditions (World Health Organization, 2016). Ambient (outdoor) air pollution is commonly considered a combination of gaseous and particulate components, such as sulfur dioxide (SO2), particulate matter (PM), ozone, nitric oxide (NO2), carbon monoxide (CO), and lead, which have been individually demonstrated to have adverse impacts on health (Bose and Diette, 2016). Exposure to ambient air pollution alone is responsible for approximately 3 million deaths each year, mainly from cardiovascular diseases such as stroke and ischemic heart disease, as well as from respiratory conditions (24%) and lung cancer (6%) (World Health Organization, 2016). Importantly, this mortality is primarily due to exposure to particulate matter smaller than 10 microns in diameter (PM10). Long-term exposure PM10 or smaller (e.g., < 2.5 microns, PM2.5), has also been associated with increased risk of adverse birth outcomes, respiratory disease, diabetes, and atherosclerosis, as well as poor neurodevelopment and cognitive function (Table 4) (Bobak, 2000; Bowatte et al., 2017; Calderón-Garcidueñas et al., 2015; Campen et al., 2012; Esposito et al., 2016; Malmqvist et al., 2011; Morgenstern et al., 2007; Thiering and Heinrich, 2015; Wang et al., 2016). Conversely, reductions in ambient PM2.5 concentrations across U.S. cities have been associated with significant increases in life expectancy, even after adjusting for changes in socioeconomic and demographic variables (Pope et al., 2009).
Table 4.
Associations between particle air pollution exposure, inflammation, and inflammation-related diseases in humans
| Citation | Cited association | Type of study | Population | Sample size | Key metrics |
|---|---|---|---|---|---|
| (Akintoye et al., 2016) | Association between fine particulate matter exposure and subclinical atherosclerosis | Meta-analysis | Adults | 11,947–6,497 | Exposure to PM2.5, carotid intima media thickness, arterial calcification, ankle-brachial index |
| (Balti et al., 2014) | Association between exposure to air pollution and increased risk of type 2 diabetes | Meta-analysis | Adults | 385,395 | Exposure to nitrous oxides (NOx), NO2, PM10, and PM2.5, risk of type 2 diabetes |
| (Behndig et al., 2006) | Increased interleukin (IL)-8 concentration in bronchial lavage was induced by diesel exhaust exposure | Experimental | Adults | 15 | Two-hour controlled exposure to PM from diesel exhaust and clean air, bronchoscopy performed 18 hours post exposure, IL-6, IL-8, lung function, endothelial adhesion molecules, vascular adhesion molecule, intracellular adhesion molecule |
| (Bernatsky et al., 2016) | Association between exposure to PM2.5 and systemic autoimmune rheumatic diseases (SARDs) | Cross-sectional | Adults | > 1,000,000 | Regional PM2.5, systemic lupus, erythematous, Sjogren’s syndrome, scleroderma, polymyositis, dermatomyositis, and undifferentiated connective tissue disease |
| (Bobak, 2000) | Association between prenatal exposure to sulfur dioxide (SO2) and low birth weight | Cross-sectional | Mother and child | 108,173 | Exposure to SO2, NOx, total suspended particles (TSP) during each trimester of pregnancy, and low birth weight, prematurity, intrauterine growth retardation |
| (Bowatte et al., 2017) | Association between exposure to traffic related air pollution (TRAP) and increased risk of atopy and wheezing | Cross-sectional | Children | 1,405 | Mean annual residential exposure to NOx, distance to nears major road, lung function, asthma, atopy |
| (J.-C. Chen and Schwartz, 2009) | Association between chronic exposure to PM10 and ozone and cognitive function | Cross-sectional | Adults | 1,764 | Exposure to PM10 and ozone, simple reaction time test (SRTT), basic measure of visuomotor speed, symbol-digit substitution test (SDST) on coding ability, serial-digit learning test (SDLT) on attention and short-term memory, age, sex, race/ethnicity, SES |
| (Dobreva et al., 2013) | Association between regional PM2.5 ambient levels and tumor necrosis factor alpha (TNF-α) and IL-10 in adolescents | Cross-sectional | Adolescents (18 years) | 40 | Regional PM10, PM2.5, SO2, and NO2 levels, TNF-α, IL-6, IL12p40, IL-10 |
| (Ghio et al., 2000) | Acute exposure to concentrated ambient particles was associated with mild inflammation in lower respiratory tract as well as higher circulatory fibrinogen | Experimental | Adults | 38 | Two-hour exposure to PM2.5 (0.1–2.5 μm), cell count via bronchoalveolar lavage, fibrinogen, lung function |
| (Gruzieva et al., 2017) | Long-term exposure to PM10 and NO2 was associated with IL-6 in healthy children, and also with IL-10 in asthmatic children | Longitudinal | Children | 670 | Exposure to PM10 and NO2, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α |
| (K.-N. Kim et al., 2016) | Association between long-term PM2.5 exposure and major depressive disorder | Longitudinal | Adolescents and adults | 27,270 | Long-term exposure to PM2.5, major depressive disorder, household income, smoking, alcohol consumption, exercise |
| (Kipen et al., 2011) | Association between exposure to fresh diesel-engine exhaust and reduced white and red blood cell proteasome activity | Experimental | Adult | 38 | Exposure to diesel exhaust, secondary organic aerosol, and clean air, platelet activation, nitric oxide metabolites, proteasome activity, endothelial function, heart rate variability |
| (Lamichhane et al., 2015) | Association of exposure to particulate matter and adverse birth outcomes | Meta-analysis | Mother and child | > 1,000,000 | Exposure to PM10, and PM2.5, birth weight, preterm birth |
| (Ostro et al., 2014) | Association between prior-year exposure to PM2.5 and C-reactive protein (CRP) | Longitudinal | Adult women | 1,923 | Exposure to PM2.5, CRP, diabetes, smoking, unmarried, income |
| (Peters et al., 2001) | A high ambient-PM episode was associated with increased odds of observing increases in CRP in men | Prospective | Adult men | 631 | Exposure to total suspended particles, SO2, and carbon monoxide (CO), CRP, body mass index (BMI), smoking |
| (Pourazar et al., 2004) | Significant increase of IL-13 expression in bronchial epithelium cells induced by diesel exhaust exposure | Experimental | Adult | 15 | One-hour controlled exposure to diesel exhaust and clean air, bronchoscopy, IL-10, IL-13, IL-18 |
| (Requia et al., 2017) | Associations between respiratory diseases and PM2.5 exposure | Meta-analysis | Adults and children | > 1,000,000 | Exposure to PM10, PM2.5, CO, SO2, NO2, ozone, mortality, hospital admissions, respiratory diseases, cardiovascular diseases |
| (Rich et al., 2012) | Changes in air pollution levels during the Beijing Olympics were associated with acute changes in biomarkers of inflammation in healthy young persons | Quasi-experimental | Adults | 125 | Exposure to PM2.5, CRP, fibrinogen, von Willebrand factor, soluble CD40 ligand (sCD40L), soluble P-selectin (sCD62P) concentrations, white blood cell count (WBC), heart rate, and blood pressure |
| (Rueckerl et al., 2007) | Association between increased circulatory IL-6 levels and acute exposure to ambient air particle levels in myocardial infraction survivors | Longitudinal | Adults | 1,003 | Exposure to PM10, PM2.5, ultrafine particles, black smoke, black carbon, CO, SO2, NOx, and ozone, IL-6, CRP, blood pressure, BMI serum lipids, HbA1c |
| (Seaton et al., 1999) | Association between personal three-day PM10 exposure and CRP | Cross-sectional | Older adult men | 112 | Personal exposure to PM10, CRP, fibrinogen, hemoglobin, packed cell volume, platelets |
| (Stieb et al., 2012) | Association of exposure to particulate matter and adverse birth outcomes | Meta-analysis | Mother and child | > 1,000,000 | Exposure to PM10, PM2.5, CO, and NO2, birth weight, preterm birth |
| (Tomczak et al., 2016) | Increases in PM2.5 exposure were associated with elevated risk of lung cancer | Prospective | Adult women | 89,234 | Long-term exposure to PM2.5, small cell carcinoma, adenocarcinoma, smoking |
| (van Eeden et al., 2001) | Circulating levels of IL-1β and IL-6 were elevated in subjects exposed to high levels of PM10 during an episode of acute air pollution. | Longitudinal | Adult men | 30 | Exposure to PM10, SO2, NO2, ozone, and CO during a forest fire, TNF-α, IL-1β, IL-6 |
| (B. Wang et al., 2014) | Association between exposure to air pollution and increased risk of type 2 diabetes | Meta-analysis | Adults | 2,371,907 | Exposure to NO2, PM10, and PM2.5, risk of type 2 diabetes |
| (Wu et al., 2012) | Associations between PM2.5 chemical constituents and multiple biomarkers of inflammation | Longitudinal | Young adults | 40 | Exposure to PM2.5, chemical composition, CRP, TNF-α, fibrinogen, plasminogen activator inhibitor type 1, tissue-type plasminogen activator, von Willebrand factor, soluble platelet selectin |
A recent study found significant evidence of adverse effects related to exposure to two common urban air pollutants (i.e., PM2.5 and ozone) even at concentrations below national standards and noted that these effects appeared most potent among socially disadvantaged individuals, such as racial minorities and people with low income (Di et al., 2017). Such findings hint that socially disadvantaged individuals may in fact be more vulnerable to air pollution. Throughout their lifespan, socially disadvantaged individuals tend to experience worse air quality (Bose and Diette, 2016; Frieden, 2011). However, although studies rarely account for exposure to both poor air quality and social disadvantage, the health impact of chronic exposure to poor air quality is closely intertwined with the negative health effects of social stressors that are also more concentrated among those who are socially disadvantaged, such as family dysfunction, violence, discrimination, and poverty (Evans and P. Kim, 2007; Kristiansson et al., 2015; Miller and Chen, 2013). Therefore, research is needed to understand how social and environmental factors interact in order to evaluate if and how synergy among these factors leads to social disparities in health. Understanding these interactions is essential for developing more effective interventions and policies to better protect susceptible populations, and in turn reduce health disparities and improve public health.
