Abstract
Background
Schizophrenia (SZ) studies suggest that neurocognition predicts functional outcome and that social cognition mediates this relationship. Bipolar disorder (BD) patients also have cognitive, social, and functional impairments but the relationship among these factors in BD is not well established. We assessed whether social cognition modulates the influence of neurocognition on community functioning in BD, as found in SZ.
Methods
200 BD patients and 49 healthy controls (HC) were administered and compared on a battery of tests assessing neurocognition, social cognition, and community functioning. We conducted a series of regression analyses to investigate potential mediation or moderation of social cognition on the relationship between neurocognition and community functioning.
Results
BD patients performed worse on neurocognitive domains of processing speed, attention, verbal learning, and global neurocognition. Also, BD patients performed worse on theory of mind, the social cognition composite score, and community functioning. Neurocognition did not significantly predict functional outcome in our BD sample. However, we found a moderating effect of social cognition: among patients with poor social cognition, better neurocognition was associated with better community functioning, a relationship not seen in BD patients with good social cognition.
Limitations
The study was limited by a relatively small HC group and assessing one subtype of functioning status.
Conclusions
The relationship between neurocognition and community functioning in BD may be dependent on social cognition status, implying the presence of social cognitive heterogeneity. Results may be relevant to choosing proper treatment interventions depending on the patient’s social cognitive level.
Keywords: bipolar disorder, neurocognition, social cognition, functioning, moderator
I. INTRODUCTION
Bipolar disorder (BD) is characterized by chronic and recurrent affective symptomatology that negatively impacts functional outcome in at least two-thirds of patients (Sanchez-Moreno et al., 2009; Huxley and Baldessarini, 2007). Mounting evidence also suggests that BD patients present with neurocognitive dysfunction. For example, a number of meta-analyses report that BD patients perform worse on neurocognitive processing compared to healthy controls (HC), particularly on neurocognitive domains of processing speed, attention, working memory, verbal learning, and visual learning, with medium to large effect sizes (Bo et al., 2017; Tsitsipa and Fountoulakis, 2015; Mann-Wrobel et al., 2011). Furthermore, recent studies suggest that poorer neurocognition in BD may be associated with worse functional outcome, disability, and psychosocial functioning (Depp et al., 2012; Sanchez-Moreno et al., 2009; Wingo et al., 2009; Tabarés-Seisdedos et al., 2008; Martinez-Aran et al., 2007; Robinson et al., 2006). though not always (Malhi et al., 2007; Martinez-Aran et al., 2002). These deficits appear to remain even after affective remission and pharmacological treatment (Wingo et al., 2009), indicating that they are a central component of the illness.
Likewise, schizophrenia (SZ) patients also demonstrate substantial neurocognitive impairment, deficits that have consistently been shown to contribute to their poor functional outcome (Green, 1996; Martinez-Aran et al., 2007; Tabarés-Seisdedos et al., 2008). However, recent research in SZ proposes a more complicated relationship, such that neurocognition and functioning may be mediated by social cognition (Schmidt et al., 2011). Social cognition is a multi-dimensional construct which encompasses mental processes underlying social behavior such as: 1) emotion recognition, 2) theory of mind, 3) social perception, 4) social knowledge, and 5) causal attribution style (Ochsner, 2008). This mediation effect found in SZ patients proposes the existence of social cognitive deficits (Savla et al., 2012), which subsequently predict functioning level (Couture et al., 2006). Currently, consensus exists supporting the idea that neurocognition and social cognition are two distinct constructs that do overlap, yet contribute in a non-redundant way to functional outcome (Allen et al., 2007; Sergi et al., 2007). However, these same relationships in BD patients are not as clear. While one study demonstrated that BD patients are indistinguishable from HCs on social cognition (Lee et al., 2013), most studies have reported impaired performance on social cognition, particularly domains of emotion recognition and theory of mind in BD relative to HCs (Bora, et al., 2016; Cusi et al., 2012; Samamé et al., 2012). One recent meta-analysis also demonstrated impairment in similar social cognitive domains for manic, depressed and euthymic BD patients (Samamé, 2013). Results regarding the association between social cognition and functioning in BD are mixed; while one BD study demonstrated no change in functional outcome in relation to social cognitive interventions (Lahera et al., 2012), other groups found a significant relationship between social cognition and community functioning (Fett et al., 2013), as well as a significant relationship between emotional processing and functioning only in BD patients with a history of psychosis (Thaler et al., 2014). It remains unclear whether these social cognitive deficits in BD are due to neurocognitive dysfunction, an underlying attentional bias, and/or other confounding effects such as medications.
Aspects of social cognition, such as social knowledge and emotion recognition, have been shown to mediate the relationship between neurocognition and community functioning in SZ (Schmidt et al., 2011). Mediation is a theoretical model that attempts to explain the process of how or why a cause-effect relationship occurs (Baron and Kenny, 1986). Rather than a direct causal relationship between the independent and dependent variables, mediation proposes that the independent variable influences a mediator variable, which in turn, influences the dependent variable. Importantly, when the relationships between the independent-mediator and mediator-dependent variables are statistically controlled, the relationship between the independent and dependent variables is no longer significant. In other words, neurocognition, which generally predicts functioning in SZ, does so partially through underlying substrates related to social cognitive mechanisms. A similar model may also explain the influence of neurocognition on community functioning in BD, though neurocognition has not always been observed to predict functional outcome. If social cognition does not mediate the relationship of neurocognition on functioning, social cognition may still influence this relationship through moderation. Moderation, better known as an interaction, explains when or for whom an independent variable most strongly (or weakly) influences a dependent variable (Baron and Kenny, 1986). Here, the strength or direction of the effect that an independent variable has on the dependent variable varies as a function of the level of the moderator variable. Also, moderation is typically used when a hypothesized causal relationship is weak or not found empirically. Even if social cognition does not mediate the relationship of neurocognition on community functioning in BD, social cognition may still impact this relationship as a function of the level of social cognition observed in BD patients. To date, no studies have assessed how social cognition affects the neurocognition-functioning association in a BD sample. Understanding the potential role social cognition plays on this relationship in BD, particularly when considering proper interventions and remediation strategies, may prove fruitful in improving functional outcome.
While social cognition appears to partially mediate the association between neurocognition and community functioning in SZ, it is unknown whether and in what manner social cognition impacts this same relationship in BD. Therefore, the current study aimed to assess the potential mediation or moderation of social cognition on the neurocognition-functioning relationship. First, we compared BD patients and HCs on demographics, cognition, and community functioning. Next, we assessed possible mediation by conducting a series of step-wise linear regressions to assess the relationship of social cognition on the neurocognition-functioning relationship (Hayes, 2009). In the event of no mediation, an exploratory moderator analysis would be conducted to ascertain whether the level of social cognition differentially influenced the relationship between neurocognition and functioning in BD patients. Given commonalities (i.e. clinical, genetic, and neurobiological) between SZ and BD, we anticipated that neurocognition would predict community functioning in our BD sample, and that social cognition would at least partially mediate this relationship.
II. METHODS
Participants
The sample included 200 BD patients and 49 HCs recruited from Icahn School of Medicine at Mount Sinai. All procedures were approved by the Institutional Review Board and written informed consent was obtained from all participants. Inclusion criteria were: 1) diagnosis of BD I or BD II from the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 2002), 2) 18–65 years of age, and 3) a score < 8 on the Clinician Administered Rating Scale for Mania (CARS-M) (Altman et al., 1994) and < 15 on the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). HCs were recruited separately as presenting without evidence of an Axis I disorder. Exclusion criterion for HCs included a family history of an Axis I disorder among first-degree relatives based on self-report. Exclusion criteria for all participants included: 1) history of central nervous system trauma, neurological disorder, or attention-deficit hyperactivity disorder, 2) recent substance use/dependence disorder (past three months), 3) electroconvulsive therapy (ECT) in the past 12 months, 4) active, unstable medical problem, and 5) estimated premorbid IQ < 70 (from the Wide Range Achievement Test-3rd edition [WRAT-3] Reading (Wilkinson, 1993)).
Clinical Measures
DSM-IV BD diagnosis (or lack of Axis I diagnosis in HCs), presence of lifetime psychotic features, length of illness in years, and psychiatric medication use were derived from the SCID-IV by highly trained clinical coordinators and postdoctoral fellows. Manic and depressive symptoms were assessed by CARS-M and HRSD, respectively.
Neurocognitive Measures
We evaluated neurocognition using the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein and Green, 2006). The MCCB includes 10 tests measuring seven domains: 1) processing speed (Brief Assessment of Cognition in Schizophrenia [BACS], Trail Making Test Part A, and semantic fluency), 2) attention and vigilance (Continuous Performance Test-Identical Pairs [CPT-IP], 3) working memory (Weschler Memory Scale spatial and letter number span), 4) verbal learning (Hopkins Verbal Learning Test-Revised [HVLT-R]), 5) visual learning (Brief Visuospatial Memory Test-Revised [BVMT-R]), 6) reasoning and problem-solving (Neuropsychological Assessment Battery [NAB] Mazes subtest), and 7) social cognition (Mayer-Salovey-Caruso Emotional Intelligence Test [MSCEIT]). Here, we replaced the HVLT-R with the California Verbal Learning Test (CVLT), as it has demonstrated better sensitivity in detecting verbal learning difficulties, particularly in less impaired BD patients (Yatham et al., 2010). BD patient scores were standardized based upon the HCs’ performance (z scores: mean=0, SD=+/− 1). Global neurocognitive composite scores were calculated as mean z-scores of MCCB domains (excepting social cognition) and CVLT, with larger scores indicating better neurocognitive performance.