6. Inflammatory mechanisms linking air pollution and health
Toxicology research in humans and animal models shows that exposure to particle air pollution can result in both local and systemic inflammation (Table 4) (Ogino et al., 2017; Ostro et al., 2014; Riva et al., 2011). For example, inhaled traffic-related PM enters the lungs and causes a local inflammatory response from alveolar macrophage and bronchial epithelial cells (Bai and Sun, 2015); it is noteworthy that this response can be indexed by the production of the same pro-inflammatory cytokines that are triggered by life stress (Ghio et al., 2000; Peters et al., 2001; Pope et al., 1999; Seaton et al., 1999). At a cellular level, onset of this inflammatory response is initiated by the release of IL-1β and TNF-α, which tend to be expressed as inactive proforms in resting cells and released without the activation of the transcriptional machinery (Schwarze et al., 2013). Subsequently, IL-1β and TNF-α regulate the expression of various secondary cytokines and chemokines, including IL-6 and IL-8.
Release of these secondary cytokines and chemokines can also be activated directly (i.e., independent of IL-1β and TNF-α) by traffic-related particle pollution through stimulation of the intracellular pro-inflammatory signaling pathways. One such pathway begins with oxidative stress that is induced by inhaled particle air pollution (Pope et al., 1999). In rats, for example, a three-hour exposure to particle matter smaller than 2.5 μm (PM2.5) has been shown to lead to a rapid increase of reactive oxygen species (ROS) generation in the heart and lung (Gurgueira et al., 2002). Oxidative stress can then activate transcription factors such as nuclear factor (NF)-κB and activator protein (AP)-1, which in turn upregulate the expression of genes coding for cytokines, chemokines, and other pro-inflammatory mediators (see Fig. 1) (Bennett et al., 2012). Notably, NF-κB signaling is central to the human inflammatory machinery and can also be activated by toll-like receptor ligands of LPS (some of which can be attached to PM), hypoxic condition, and TNF-α. The localized pro-inflammatory mediators that result from NF-κB activation then spill into the circulatory system and fuel a low-grade peripheral inflammatory state.
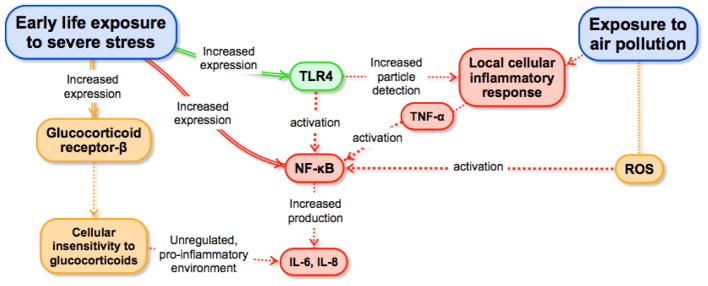
Fig. 1.
An integrated multi-level model of early life stress, air pollution, and health. Depicted are the mechanisms through which early life stress exposure may affect the inflammatory response to air pollution exposure, leading in turn to poor lifespan health. Early life exposure to severe stress increases the expression of toll-like receptor 4 (TLR4), glucocorticoid receptor-β, and nuclear factor (NF)-κB (Bennett et al., 2012; Fiordelisi et al., 2017) (G. E. Miller et al., 2009). TLR4 is part of the air pollution recognition process that leads to the production of tumor necrosis factor alpha (TNF-α) and culminates in the activation of NF-κB (Kampfrath et al., 2011) (Kawai and Akira, 2007; Lund et al., 2011)(Becker et al., 2002; Shoenfelt et al., 2009). Exposure to air pollution increases reactive oxygen species (ROS) generation in the heart and lung (Gurgueira et al., 2002), which in turn also activates NF-κB. Activation of NF-κB upregulates the expression of genes coding for cytokines, chemokines, and other pro-inflammatory mediators such as interleukin-6 (IL-6) and interleukin-8 (IL-8) (Gurgueira et al., 2002)(Bennett et al., 2012). Finally, increased expression of glucocorticoid receptor- β leads to insensitivity to glucocorticoids that creates a pro-inflammatory environment, which culminates in increased production of IL-6 and IL-8 (Cain and Cidlowski, 2015; Hamid et al., 1999).
Activation of toll-like receptor 4 (TLR4) and NADPH oxidase in monocyte/macrophages by oxidized phospholipids may represent another potential pathway through which PM2.5 mediates systemic inflammation (Kampfrath et al., 2011). Toll-like receptors and related scavenger receptors, such as CD36 and lectin-like oxidized LDL receptor-1 (LOX-1), are the major immune sensors that recognize pathogen-associated and damage-associated molecular patterns arising from inflammation, infection, or cell stress, and these receptors have been mechanistically implicated as drivers of systemic inflammation following air pollution exposure (Aragon et al., 2017; Nergiz-Unal et al., 2011; Rao et al., 2014; Robertson et al., 2013). The scavenger receptor signaling pathways culminate in activation of NF-κB (Kawai and Akira, 2007; Lund et al., 2011). TLR4 has also been involved in the recognition of PM and, similar to the scavenger receptors, has been shown to mediate the particle-induced production of TNF-α (Becker et al., 2002; Shoenfelt et al., 2009).