Social Cognitive Measures
The MCCB includes the MSCEIT Managing Emotions subtest, which measures emotion management and emotion regulation through the presentation of vignettes of various social situations with participants selecting the most appropriate social response to achieve preferred outcomes. Additionally, we administered the Reading the Mind in the Eyes test (RMET) (Baron-Cohen et al., 2001), a measure of theory of mind. The RMET presents participants with 36 black-and-white photographs of pairs of eyes, with the rest of the face obscured; each associated with one of four forced choice emotion labels. Z-scores and composite scores were calculated in the same way as above, with larger scores reflecting better social cognition.
Community Functioning
We assessed community functioning using the World Health Organization Disability Assessment Schedule (WHODAS 2.0) (Üstün, 2010), a 36-item questionnaire measuring disability severity across 6 domains in the past 30 days: understanding and communicating; getting around; self-care; getting along with others; life activities; participation in society. We used the alternate 32-item calculation omitting employment, as the WHODAS combines work and school, which confounded the reports in our sample. Employment status (employed vs. unemployed) was derived from our demographics questionnaire. Domain scores were computed by adding the relevant item responses; a global score was calculated by summing all items, with greater WHODAS scores representing worse functioning.
Statistical Analyses
We first compared BDs and HCs on demographics, symptoms, premorbid IQ, neurocognition, social cognition, and community functioning using Chi-square and independent samples t-tests, as appropriate. Next, we calculated Pearson’s partial correlations between all neurocognitive, social cognitive, and functioning domains. To assess for potential mediation of social cognition on neurocognition and functioning, we used Hayes’ (2009) PROCESS mediator analysis. This analysis estimates total effects, direct effects and indirect effects using a series of means of ordinary least squares (OLS) regression analyses separately for functional outcome. The effect of the independent variable (i.e., the neurocognition composite score) is presented in the total effect, while the direct effect demonstrates this relationship controlling for the mediator (i.e., the social cognition composite score). The indirect effect includes the total path over social cognition. Furthermore, PROCESS utilizes a boot-strapping approach (10,000 samples tested here) to assess the 95% confidence limits of the model’s indirect effects, thereby bias-correcting non-normally distributed variables and increasing power, thus offering a more reliable estimation of the indirect effect (Hayes and Preacher, 2014). If this analysis yielded no significant total or partial mediation, we would employ an exploratory moderator model by conducting a hierarchical multiple regression. In this analysis, the two predictor variables (i.e., global neurocognition and social cognition composite scores) were centered (i.e., each score was subtracted from that variable’s average), and interaction term was created by multiplying the centered predictor variables; this procedure is commonly used to avoid multicollinearity with the predictors and their interaction term (Aiken et al., 1991). All regression predictors were entered hierarchically (i.e., covariates in block 1, predictor variables in block 2, and the interaction term in block 3) to assess whether the introduction of subsequent predictors improved the model. A significant interaction term was followed up using a simple-slopes test to determine whether the slopes of two regression lines differ from zero (Aiken et al., 1991). The simple slopes test includes arbitrarily dichotomizing the moderator variable, generally grouping individuals who score below and above 1 standard deviation from the mean of the moderator variable. Covariates for correlations and all regression analyses (both mediator and moderator models) included: premorbid IQ, CARS-M and HRSD, presence of lifetime psychosis, number of current total medications, age, sex, race (Caucasian vs. non-Caucasian), and length of illness. Statistical analyses were performed using IBM SPSS Statistics 23 (IBM corp., Armonk, NY, USA), the SPSS macro PROCESS (Hayes, 2012), and Interaction (Soper, 2013). For all analyses, alpha level was set at .05, and all tests were two-tailed.
III. RESULTS
Sample Characteristics
Diagnostic group comparisons for demographics and clinical characteristics are presented in Table 1, while comparisons for neurocognitive, social cognitive and functioning data are shown in Table 2. BD patients and HCs performed comparably on almost all demographics, although BD patients were significantly more likely to be unemployed. BD patients had more symptoms and a lower premorbid IQ score than HCs. Furthermore, BD patients performed significantly worse than HCs on domains of processing speed, attention, verbal learning, and the neurocognitive composite score. For social cognition, BD patients performed worse than HCs on the RMET and the social cognitive composite score but did not differ on the MSCEIT. Finally, BD patients reported poorer overall functioning based on the WHODAS composite score compared to HCs.
Table 1.
Diagnostic Comparisons of Demographic and Clinical Characteristics
| Statistics
|
|||||
|---|---|---|---|---|---|
| BD (n = 200) | HC (n = 49) | t or χ² | p | Ф or η2p | |
| Diagnosis, n | |||||
| BD I | 157 | - | |||
| BD II | 43 | - | |||
| Sex, n (%) | |||||
| Males | 107 (53.5) | 22 (44.9) | 1.23 | 0.27 | 0.07 |
| Females | 93 (46.5) | 27 (55.1) | |||
| Race, n (%) | |||||
| Caucasian | 69 (34.5) | 14 (28.6) | 0.59 | 0.44 | -0.05 |
| Non-Caucasian | 131 (65.5) | 35 (71.4) | |||
| Employment status, n (%) | |||||
| Not employed | 140 (70.0) | 14 (29.2) | 27.42 | <0.001 | 0.33 |
| Employed | 60 (30.0) | 34 (70.8) | |||
| Age, years | 43.64 (11.96) | 40.02 (13.68) | 1.85 | 0.07 | 0.01 |
| Age of onset, years | 20.53 (8.32) | - | |||
| Education, years | 14.22 (2.41) | 15.51 (1.78) | −4.20 | <0.001 | 0.05 |
| Depressive symptoms; HRSD | 7.50 (6.44) | 0.57 (1.29) | 14.10 | <0.001 | 0.19 |
| Manic symptoms; CARS-M | 3.41 (4.36) | 0.25 (0.80) | 9.62 | <0.001 | 0.09 |
| Premorbid IQ; WRAT-3 | 101.65 (13.73) | 106.02 (12.94) | −2.02 | 0.04 | 0.02 |
Note: Data are given as mean (standard deviation).
BD, Bipolar Disorder; HC, Healthy Control; HRSD, Hamilton Rating Scale for Depression; CARS-M, Clinician Administered Rating Scale for Mania; IQ, intelligence quotient; WRAT-3, Wide Range Achievement Test.
Table 2.
Diagnostic Comparisons of Cognitive and Functioning Data
| Statistics
|
|||||
|---|---|---|---|---|---|
| BD (n = 200) | HC (n = 49) | t or χ² | p | Ф or η2p | |
| Cognitive Domains (Z score) | |||||
| Processing Speed | −0.3003 (0.54) | −0.0002 (0.51)a | −3.52 | 0.001 | 0.05 |
| Attention/Vigilance | −0.5642 (1.05) | 0.0000 (1.00)b | −3.35 | 0.001 | 0.04 |
| Working Memory | −0.2693 (0.88) | 0.0006 (0.82)a | −1.93 | 0.06 | 0.02 |
| Verbal Learning | −0.3280 (0.97) | −0.0007 (0.94)a | −2.12 | 0.04 | 0.02 |
| Visual Learning | −0.2904 (0.94) | −0.0005 (1.00)a | −1.89 | 0.06 | 0.01 |
| Reasoning/Problem-Solving | −0.0999 (1.06) | −0.0002 (1.00)a | −0.59 | 0.56 | 0.00 |
| Cognitive Composite Score | −0.3111 (0.61) | 0.0059 (0.64)b | −3.17 | 0.002 | 0.04 |
|
| |||||
| Social Cognitive Domains (Z-Score) | |||||
| MSCEIT | −0.2070 (1.07) | 0.0000 (1.00)a | −1.22 | 0.22 | 0.01 |
| RMET | −0.3192 (0.87) | −0.0008 (1.00) | −2.24 | 0.03 | 0.02 |
| Social Cognitive Composite Score | −0.2630 (0.80) | −0.0085 (0.83)a | −1.97 | 0.05 | 0.02 |
|
| |||||
| WHODAS Overall without work | 21.39 (14.09) | 4.88 (6.62)b | 11.90 | <0.001 | 0.20 |
Note: Data are given as mean (standard deviation).
n = 48;
n = 47.
BD, Bipolar Disorder; HC, Healthy Control; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; RMET, Reading the Mind in the Eyes Test; WHODAS, World Health Organization Disability Assessment Schedule, η2p, partial eta-squared.
Relationships between Neurocognition, Social Cognition and Community Functioning
Pearson’s partial correlations between neurocognition, social cognition and functioning are displayed in Table 3. None of the neurocognitive or social cognitive domains significantly correlated with the WHODAS composite score. The neurocognitive composite score correlated with the social cognitive composite score; however, this relationship appears to be primarily driven by the RMET, given that the MSCEIT was not significantly associated with any of the neurocognitive subdomains or composite score.
Table 3.