7. Inflammation as a common process triggered by early life stress and air pollution
Some epidemiologic studies have directly examined the extent to which psychosocial stress and air pollution have synergistic effects, and so far, this literature has revealed evidence that such effects may occur. For instance, Chen at al., (2008) studied the interaction between chronic exposure to chronic family stress and traffic-related air pollution in predicting biologic and clinical outcomes in 73 children with asthma (Chen et al., 2008). They found significant interactions, for instance children with high chronic family stress had elevated levels of IL-5, immunoglobulin E (IgE), and eosisophil counts as exposure to nitric dioxide decreased. Shankardass et al. (2009) studied 2,497 children aged 5–9 years old from the Children’s Health Study (McConnell et al., 2006; Shankardass et al., 2009), and looked at doctor-diagnosed new onset of asthma during a 3-year follow-up. They observed that risk of asthma attributable to exposure to traffic related air pollution (TRP) was significantly higher for children with high parental stress [Hazard Ratio (HR) = 1.51 across the interquartile range for TRP; 95% CI = 1.16–1.96] as compared to those with low parental stress (HR = 1.05, 95% CI = 0.74–1.49; interaction P value = 0.05). Although parental stress may have influenced the development of asthma in children through pathways other than psychosocial stress in these children, the pattern of susceptibility to air pollution based on stress was not explained by potentially relevant history of illness and a range of behavioral, socioeconomic, and environmental risk factors for asthma (Shankardass et al., 2009). Using the same data set, Islam et al. (2012) reported that nitrogen oxide concentrations at home and school had a greater impact on lung function (e.g., FEV1 and FVC) among children of parents reporting higher perceived stress, after adjusting for household socioeconomic status (Islam et al., 2011). These findings are also broadly consistent with evidence showing that individuals from low socioeconomic backgrounds (which can give rise to stress) have greater air-pollution related mortality than their higher socioeconomic counterparts (Forastiere et al., 2007; Jerrett et al., 2004; Krewski et al., 2003).
Here, we posit that early life stress and air pollution are likely to have joint effects on health by way of stress exposure early in life increasing individuals’ inflammatory response to particle air pollution across the lifespan. Evidence for this pathway comes from at least three lines of research. First, there is substantial overlap between the specific diseases that are strongly associated with particle air pollution and with early life stress exposure. These include disorders that cause substantial disease burden worldwide, including cardiovascular disease, autoimmune diseases, lung cancer, and depression (Bernatsky et al., 2016; Brunekreef and Holgate, 2002; Calderón-Garcidueñas et al., 2015; Lim et al., 2012). Second, many of these diseases have an underlying inflammatory component (Danese et al., 2009; O’Neill et al., 2007; Slavich and Irwin, 2014; Wellen and Hotamisligil, 2005). Moreover, psychosocial stress and particle air pollution seem to influence the same inflammatory processes, such as inducing oxidative stress, as well as activating NF-κB and TLR4 (see Fig. 1); (Bennett et al., 2012; Fiordelisi et al., 2017).
In one study that sampled caregivers of brain-cancer patients, for example, exposure to chronic stress was associated with increased expression of transcripts with response elements for NF-κB and reduced expression of transcripts bearing response elements for glucocorticoids (Miller et al., 2008). Another study with female adolescents found that lower socioeconomic status early in life was associated with reduced expression of genes coding for glucocorticoid receptor and increased expression of TLR4 (Miller and Chen, 2007). The observed changes in the expression of these genes can lead to the improper regulation of inflammatory response.
Given that the inflammatory response to particle air pollution is mediated by the activation of NF-κB via oxidative stress, a psychosocially induced increase in expression of NF-κB could potentiate the inflammatory response to air pollution (Fiordelisi et al., 2017). Additionally, increased expression of glucocorticoid receptor-β can lead to cellular insensitivity to glucocorticoids, including in airway cells, creating a physiologic environment that favors the production of pro-inflammatory cytokines and increases systemic inflammation (Cain and Cidlowski, 2015; Hamid et al., 1999). Neither study evaluated possible interactions with air pollution. However, it is possible that risk of adverse outcomes is even greater than initially considered given the inflammatory response to fine particle air pollution, which is mediated by TLR4, is exacerbated by exposure to early life stress because such exposure increases expression of TLR4 (Kampfrath et al., 2011).
In sum, exposure to early life stress and to early life stress are socially patterned in very similar ways. Consequently, the aforementioned inflammatory pathway that links early life stress and air pollution exposure could help explain disparities in inflammation-related health problems during the lifespan for individuals in different socially disadvantaged groups.
8. An integrated multi-level model of early life stress, air pollution, and health
Based on the literatures synthesized in prior sections, we propose an integrated, multilevel immunologic model of how severe early life stress exposure combines with exposure to air pollution to structure social disparities in inflammation-related health outcomes across the lifespan. Fixed factors that determine social disadvantage such as gender, race, and ethnicity are present from birth and persist across life. Factors such as poverty and SES although unfixed, also tend to persist across life (Chetty et al., 2014). Because individuals born into social disadvantage tend to remain in those circumstances into adulthood, they experience exposures across the lifespan that can interact to affect health and promote health disparities. One example of this is when individuals born into social disadvantage experience stress early in life and develop a sensitivity to air pollution characterized by a pro-inflammatory phenotype, while also being more likely to experience higher exposure to air pollution across the lifespan (Boehmer et al., 2013; Cicchetti and Toth, 2005; Evans, 2004). The model we propose in Figure 2 describes this interaction and how it can promote health disparities by increasing a person’s risk of developing health problems with an inflammatory component (e.g., depression, cardiovascular disease, diabetes).
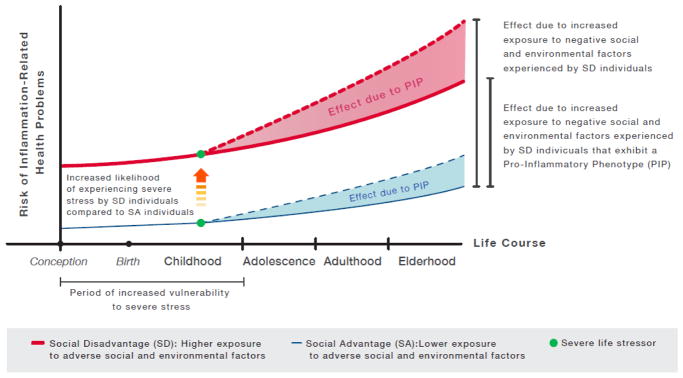
Fig. 2.
The immunologic model illustrates a pathway through which specific social factors (i.e., early life stress exposure) and environmental factors (i.e., air pollution exposure) interact to increase the risk of developing inflammation-related health problems. It also describes how the accumulation of risk across the lifespan occurring as a function of higher exposure to these factors drives disparity across social strata. First, socially disadvantaged individuals are shown to be at particularly high risk for poor health, as compared to individuals in better social standing, due to a greater chance of experiencing severe stress during childhood (orange arrow) and greater exposure to air pollution -and other inflammation-inducing triggers- over the lifetime (shift between solid lines). Second, regardless of social circumstances, early life stress can program, via epigenetic mechanisms, a pro-inflammatory phenotype that ultimately results in vulnerability to inflammation-inducing triggers. As a consequence, individuals with this phenotype exhibit greater inflammatory reactivity to inflammatory triggers as compared to individuals without this phenotype. Over time, this pro-inflammatory phenotype increases individuals’ risk of developing inflammation-related health problems as a function of exposure to inflammation-inducing triggers (shaded areas between solid and dotted lines).
As depicted in Figure 2, our formulation highlights why compared to individuals in socially advantageous circumstances (blue lines), those in socially disadvantageous circumstances (red lines) are at particularly high risk of experiencing more inflammation-related health problems across the lifespan—namely, because:
Individuals in socially disadvantageous circumstances have greater risk of developing inflammation-related health problems due to exposure to higher levels of air pollution over the lifetime, as compared to those in socially advantageous circumstances (shift between solid blue and red lines).
Individuals in socially disadvantageous circumstances have a greater chance of experiencing severe stress early in life (orange arrow), and therefore of developing a pro-inflammatory phenotype that increases their inflammatory reactivity to air pollution exposure, as compared to individuals in socially advantageous circumstances (see also Fig. 1).
Independent of social condition, individuals who develop a pro-inflammatory phenotype experience greater risk of inflammation-related health problems due to increased inflammatory responsivity to air pollution exposure (shift between solid and dotted lines).
Disparity of inflammation-related health problems between individuals in socially advantageous circumstances and those in disadvantageous circumstances (shift between solid blue line and dotted red line), can result from differences in experiences of early life stress, sensitivity to air pollution due to the development of a pro-inflammatory phenotype, and exposure to higher levels of air pollution over the lifetime.