Partial Correlations between Neurocognition, Social Cognition, and Community Functioning in BD (N = 200)
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Processing Speed | - | .19** | .17* | .11 | .17* | .09 | .40*** | .09 | .18* | .17* | −.05 |
| 2. Attention/Vigilance | - | .31*** | .18* | .15* | .13 | .58*** | .13 | .11 | .16* | −.02 | |
| 3. Working Memory | - | .26*** | .43*** | .36*** | .70*** | .06 | .06 | .08 | −.00 | ||
| 4. Verbal Learning | - | .40*** | .06 | .58*** | .10 | .21** | .19** | −.08 | |||
| 5. Visual Learning | - | .28*** | .69*** | .13 | .14 | .17* | .02 | ||||
| 6. Reasoning/Problem Solving | - | .56*** | −.02 | −.04 | −.04 | −.03 | |||||
| 7. Neurocognition Composite | - | .13 | .18* | .20** | −.05 | ||||||
|
| |||||||||||
| 8. MSCEIT | - | .18* | .84*** | −.01 | |||||||
| 9. RMET | - | .68*** | −.07 | ||||||||
| 10. Social Cognition Composite | - | −.05 | |||||||||
|
| |||||||||||
| 11. WHODAS Overall without work | - | ||||||||||
Note:
p < .05,
p < .01,
p < .001.
Covariates include: Wide Range Achievement Test (WRAT), Clinician Administered Rating Scale for Mania (CARS-M), Hamilton Rating Scale for Depression (HRSD), history of psychosis, total medications, age, sex, race, and length of illness.
Mediator Model Results
Results for the regression assessing the predictive ability of neurocognition on social cognition revealed an overall significant model (F(10, 187) = 11.36, p < 0.001). Significant predictors for this analysis included neurocognition (β = 0.28, p < 0.01), premorbid IQ (β = 0.02, p < 0.001), and sex (β = 0.33, p < 0.001). Results for the regression assessing the predictive ability of both neurocognition and social cognition on functioning demonstrated a significant overall model (F(11, 186) = 4.18, p < 0.001), and accounted for 19.8% of the variance. However, the only significant predictor in this model included depressive symptomatology (HRSD) (β = 0.87, p < 0.001).
Results for the total effect (i.e., the relationship of global neurocognition on functioning) showed an overall significant model (F(10, 187) = 4.60, p < 0.001), and accounted for 19.7% of the variance. However, the neurocognitive composite score did not significantly predict the WHODAS score (β = −1.03, p = 0.61). The only significant predictor of functioning for this model included depressive symptoms (β = 0.87, p < 0.001). (Secondary analyses assessing predictive ability of each individual neurocognitive domain on functioning found that no specific neurocognitive domain significantly predicted functioning.) The direct effect of the neurocognition composite score on functioning (controlling for the social cognition composite) indicated no significant influence of social cognition on the neurocognition-functioning relationship (β = −0.87, p = 0.68). Finally, the results for the indirect effect (assessing the overall pathway of the mediator model) demonstrated a non-significant indirect coefficient (β = −0.17, 95% CI = −1.33, 0.51). Overall, these results suggest no total or partial mediation of social cognition on neurocognition and functioning.
Moderator Model Results
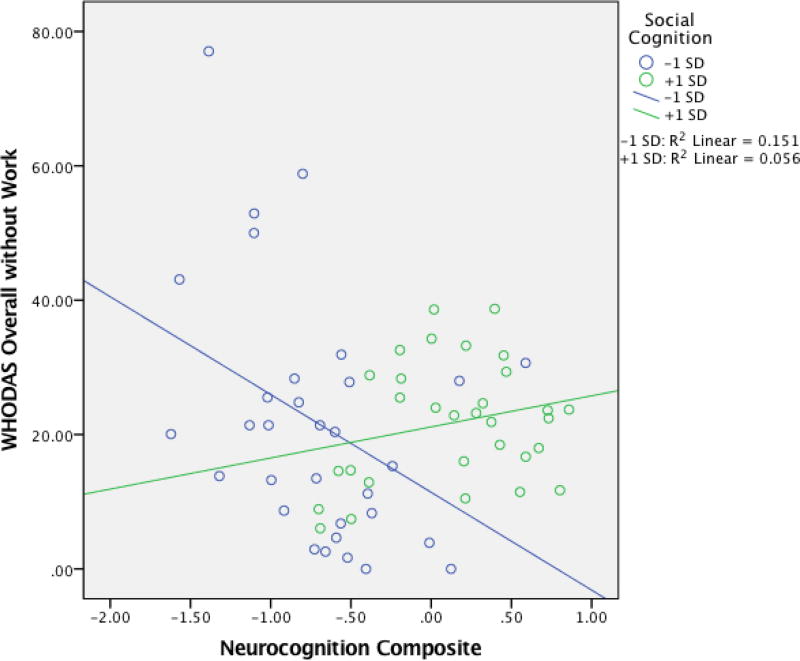