Our model describes how among socially disadvantaged individuals, an interaction between stress in childhood and air pollution across the lifespan, that is mediated by a pro-inflammatory phenotype, can result in a greater and compounded risk of developing inflammation-related health problems. The evidence discussed in previous sections also suggests that the pro-inflammatory phenotype results in increased inflammatory reactivity to inflammation-inducing factors, other than air pollution, such as psychosocial stress and pathogens (Baumeister et al., 2016; Carpenter et al., 2010; 2009; 2007; Elzinga et al., 2008; Gouin et al., 2012), to which socially disadvantaged individuals are also more likely to be disproportionately exposed during their life, compared to individuals in better social circumstances. For this reason, our model (shown in Figure 2) was generalized to describe how severe stress experienced during childhood can drive disparity in inflammation-related health problems by programming a pro-inflammatory phenotype that increases the inflammatory response to social and environmental factors (that induce inflammation) and for which exposure is patterned by social advantage.
The immunological model proposed here is in alignment with the allostatic load model as it posits that historical factors (trauma/abuse, stressful environments during childhood) induce a vulnerability to certain stressors by compromising allostatic mechanisms which result in the dysregulation of primary mediators; over-production of pro-inflammatory cytokines (e.g., IL-6, TNFα) and under-production of stress hormones (e.g., cortisol). Allostatic load as described by (McEwen and Stellar, 1993) refers to the “wear and tear” the body experiences in response to repeated allostatic activations caused by persistent perception of psychosocial stressors. The allostatic load model delineates that an individual’s vulnerability or resiliency to stress is determined by their perception of threats and the subsequent activation of allostatic mechanisms, which in turn can be compromised by synergistic effects of primary mediators -stress hormones (epinephrine, norepinephrine, and cortisol) their antagonists, as well as pro- and anti- inflammatory cytokines (e.g., IL-6, TNFα)- on cellular activities (enzyme, receptor, ion channel, genomic) (Juster et al., 2010; McEwen, 1998; McEwen and Wingfield, 2003). Also, our model expands the allostatic load model by suggesting that allostatic load can result in vulnerability to environmental stressors such as air pollution.
Finally, our model emphasizes an interaction between stress early in life and air pollution exposure across the lifespan. However, there is also evidence of additive effects between psychosocial stress and environmental exposures (e.g., bacteria, virus, Pb) (Clougherty et al., 2014). The model also delineates a different risk of developing inflammation-related health problems at conception based on potential genetic susceptibilities passed on by parents, due to exposure to social and environmental factors associated with social advantage.
9. Future directions
Despite an evident overlap of social and environmental risk factors in society that is patterned by social disadvantage, insufficient research has focused on studying the interaction of these factors. Interdisciplinary research is urgently needed to determine how exposures, as they occur in the real world rather than in the siloed world of epidemiology, contribute to social disparities in health and, most importantly, how data along these lines can help scientists identify the factors that can be modified to prevent or manage these problems. To this end, we recently proposed a broad framework that accounts for a wide range of overlapping social and environmental exposures across the lifespan and delineates underlying biobehavioral pathways that lead to chronic illness (Olvera Alvarez et al., 2018). Along with this framework, a roadmap for research in this area was also delineated. Although this framework may generally guide research in this area, different combinations of exposures may require their own specific consideration to identify appropriate mechanisms and processes at play. For that reason, here we focus on a set of specific commonly co-occurring exposures, early life stress and air pollution, and consider how they may interact to affect health.
Research along several lines is needed to test and advance the hypothesis that a pro-inflammatory phenotype may help to understand the greater susceptibility of individuals who experience high levels of early life adversity, to adverse health effects of air pollution. First, research is necessary to understand which specific types of life stress most strongly trigger inflammation and lead to the development of a pro-inflammatory phenotype (Epel et al., in press; Irwin and Slavich, 2017; Slavich et al., 2010). Second, there may be periods of vulnerability during childhood when stress exposure is more likely to promote a pro-inflammatory phenotype, but these periods presently remain unknown. Third, studies on the biological and physiological processes that characterize the pro-inflammatory phenotype, including the epigenetic markers such as the changes in methylation of specific genes at specific sites in specific cells, should be conducted to establish effective ways to assess the presence, persistence, and potential reversibility (if any) of this vulnerability. Such information may be used to identify individuals at risk earlier in the life course and may provide earlier opportunities for intervention.
Fourth, broad research efforts are necessary to determine the most pervasive factors that may induce inflammation to which individuals with a pro-inflammatory phenotype are vulnerable. Understanding these factors (e.g., certain foods, or specific forms of stress) could help direct efforts to minimize deleterious exposures. Relatedly, greater understanding of the social and environmental contexts in which exposure to factors that induce inflammation occurs is also important. Although it is clear that social disadvantage is broadly associated with greater exposure to harmful social and environmental risk factors, it is likely that substantial variation in vulnerability and risk also exists among those who are socially disadvantaged. For example, being part of an ethnic minority group or in low-SES does not imply an automatic constant risk. In this regard, future research should consider alternative pathways that may confound the associations between early life stress and adult health outcomes, such as access and quality of health care. Similarly, among socially disadvantaged populations, subgroups of intense risk also exist (e.g., very poor, individuals in isolation, minorities within very segregated communities), which need to be identified and prioritized in intervention efforts. In this regard, research efforts should focus primarily on identifying modifiable factors that could help reduce exposure to factors that induce inflammation among vulnerable populations.
Fifth, research is needed to explore potential protective factors (e.g., social, psychological, immunologic) against severe early life stress and the programming of the pro-inflammatory phenotype. There is evidence for instance that positive parental attachment could protect children against some of the outcomes associated with early life stress exposure (Cameron et al., 2017; Okello et al., 2014), but this research has generally not considered the potential role of air pollution or inflammatory processes. Research on the availability or prevalence of protective factors across social groups could further reveal opportunity for interventions is also necessary.
In general, despite growing interest in examining the joint contribution of social and environmental determinants of health, conducting interdisciplinary research on this topic can be challenging, and a critical limiting factor presently involves a lack of knowledge about how to measure exposures outside of one own’s primary discipline. As a starting point, therefore, stress researchers are referred toa set of reviews summarizing air pollution exposure assessment methods (Krzyzanowski, 1997; Mirowsky and Gordon, 2015; Zhang and Lioy, 2002; Zou et al., 2009), as well as the USEPA’s online resources for human air pollution exposure. Environmental health researchers, in turn, are referred to a set of reviews summarizing early life stress measures (Appleton et al., 2017; Burgermeister, 2007), as well as lifetime stress exposure measurement (Shields and Slavich, 2017; Slavich, 2016; Slavich and Shields, 2018).
10. Conclusion
In summary, we believe there is relatively strong evidence, emerging from different lines of research, to suggest that social factors interact with environmental factors via biobehavioral pathways that in turn affect health. With the rich separate bodies of evidence linking early adversity and air pollution with both inflammation and inflammation-related chronic diseases, considering the combined effects of early adversity and air pollution provides a potent example of how these effects might play out. To date, empirical research exploring the full range of factors that may be relevant for developing a comprehensive understanding of this interplay is limited. To illustrate the promise of this work and more directly explore a plausible pathway by which social disadvantage gets under the skin to influence lifelong health, we integrated evidence from epidemiology, psychoneuroimmunology, toxicology, and genomicsresearch to support the hypothesis that early life stress activates a pro-inflammatory phenotype, which in turn increases individuals’ susceptibility to developing inflammation-related diseases over the lifespan. Because individuals in socially disadvantaged circumstances are more likely to be exposed to major life stress during childhood, as well as stressors that may induce inflammation across the lifespan, we posit that the activation of this phenotype is a key determinant of social disparities in health. To the extent that this is true, a better understanding of how this pro-inflammatory phenotype develops, as well as what interventions might help reduce inflammation-related health risks, will be important for reducing health disparities and improving population health worldwide (Slavich, 2015).
Highlights.