Hierarchical linear regression results investigating potential moderation of social cognition on the neurocognition-functioning relationship are presented in Table 4. In block 1, HRSD alone significantly predicted functional outcome. However, neither the inclusion of the neurocognitive composite or social cognitive composite score in block 2 significantly predicted WHODAS scores. In block 3, the addition of the interaction between the neurocognitive composite and social cognitive composite scores was significant (β = 0.18, p = 0.009), indicating that the relationship between total neurocognition and community functioning depends on one’s level of social cognition; the overall model was significant, and accounted for 22.9% of the variance. Results from the follow-up simple slopes test revealed no significant relationship between neurocognition and functioning for patients with good social cognition (i.e., scoring 1 S.D. above the mean; n = 31) (B = 3.04, t(185) = 1.51, p = 0.13). However, patients with poor social cognition (i.e., scoring 1 S.D. below the mean; n = 32) demonstrated a significant negative relationship (B = −5.39, t(185) = -2.62, p = 0.01), such that WHODAS score increased (i.e., worse functioning) with lower neurocognitive composite scores (see Figure 1). Secondary moderator model analyses assessing the social cognitive tests as separate moderators (i.e., separate models using MSCEIT and RMET as moderators) yielded similar moderation effects, with the RMET moderator reaching significance (β = .24, p = .01), and the MSCEIT moderator achieving trend-level effects (β = . 159, p = .06). Supplemental analyses assessing separate moderator models for groups with and without depressive symptomatology (using the HRSD cutoff score = 7), determined that a significant interaction between neurocognition and social cognition held only for the group with depressive symptoms (F(12, 84), = 1.97, p = 0.04, β = .28, p = .02). There was no evidence for moderation in the group without depressive symptoms.
Table 4.
Hierarchical Linear Regression Analysis Assessing Moderation of Social Cognition on the Relationship between Neurocognition and Community Functioning in BD
| Block 1 | Block 2 | Block 3 | ||||
|---|---|---|---|---|---|---|
| Predictors | B (SE) | β (p) | B (SE) | β (p) | B (SE) | β (p) |
| WRAT-3 | 0.01 (0.08) | 0.01 (0.93) | 0.04 (0.09) | 0.04 (0.65) | 0.05 (0.09) | 0.05 (0.56) |
| CARS-M | 0.22 (0.23) | 0.07 (0.33) | 0.23 (0.23) | 0.07 (0.31) | 0.19 (0.22) | 0.06 (0.86) |
| HRSD | 0.87 (0.15) | 0.40 (<0.001) | 0.87 (0.15) | 0.40 (<0.001) | 0.87 (0.15) | 0.39 (<0.001) |
| Psychotic history | 2.94 (1.95) | 0.10 (0.13) | 2.84 (1.97) | 0.10 (0.15) | 3.39 (1.95) | 0.12 (0.08) |
| # of medications | −0.06 (0.74) | −0.01 (0.94) | −0.05 (0.76) | −0.01 (0.95) | −0.12 (0.74) | −0.01 (0.87) |
| Age | 0.01 (0.12) | 0.01 (0.91) | −0.01 (0.13) | −0.01 (0.96) | 0.03 (0.13) | 0.03 (0.80) |
| Sex | 1.32 (1.91) | 0.05 (0.49) | 1.63 (1.98) | 0.06 (0.41) | 1.39 (1.95) | 0.05 (0.48) |
| Race | 1.03 (2.18) | 0.04 (0.64) | 1.37 (2.24) | 0.05 (0.54) | 1.34 (2.21) | 0.05 (0.54) |
| Length of illness | 0.02 (0.12) | 0.01 (0.90) | 0.01 (0.12) | 0.01 (0.92) | 0.02 (0.12) | 0.02 (0.86) |
| Neurocognition | −0.90 (2.06) | −0.04 (0.66) | −1.15 (2.03) | −0.05 (0.57) | ||
| Social Cognition | −0.72 (1.47) | −0.04 (0.62) | −0.03 (1.47) | −0.00 (0.99) | ||
| NC* SC | 5.28 (1.99) | 0.18 (0.009) | ||||
|
| ||||||
| F | 5.15*** | 4.23*** | 4.59*** | |||
| ΔF | - | 0.27 | 7.01** | |||
| R2 | 0.20 | 0.20 | 0.23 | |||
| ΔR2 | - | 0.002 | 0.03 | |||
Note: N=198. WRAT-3, Wide Range Achievement Test; ; CARS-M, Clinician Administered Rating Scale for Mania; Hamilton Rating Scale for Depression; NC*SC, Neurocognition x Social Cognition interaction; SE, Standard error of B; β, Standardized regression coefficient; ΔF, Change in contribution of added predictors; R2, Variance; ΔR2, Change in variance.
p <.05;
p<0.01;
p<0.001.