A neuroimmunologic link between stress, inflammation, air pollution, was identified
A model of social-environmental adversity and lifespan health was proposed
We posit that early life stress and air pollution jointly drive health disparity
Acknowledgments
Preparation of this review was supported by a JPB Environmental Health Fellowship and Grant 5G12MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH) to Hector Olvera; by NIEHS Virtual Consortium for Translational/Transdisciplinary Environmental Research (ViCTER) grant 3R01ES014639-09S1 and grant R01 ES014639 to Matthew Campen; and by a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation, and NIH grant K08 MH103443 to George Slavich. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NE, Stewart J. Health disparities across the lifespan: Meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- Akintoye E, Shi L, Obaitan I, Olusunmade M, Wang Y, Newman JD, Dodson JA. Association between fine particulate matter exposure and subclinical atherosclerosis: A meta-analysis. Eur J Prev Cardiol. 2016;23:602–612. doi: 10.1177/2047487315588758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34:396–403. doi: 10.1016/j.amepre.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JP, Blosnich J. Disparities in adverse childhood experiences among sexual minority and heterosexual adults: Results from a multi-state probability-based sample. PLoS One. 2013;8:e54691–e54697. doi: 10.1371/journal.pone.0054691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Holdsworth E, Ryan M, Tracy M. Measuring childhood adversity in life course cardiovascular research: A systematic review. Psychosom Med. 2017;79:434–440. doi: 10.1097/PSY.0000000000000430. [DOI] [PubMed] [Google Scholar]
- Aragon MJ, Topper L, Tyler CR, Sanchez B, Zychowski K, Young T, Herbert G, Hall P, Erdely A, Eye T, Bishop L, Saunders SA, Muldoon PP, Ottens AK, Campen MJ. Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood–brain barrier impairment. PNAS. 2017;114:E1968–E1976. doi: 10.1073/pnas.1616070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL. Influence of socioeconomic status trajectories on innate immune responsiveness in children. PLoS One. 2012;7:e38669. doi: 10.1371/journal.pone.0038669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev. 2015;16:127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Arseneault L, Caspi A, Fisher HL, Moffitt TE, Odgers CL, Pariante C, Ambler A, Dove R, Kepa A, Matthews T, Menard A, Sugden K, Williams B, Danese A. Childhood victimization and inflammation in young adulthood: A genetically sensitive cohort study. Brain Behav Immun. 2018;67:211–217. doi: 10.1016/j.bbi.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balti EV, Echouffo-Tcheugui JB, Yako YY, Kengne AP. Air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106:161–172. doi: 10.1016/j.diabres.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Resp Cell Mol Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, Wilson SJ, Boman C, Cassee FR, Frew AJ, Kelly FJ, Sandstrom T, Blomberg A. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27:359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- Bennett JM, Gillie BL, Lindgren ME, Fagundes CP, Kiecolt-Glaser JK. Handbook of Systems and Complexity in Health. Springer New York; New York, NY: 2012. Inflammation through a psychoneuroimmunological lens; pp. 279–299. [DOI] [Google Scholar]
- Bernatsky S, Smargiassi A, Barnabe C, Svenson LW, Brand A, Martin RV, Hudson M, Clarke AE, Fortin PR, van Donkelaar A, Edworthy S, Bélisle P, Joseph L. Fine particulate air pollution and systemic autoimmune rheumatic disease in two Canadian provinces. Environ Res. 2016;146:85–91. doi: 10.1016/j.envres.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch SJ, Ford JL. C-reactive protein levels among U.S. adults exposed to parental incarceration. Biol Res Nurs. 2015;17:574–584. doi: 10.1177/1099800414564011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer TK, Foster SL, Henry JR, Woghiren-Akinnifesi EL, Yip FY. CDC Health disparities and inequalities report: United States, 2013. MMWR. Surveillance summaries: Morbidity and mortality weekly report. Surveillance summaries/CDC 2013 [Google Scholar]
- Bose S, Diette GB. Health disparities related to environmental air quality. In: Gerald LB, Berry CE, editors. Health Disparities in Respiratory Medicine, Respiratory Medicine. Springer International Publishing; Switzerland: 2016. pp. 41–58. [DOI] [Google Scholar]
- Bowatte G, Lodge CJ, Knibbs LD, Lowe AJ, Erbas B, Dennekamp M, Marks GB, Giles G, Morrison S, Thompson B, Thomas PS, Hui J, Perret JL, Abramson MJ, Walters H, Matheson MC, Dharmage SC. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol. 2017;139:122–129.e1. doi: 10.1016/j.jaci.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Felitti VJ, Edwards VJ, Malarcher AM, Croft JB, Giles WH. Adverse childhood experiences are associated with the risk of lung cancer: A prospective cohort study. BMC Public Health. 2010;10:20. doi: 10.1186/1471-2458-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Thacker LR, Cohen SA. Association between adverse childhood experiences and diagnosis of cancer. PLoS One. 2013;8:e65524. doi: 10.1371/journal.pone.0065524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Burgermeister D. Childhood adversity: A review of measurement instruments. J Nurs Measure. 2007;15:163–176. doi: 10.1891/106137407783095766. [DOI] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29:545–556. doi: 10.1016/j.beem.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Calderón-Garcidueñas A, Torres-Jardón R, Avila-Ramírez J, Kulesza RJ, Angiulli AD. Air pollution and your brain: What do you need to know right now. Prim Health Care Res Dev. 2015;16:329–345. doi: 10.1017/S146342361400036X. [DOI] [PubMed] [Google Scholar]
- Cameron CA, McKay S, Susman EJ, Wynne-Edwards K, Wright JM, Weinberg J. Cortisol stress response variability in early adolescence: Attachment, affect and sex. J Youth Adolesc. 2017;46:104–120. doi: 10.1007/s10964-016-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Lund A, Rosenfeld M. Mechanisms linking traffic-related air pollution and atherosclerosis. Curr Opin Pulm Med. 2012;18:155–160. doi: 10.1097/MCP.0b013e32834f210a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. PNAS. 2013;110:17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chen E, Schreier HMC, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008;116:970–975. doi: 10.1289/ehp.11076. doi:110.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in U.S. adults. Neurotoxicology. 2009;30:231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Chen M, Lacey RE. Adverse childhood experiences and adult inflammation: Findings from the 1958 British birth cohort. Brain Behav Immun. 2018;69:582–590. doi: 10.1016/j.bbi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Chetty R, Hendren N, Kline P, Saez E. Where is the land of opportunity? The geography of intergenerational mobility in the United States. Q J Econ. 2014;129:1553–1623. doi: 10.1093/qje/qju022. [DOI] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annu Rev Clin Psychol. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Shmool JLC, Kubzansky LD. The role of non-chemical stressors in mediating socioeconomic susceptibility to environmental chemicals. Curr Environ Health Rep. 2014;1:302–313. doi: 10.1007/s40572-014-0031-y. [DOI] [Google Scholar]
- Cohen-Woods S, Fisher HL, Ahmetspahic D, Douroudis K, Stacey D, Hosang GM, Korszun A, Owen M, Craddock N, Arolt V, Dannowski U, Breen G, Craig IW, Farmer A, Baune BT, Lewis CM, Uher R, McGuffin P. Interaction between childhood maltreatment on immunogenetic risk in depression: Discovery and replication in clinical case-control samples. Brain Behav Immun. 2018;67:203–210. doi: 10.1016/j.bbi.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Lereya ST, Shanahan L, Worthman C, Costello EJ. Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. PNAS. 2014;111:7570–7575. doi: 10.1073/pnas.1323641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, Freeman J, Pariante CM, Moffitt TE, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–17. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD. Air pollution and mortality in the medicare population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Meng H, Goldberg R, Schneiderman N, Delamater A. Stress and body mass index each contributes independently to tumor necrosis factor-α production in prepubescent latino children. J Pediatr Nurs. 2009;24:378–388. doi: 10.1016/j.pedn.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva ZG, Kostadinova GS, Popov BN, Petkov GS, Stanilova SA. Proinflammatory and anti-inflammatory cytokines in adolescents from Southeast Bulgarian cities with different levels of air pollution. Toxicol Ind Health. 2013;31:1210–1217. doi: 10.1177/0748233713491812. [DOI] [PubMed] [Google Scholar]
- Dong M, Dube SR, Felitti VJ, Giles WH, Anda RF. Adverse childhood experiences and self-reported liver disease: New insights into the causal pathway. Arch Intern Med. 2003;163:1949–1956. doi: 10.1001/archinte.163.16.1949. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016;8:E8–E19. doi: 10.3978/j.issn.2072-1439.2015.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the adverse childhood experiences study. JAMA. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB. More than a feeling: A unified view of stress measurement for population science. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2018.03.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Petrizzo M, Maiorino MI, Bellastella G, Giugliano D. Particulate matter pollutants and risk of type 2 diabetes: a time for concern? Endocrine. 2016;51:32–37. doi: 10.1007/s12020-015-0638-2. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Way B. Early-life stress and adult inflammation. Curr Dir Psychol Sci. 2014;23:277–283. doi: 10.1177/0963721414535603. [DOI] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fiordelisi A, Piscitelli P, Trimarco B, Coscioni E, Iaccarino G, Sorriento D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev. 2017;22:337–347. doi: 10.1007/s10741-017-9606-7. [DOI] [PubMed] [Google Scholar]
- Forastiere F, Stafoggia M, Tasco C, Picciotto S, Agabiti N, Cesaroni G, Perucci CA. Socioeconomic status, particulate air pollution, and daily mortality: Differential exposure or differential susceptibility. Am J Ind Med. 2007;50:208–216. doi: 10.1002/ajim.20368. [DOI] [PubMed] [Google Scholar]
- Frieden TR. CDC Health disparities and inequalities report: United States, 2011. MMWR Surveill Summ. 2011;60(Suppl):1–2. [PubMed] [Google Scholar]
- Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162:981–988. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- Goodman E, McEwen BS, Dolan LM, Schafer-Kalkhoff T, Adler NE. Social disadvantage and adolescent stress. J Adolesc Health. 2005;37:484–492. doi: 10.1016/j.jadohealth.2004.11.126. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med. 2012;44:287–292. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L, Ambrée O, Jörgens S, Jawahar MC, Singhal G, Stacey D, Arolt V, Baune BT. Cytokine levels in major depression are related to childhood trauma but not to recent stressors. Psychoneuroendocrinology. 2016;73:24–31. doi: 10.1016/j.psyneuen.2016.07.205. [DOI] [PubMed] [Google Scholar]
- Gruzieva O, Merid SK, Gref A, Gajulapuri A, Lemonnier N, Ballereau S, Gigante B, Kere J, Auffray C, Melén E, Pershagen G. Exposure to traffic-related air pollution and serum inflammatory cytokines in children. Environ Health Perspect. 2017;125:1–8. doi: 10.1289/EHP460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy G, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Stenholm S, Pentti J, Kawachi I, Subramanian SV, Kivimäki M, Vahtera J. Childhood psychosocial adversity and adult neighborhood disadvantage as predictors of cardiovascular disease: A cohort study. Circulation. 2015;132:371–379. doi: 10.1161/circulationaha.115.015392. [DOI] [PubMed] [Google Scholar]
- Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 1999;159:1600–1604. doi: 10.1164/ajrccm.159.5.9804131. [DOI] [PubMed] [Google Scholar]
- Hartwell KJ, Maria MMMS, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL, Brady KT. Association of elevated cytokines with childhood adversity in a sample of healthy adults. J Psychiatr Res. 2013;47:604–610. doi: 10.1016/j.jpsychires.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heleniak C, McLaughlin KA, Ormel J, Riese H. Cardiovascular reactivity as a mechanism linking child trauma to adolescent psychopathology. Biol Psychol. 2016;120:108–119. doi: 10.1016/j.biopsycho.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung OP, Heim CM. Gene-environment interactions and intermediate phenotypes: Early trauma and depression. Front Endocrinol. 2014;5:14. doi: 10.3389/fendo.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AV. Outcomes in the sociology of mental health and illness: Where have we been and where are we going? J Health Soc Behav. 2002;43:143–151. doi: 10.2307/3090193. [DOI] [PubMed] [Google Scholar]
- Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, Gao H, Hao L, Liu L. Adverse childhood experiences and risk of type 2 diabetes: A systematic review and meta-analysis. Metabolism. 2015;64:1408–1418. doi: 10.1016/j.metabol.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, PhD, CM, Jones L, Dunne MP. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- Infurna MR, Reichl C, Parzer P, Schimmenti A, Bifulco A, Kaess M. Associations between depression and specific childhood experiences of abuse and neglect: A meta-analysis. J Affect Disord. 2016;190:47–55. doi: 10.1016/j.jad.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Slavich GM. Psychoneuroimmunology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 4. New York: Cambridge University Press; 2017. pp. 377–398. [DOI] [Google Scholar]
- Islam T, Urman R, Gauderman WJ, Milam J, Lurmann F, Shankardass K, Avol E, Gilliland F, McConnell R. Parental stress increases the detrimental effect of traffic exposure on children’s lung function. Am J Respir Crit Care Med. 2011;184:822–827. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusek LW, Tell D, Gaylord-Harden N, Mathews HL. Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: An epigenetic link. Brain Behav Immun. 2017;60:126–135. doi: 10.1016/j.bbi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Brook J, Kanaroglou P, Giovis C, Finkelstein N, Hutchison B. Do socioeconomic characteristics modify the short term association between air pollution and mortality? Evidence from a zonal time series in Hamilton, Canada. J Epidemol Community Health. 2004;58:31–40. doi: 10.1136/jech.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juonala M, Viikari JSA, Rönnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. Arterioscler Thrombo Vasc Biol. 2006;26:1883–1888. doi: 10.1161/01.atv.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–726. doi: 10.1161/circresaha.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-κB by toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Khulan B, Manning JR, Dunbar DR, Seckl JR, Raikkonen K, Eriksson JG, Drake AJ. Epigenomic profiling of men exposed to early-life stress reveals DNA methylation differences in association with current mental state. Trans Psychiatry. 2014;4:e448–e448. doi: 10.1038/tp.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Lim YH, Bae HJ, Kim M, Jung K, Hong YC. Long-term fine particulate matter exposure and major depressive disorder in a community-based urban cohort. Environ Health Perspect. 2016;124:1547–1553. doi: 10.1289/ehp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipen HM, Gandhi S, Rich DQ, Ohman-Strickland P, Laumbach R, Fan ZH, Chen L, Laskin DL, Zhang J, Madura K. Acute decreases in proteasome pathway activity after inhalation of fresh diesel exhaust or secondary organic aerosol. Environ Health Perspect. 2011;119:658–663. doi: 10.1289/ehp.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Smith GD, Juonala M, Ferrie JE, Keltikangas-Järvinen L, Elovainio M, Pulkki-Råback L, Vahtera J, Leino M, Viikari JSA, Raitakari OT. Socioeconomic position in childhood and adult cardiovascular risk factors, vascular structure, and function: Cardiovascular Risk in Young Finns Study. Heart. 2006;92:474–480. doi: 10.1136/hrt.2005.067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TWW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkeila J, Vahtera J, Korkeila K, Kivimäki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96:298–303. doi: 10.1136/hrt.2009.188250. [DOI] [PubMed] [Google Scholar]
- Krewski D, Burnett RT, Goldberg MS, Hoover BK, Siemiatycki J, Jerrett M, Abrahamowicz M, White WH. Overview of the reanalysis of the Harvard six cities study and American Cancer Society study of particulate air pollution and mortality. J Toxicol Environ Health Part A. 2003;66:1507–1551. doi: 10.1080/15287390306424. [DOI] [PubMed] [Google Scholar]