All analyses are two-tailed.
Figure 1. Interaction between Neurocognition and Social Cognition on Functional Outcome in a BD Cohort.
Note: WHODAS, World Health Organization Disability Assessment Schedule; +1 S.D., good social cognition scorers (n = 31); −1 S.D., poor social cognition scorers (n = 32).
IV. DISCUSSION
The current study investigated whether neurocognition predicted community functioning in a BD sample. Additionally, we sought to determine whether and in what manner social cognition influenced this relationship. We used the MCCB domains and CVLT to assess neurocognition, the MSCEIT and RMET to measure social cognition, and the WHODAS to quantify community functioning. BD patients performed significantly worse than HCs on neurocognitive domains of processing speed, attention, verbal learning, and the neurocognitive composite score and reported worse community functioning. BD patients also performed worse on the RMET and the social cognitive composite score relative to HC. Contrary to our prediction, we discovered that neurocognition did not predict community functioning (nor did social cognition) in our sample, nor did social cognition totally or partially mediate the relationship of neurocognition and functional outcome. However, the moderator model revealed a significant moderation effect of social cognition; specifically, we observed that for patients with poor social cognition, better neurocognitive performance was significantly associated with better community functioning. There was no significant relationship between neurocognition and functioning in patients with good social cognition.
Research has found neurocognitive deficits in SZ, and to a lesser degree, BD (Altshuler et al., 2004). Compared to HCs, SZ patients usually present with global impairment while BD patients exhibit deficits across specific domains, such as attention, verbal learning and working memory, even after controlling for demographic and clinical variables (Arts et al., 2008; Mann-Wrobel et al., 2011). Current results are consistent with data indicating that most neurocognitive domains are impaired in BD even during affective remission, compared to HCs. Social cognitive deficits have also been reliably reported in SZ (Schmidt et al., 2011); however, studies in BD have been more inconsistent. While BD patients have shown no impairments in social cognition relative to HCs (Lee et al., 2013), recent meta-analyses have reported social cognitive impairment in BD, with deficits in theory of mind and emotion recognition (Bora et al., 2016; Samamé et al., 2012). However, many of the studies included in these meta-analyses did not account for confounds that may influence social cognition, such as affective symptoms, neurocognition, psychosis history, and medications. Our results support the meta-analytic findings, with our BD patients performing significantly worse than controls on the theory of mind task. Overall, our results are in line with previous work, providing convergent evidence of neurocognitive and social cognitive deficits in BD.
SZ research has been largely consistent in reporting a significant association between neurocognition and functional outcome (Green, 1996; Fett et al., 2011). One meta-analysis does suggest a similar relationship in BD patients (Depp et al., 2012); however, the effect of neurocognitive ability on community functioning is small to moderate at best, which could also be explained by other factors such as symptoms or motivation. Indeed, there are some studies that suggest no association between neurocognition and community functioning in BD (Martinez-Aran et al., 2001), especially in euthymic patients (Malhi et al., 2007). Also, one meta-analysis indicates inconsistent relationships between cognitive performance and measures of general functioning, while domain-specific functioning measures present relationships specifically between cognition and social and occupational functioning (Baune and Malhi, 2015). Similarly, inconsistent results have also been demonstrated regarding social cognition and functioning in BD (Lahera et al., 2012; Thaler et al., 2014). In the current study, neither neurocognition nor social cognition appeared to predict functional outcome. Interestingly, subthreshold depressive symptomatology was the only significant predictor, highlighting the importance of treating depressive symptoms to the point of full recovery in order to improve functional outcome in BD. One possibility for these contradictory results in BD concerns the presence of cognitive heterogeneity (Altshuler et al., 2004; Burdick et al., 2014). Several recent studies propose that BD may be characterized by several cognitive subgroups, with some patients presenting with severe impairment in most domains (including social cognition), some patients with impairment on a subset of domains, and other patients with intact cognition that is indistinguishable from HCs. Furthermore, our prior work (Burdick et al., 2014) also demonstrated that these distinct neurocognitive subgroups differ with regard to community function. While we did not assess neurocognitive heterogeneity in the current study, our significant moderator model does provide further support for heterogeneity in the social cognition domain, which affects the neurocognitive-functioning relationship differently in discrete subgroups (patients with poor vs. good social cognition).