- Kristiansson M, Sörman K, Tekwe C, Calderón-Garcidueñas L. Urban air pollution, poverty, violence and health: Neurological and immunological aspects as mediating factors. Environ Res. 2015;140:511–513. doi: 10.1016/j.envres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Krzyzanowski M. Methods for assessing the extent of exposure and effects of air pollution. Occup Environ Med. 1997;54:145–151. doi: 10.1007/978-1-4684-5484-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, Bureau A, Mechawar N, Szyf M, Meaney MJ, Turecki G. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69:722–731. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey RE, Kumari M, Bartley M. Social isolation in childhood and adult inflammation: Evidence from the National Child Development Study. Psychoneuroendocrinology. 2014;50:85–94. doi: 10.1016/j.psyneuen.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Lacey RE, Kumari M, McMunn A. Parental separation in childhood and adult inflammation: The importance of material and psychosocial pathways. Psychoneuroendocrinology. 2013;38:2476–2484. doi: 10.1016/j.psyneuen.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Lam LL, Emberly E, Fraser HB, Neumann SM, Chen E, Miller GE, Kobor MS. Factors underlying variable DNA methylation in a human community cohort. PNAS. 2012;109:17253–17260. doi: 10.1073/pnas.1121249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, Kim HC. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol. 2015;30:e2015011. doi: 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SM, Elomaa AP, Niskanen L, Herzig KH, Tolmunen T, Viinamäki H, Koivumaa-Honkanen H, Huotari A, Honkalampi K, Valkonen-Korhonen M, Sinikallio S, Ruotsalainen H, Hintikka J. Serum adipokine levels in adults with a history of childhood maltreatment. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:217–221. doi: 10.1016/j.pnpbp.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Levine ME, Cole SW, Weir DR, Crimmins EM. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med. 2015;130:16–22. doi: 10.1016/j.socscimed.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YH, Kim H, Kim JH, Bae S, Park HY, Hong YC. Air pollution and symptoms of depression in elderly adults. Environ Health Perspect. 2012;120:1023–1028. doi: 10.1289/ehp.1104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JE, Neylan TC, Epel E, O’Donovan A. Associations of childhood adversity and adulthood trauma with C-reactive protein: A cross-sectional population-based study. Brain Behav Immun. 2016;53:105–112. doi: 10.1016/j.bbi.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindert J, von Ehrenstein OS, Grashow R, Gal G, Braehler E, Weisskopf MG. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: Systematic review and meta-analysis. Int J Public Health. 2014;59:359–372. doi: 10.1007/s00038-013-0519-5. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC, Campen MJ. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am J Respir Crit Care Med. 2011;184:82–91. doi: 10.1164/rccm.201012-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist E, Rignell-Hydbom A, Tinnerberg H, Björk J, Stroh E, Jakobsson K, Rittner R, Rylander L. Maternal exposure to air pollution and birth outcomes. Environ Health Perspect. 2011;119:553–558. doi: 10.1289/ehp.1002564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marzi SJ, Sugden K, Arseneault L, Belsky DW, Burrage J, Corcoran DL, Danese A, Fisher HL, Hannon E, Moffitt TE, Odgers CL, Pariante C, Poulton R, Williams BS, Wong CCY, Mill J, Caspi A. Analysis of DNA methylation in young people: Limited evidence for an association between victimization stress and epigenetic variation in blood. Am J Psychiatry. 2018 doi: 10.1176/appi.ajp.2017.17060693. appi.ajp.2017.1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Künzli N, Gauderman J, Avol E, Thomas D, Peters J. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: A review of neurobiological and genetic factors. Front Psychiatry. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/nejm199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. doi: 10.1001/archinte.1993.00410180039004. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA. Future directions in childhood adversity and youth psychopathology. J Clin Child Adolesc Psychol. 2016;45:361–382. doi: 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M, Loeschner A, Gonik M, Mercer KB, Bradley B, Mueller-Myhsok B, Ressler KJ, Binder EB. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. PNAS. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–409. doi: 10.1097/psy.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. The biological residue of childhood poverty. Child Dev Perspect. 2013;7:67–73. doi: 10.1111/cdep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. PNAS. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole Steve W. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J, Gordon T. Noninvasive effects measurements for air pollution human studies: methods, analysis and implications. J Expo Sci Environ Epidemiol. 2015;25:354–380. doi: 10.1038/jes.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE E-Risk Study Team. Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry. 2002;43:727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM. Psychological stressors: Overview. In: Fink G, editor. Stress: Concepts, Cognition, Emotion, and Behavior. Elsevier; Cambridge, MA: 2016. pp. 109–115. [DOI] [Google Scholar]
- Montez JK, Hayward MD. Cumulative childhood adversity, educational attainment, and active life expectancy among U.S. adults. Demography. 2014;51:413–435. doi: 10.1007/s13524-013-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FP, Wiener CD, Jansen K, Portela LV, Lara DR, de Mattos Souza LD, da Silva RA, Oses JP. Childhood trauma and increased peripheral cytokines in young adults with major depressive: Population-based study. J Neuroimmunol. 2018:1–0. doi: 10.1016/j.jneuroim.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Morgenstern V, Zutavern A, Cyrys J, Brockow I, Gehring U, Koletzko S, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med. 2007;64:8–16. doi: 10.1136/oem.2006.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi N, Vlaanderen J, Chadeau-Hyam M, Beelen R, Modig L, Palli D, Bergdahl IA, Vineis P, Hoek G, Kyrtopoulos SA, Vermeulen R. Inflammatory markers in relation to long-term air pollution. Environ Int. 2015;81:1–7. doi: 10.1016/j.envint.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Munjiza A, Kostic M, Pesic D, Gajic M, Markovic I, Tosevski DL. Higher concentration of interleukin 6: A possible link between major depressive disorder and childhood abuse. Psychiatry Res. 2018;264:26–30. doi: 10.1016/j.psychres.2018.03.072. [DOI] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Chen E, Miller GE. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma. Psychol Sci. 2015;26:111–121. doi: 10.1177/0956797614556320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clin Psychol Sci. 2013;1:30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Nergiz-Unal R, Rademakers T, Cosemans JMEM, Heemskerk JWM. CD36 as a multiple-ligand signaling receptor in atherothrombosis. Cardiovasc Hematol Agents Med Chem. 2011;9:42–55. doi: 10.2174/187152511794182855. [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Med. 2012;9:e1001349–31. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurius PS, Uehara E, Zatzick DF. Intersection of stress, social disadvantage, and life course processes: Reframing trauma and mental health. Am J Psychiatr Rehabil. 2013;16:91–114. doi: 10.1080/15487768.2013.789688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: A mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino K, Nagaoka K, Okuda T, Oka A, Kubo M, Eguchi E, Fujikura Y. PM2.5-induced airway inflammation and hyperresponsiveness in NC/Nga mice. Environ Toxicol. 2017;32:1047–1054. doi: 10.1002/tox.22303. [DOI] [PubMed] [Google Scholar]
- Okello J, Nakimuli-Mpungu E, Musisi S, Broekaert E, Derluyn I. The association between attachment and mental health symptoms among school-going adolescents in northern Uganda: The moderating role of war-related trauma. PLoS One. 2014;9:e88494. doi: 10.1371/journal.pone.0088494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera Alvarez HA, Appleton AA, Fuller CH, Belcourt A, Kubzansky LD. An integrated socio-environmental model of health and well-being: A conceptual framework exploring the joint contribution of environmental and social exposures to health and disease over the life span. Curr Environ Health Rep. 2018;3:1–11. doi: 10.1007/s40572-018-0191-2. [DOI] [PubMed] [Google Scholar]
- Ostro B, Malig B, Broadwin R, Basu R, Gold EB, Bromberger JT, Derby C, Feinstein S, Greendale GA, Jackson EA, Kravitz HM, Matthews KA, Sternfeld B, Tomey K, Green RR, Green R. Chronic PM2.5 exposure and inflammation: Determining sensitive subgroups in mid-life women. Environ Res. 2014;132:168–175. doi: 10.1016/j.envres.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard CJ, Bezlyak V, McLean JS, Batty GD, Ford I, Burns H, Cavanagh J, Deans KA, Henderson M, McGinty A, Millar K, Sattar N, Shiels PG, Velupillai YN, Tannahill C. Early life socioeconomic adversity is associated in adult life with chronic inflammation, carotid atherosclerosis, poorer lung function and decreased cognitive performance: A cross-sectional, population-based study. BMC Public Health. 2011;11:42. doi: 10.1186/1471-2458-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmier-Claus JE, Berry K, Bucci S, Mansell W, Varese F. Relationship between childhood adversity and bipolar affective disorder: Systematic review and meta-analysis. Br J Psychiatry. 2018;209:454–459. doi: 10.1192/bjp.bp.115.179655. [DOI] [PubMed] [Google Scholar]
- Peters A, Fröhlich M, Döring A, Immervoll T, Wichmann HE, Hutchinson WL, Pepys MB, Koenig W. Particulate air pollution is associated with an acute phase response in men: Results from the MONICA-Augsburg Study. Eur Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur J Epidemol. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Pope CA, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/nejmsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, III, Dockery DW, Kanner RE, Villegas GM, Schwartz J. Oxygen saturation, pulse rate, and particulate air pollution: A daily time-series panel study. Am J Respir Crit Care Med. 1999;159:365–372. doi: 10.1164/ajrccm.159.2.9702103. [DOI] [PubMed] [Google Scholar]
- Pourazar J, Frew AJ, Blomberg A, Helleday R, Kelly FJ, Wilson S, Sandström T. Diesel exhaust exposure enhances the expression of IL-13 in the bronchial epithelium of healthy subjects. Respir Med. 2004;98:821–825. doi: 10.1016/j.rmed.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Pusalkar M, Suri D, Kelkar A, Bhattacharya A, Galande S, Vaidya AA. Early stress evokes dysregulation of histone modifiers in medial prefrontal cortex across the life span. Dev Psychobiol. 2015;58:198–210. doi: 10.1002/dev.21365. [DOI] [PubMed] [Google Scholar]
- Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res. 2014;115:770–780. doi: 10.1161/circresaha.115.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requia WJ, Adams MD, Arain A, Papatheodorou S, Koutrakis P, Mahmoud M. Global association of air pollution and cardiorespiratory diseases: A systematic review, meta-analysis, and investigation of modifier variables. Am J Public Health. 2017:e1–e8. doi: 10.2105/ajph.2017.303839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T, Zhang JJ. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307:2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva DR, Magalhães CB, Lopes AA, Lanças T, Mauad T, Malm O, Valença SS, Saldiva PH, Faffe DS, Zin WA. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 2011;23:257–267. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- Robertson S, Colombo ES, Lucas SN, Hall PR, Febbraio M, Paffett ML, Campen MJ. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci. 2013;134:304–311. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens SE, McDonald J, Svaren J, Pollak SD. Associations between early life stress and gene methylation in children. Child Dev. 2014;86:303–309. doi: 10.1111/cdev.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Kuechenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier HMC, Roy LB, Frimer LT, Chen E. Family chaos and adolescent inflammatory profiles: The moderating role of socioeconomic status. Psychosom Med. 2014;76:460–467. doi: 10.1097/psy.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Schwaiger M, Grinberg M, Moser D, Zang JCS, Heinrichs M, Hengstler JG, Rahnenführer J, Cole S, Kumsta R. Altered stress-induced regulation of genes in monocytes in adults with a history of childhood adversity. Neuropsychopharmacology. 2016;41:2530–2540. doi: 10.1038/npp.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze PE, Totlandsdal AI, Låg M, Refsnes M, Holme JA, Øvrevik J. Inflammation-related effects of diesel engine exhaust particles: Studies on lung cells in vitro. BioMed Res Int. 2013;2013:1–13. doi: 10.1155/2013/685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10:369–374. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, Watt M, Agius R, Stout R. Particulate air pollution and the blood. Thorax. 1999;54:1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. PNAS. 2009;106:12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Slavich GM. Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Soc Personal Psychol Compass. 2017;11:e12335. doi: 10.1111/spc3.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfelt J, Mitkus RJ, Zeisler R, Spatz RO, Powell J, Fenton MJ, Squibb KA, Medvedev AE. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J Leukoc Biol. 2009;86:303–312. doi: 10.1189/jlb.1008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81:357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–46. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Slavich GM. Understanding inflammation, its regulation, and relevance for health: A top scientific and public priority. Brain Behav Immun. 2015;45:13–14. doi: 10.1016/j.bbi.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. Life stress and health: A review of conceptual issues and recent findings. Teach Psychol. 2016;43:346–355. doi: 10.1177/0098628316662768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM. Psychoneuroimmunology of stress and mental health. In: Harkness K, Hayden E, editors. The Oxford handbook of stress and mental health. New York: Oxford University Press; in press. [Google Scholar]
- Slavich GM, Auerbach RP. Stress and its sequelae: Depression, suicide, inflammation, and physical illness. In: Butcher JN, Hooley JM, editors. APA handbook of psychopathology: Vol. 1. Psychopathology: Understanding, assessing, and treating adult mental disorders. Washington, DC: American Psychological Association; 2018. pp. 375–402. [DOI] [Google Scholar]
- Slavich GM, Cole Steven W. The emerging field of human social genomics. Clin Psychol Sci. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci Biobehav Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Shields GS. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosom Med. 2018;80:17–27. doi: 10.1097/psy.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, Williams DR. Racial disparities in child adversity in the U.S.: Interactions with family immigration history and income. Am J Prev Med. 2016;50:47–56. doi: 10.1016/j.amepre.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39:2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Feldman RJ, Kunz S, Owen N, Willemsen G, Marmot M. Stress responsivity and socioeconomic status: A mechanism for increased cardiovascular disease risk? Eur Heart J. 2002a;23:1757–1763. doi: 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun. 2002b;16:774–784. doi: 10.1016/S0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Szyf M, Bick J. DNA Methylation: A mechanism for embedding early life experiences in the genome. Child Dev. 2012;84:49–57. doi: 10.1111/j.1467-8624.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Heinrich J. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab. 2015;26:384–394. doi: 10.1016/j.tem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Tomczak A, Miller AB, Weichenthal SA, To T, Wall C, van Donkelaar A, Martin RV, Crouse DL, Villeneuve PJ. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int J Cancer. 2016;139:1958–1966. doi: 10.1002/ijc.30255. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS One. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bogaert T, De Bosscher K, Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev. 2010;21:275–286. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- van der Knaap LJ, Riese H, Hudziak JJ, Verbiest MMPJ, Verhulst FC, Oldehinkel AJ, van Oort FVA. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Trans Psychiatry. 2014;4:e381–e381. doi: 10.1038/tp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophrenia Bulletin. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: A systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171:R173–82. doi: 10.1530/eje-14-0365. [DOI] [PubMed] [Google Scholar]
- Wang F, Jia X, Wang X, Zhao Y, Hao W. Particulate matter and atherosclerosis: A bibliometric analysis of original research articles published in 1973-2014. BMC Public Health. 2016;16:348. doi: 10.1186/s12889-016-3015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/jci25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler K, Voellmin A, Hug E, Kirmse U, Helmig S, Princip M, Cajochen C, Bader K, Wilhelm FH. Adverse childhood experiences and autonomic regulation in response to acute stress: The role of the sympathetic and parasympathetic nervous systems. Anxiety Stress Coping. 2017;30:145–154. doi: 10.1080/10615806.2016.1238076. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Sewart A, Vrshek-Schallhorn S, Zinbarg R, Mineka S, Hammen C, Bobova L, Adam EK, Craske MG. The effects of childhood and adolescent adversity on substance use disorders and poor health in early adulthood. J Youth Adolesc. 2017;46:15–27. doi: 10.1007/s10964-016-0566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease. Geneva, Switzerland: 2016. [Google Scholar]
- World Health Organization. [accessed 5.7.15];Ambient (outdoor) air quality and health. 2014 www.who.int. URL http://www.who.int/mediacentre/factsheets/fs313/en/#.
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, Lee-Parritz A, Wood RA, Kattan M, Bloomberg GR, Burger M, Togias A, Witter FR, Sperling RS, Sadovsky Y, Gern JE. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Deng F, Wei H, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Hao Y, Guo X. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: A prospective panel study. Part Fibre Toxicol. 2012:9. doi: 10.1186/1743-8977-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatti C, Rosa V, Barros A, Valdivia L, Calegaro VC, Freitas LH, Ceresér KMM, da Rocha NS, Bastos AG, Schuch FB. Childhood trauma and suicide attempt: A meta-analysis of longitudinal studies from the last decade. Psychiatry Res. 2017;256:353–358. doi: 10.1016/j.psychres.2017.06.082. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Lioy PJ. Human exposure assessment in air pollution systems. Sci Worl J. 2002;2:497–513. doi: 10.1100/tsw.2002.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou B, Wilson JG, Zhan FB, Zeng Y. Air pollution exposure assessment methods utilized in epidemiological studies. J Environ Monit. 2009;11:475–490. doi: 10.1039/b813889c. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]