Social cognition is hypothesized to mediate the relationship between neurocognition and community functioning (Schmidt et al., 2011); in other words, social cognition is a significant underlying mechanism through which neurocognition impacts functional outcome in SZ, which may be better improved utilizing a combination of social and neurocognitive remediation strategies (Brekke et al., 2005). In contrast, the current results suggest that social cognition is relevant in BD in determining for whom neurocognition best predicts functional outcome. Specifically, neurocognitive deficits appear to contribute more prominently to community outcome in those with poor social cognition than it does in those with good social cognition, suggesting that neurocognitive intervention may have a bigger impact upon community functioning in this particular subgroup of patients. While not significant, the overall pattern for patients with good social cognition curiously demonstrated the opposite relationship, such that better neurocognitive performance predicted worse functioning. These results may represent an artifact of variance, the possibility of a non-linear relationship between neurocognition and functioning for good social cognition scorers, or perhaps the combination of better neurocognition and social cognition confers a greater level of insight in these individuals, who may be more sensitive to perceived functional disability, compared to individuals with poor social cognition. Further research is necessary to better understand this relationship and to develop individualized treatment strategies.
The current work has some limitations. Our HC group consisted of 49 participants, which does not equally match our BD sample; however, a sample size of 50 is typically viewed as an adequate size for appropriate normative standardization (Crawford and Howell, 1998). We used a cross-sectional design, which limits our ability to suggest causality between the investigated variables. While we assessed some aspects of social cognition, a more comprehensive assessment of social cognition (e.g., emotion recognition, attributional style) is necessary to better understand its relationship to neurocognition and community functioning in BD. Also, we assessed functioning using the WHODAS 2.0, which focuses on disability level. Other functioning measures, which assess psychosocial and adaptive functioning, may be used to acquire a more comprehensive understanding of functional status. As seen previously, considering domain-specific functioning measures may account for some inconsistency between neurocognition and functional outcome generally found in the literature (Baune and Malhi, 2015). Finally, our BD sample included both patients in remission and with mild depressive symptomatology; and as such, our models cannot account for moderate and severe symptomatology, which likely contributes to disability during episodes. It should be noted here that our results support prior work showing that even subthreshold levels of depressive symptoms contribute to functional deficits in BD.
Our results suggest that, in patients with BD, neurocognition and social cognition contribute to functional outcome in an interactive manner. While social cognition plays a larger, mediating role on this relationship in SZ, social cognition in BD may help to identify in which patients neurocognition most directly influences community functioning. While the neurocognition-functioning relationship is weaker in BD than in SZ, this is likely due to greater social cognitive heterogeneity in BD than is seen in SZ patients, who typically present with consistent and pronounced social cognitive impairments. BD patients with poor social cognition are more comparable to SZ in their cognitive performance and its relation to community functioning, while no such relationship exists for BD patients with good social cognition. Therefore, the association between neurocognition and functioning may be primarily dependent on a person’s level of social cognition, irrespective of diagnosis.
Supplementary Material
Highlights.
Bipolar group performed worse on global neurocognition compared to healthy controls.
Bipolar group performed worse on theory of mind compared to healthy controls.
Overall, neurocognition did not predict community functioning in bipolar patients.
Social cognition moderated neurocognition-functioning relationship in bipolar group.
For bipolar group with poor social cognition, neurocognition predicted functioning.
Acknowledgments
Author Disclosure
This study was funded by Grants from the National Institute of Mental Health (NIMH) to KEB (R01 MH 100125; R34 MH101267; R01 MH102257) and the Veterans Administration (VA) Health system to KEB (I01CH000995).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Burdick has served as an advisory board member for Dainippon Sumitomo Pharmaceutical, Otsuks Pharmaceuticals, Neuralstem, and Takeda Lundbeck, which had no impact upon the work presented in this manuscript. Dr. Nitzburg has received research funding from Talkspace Incorporated, which had no impact upon the work presented in this manuscript. All other authors report no competing interests regarding the present study.
Author Contribution: Dr. Ospina undertook the statistical analyses and wrote the completed draft of the manuscript. Dr. Nitzburg aided in data collection and consulted on statistical approaches. Ms. Shanahan also assisted in data collection and management. Dr. Perez-Rodriguez assisted in study design and protocol. Mr. Larsen and Ms. Latifoglu aided in literature searches and table/figure creations for the manuscript. Dr. Burdick primarily designed the study and wrote protocol. All authors contributed to and have approved the final manuscript.
References
- Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- Allen DN, Strauss GP, Donohue B, van Kammen DP. Factor analytic support for social cognition as a separable cognitive domain in schizophrenia. Schizophrenia research. 2007;93(1):325–333. doi: 10.1016/j.schres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The clinician-administered rating scale for mania (CARS-M): development, reliability, and validity. Biological Psychiatry. 1994;36(2):124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biological psychiatry. 2004;56(8):560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, Van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychological medicine. 2008;38(6):771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Baune BT, Malhi GS. A review on the impact of cognitive dysfunction on social, occupational, and general functional outcomes in bipolar disorder. Bipolar disorders. 2015;17(S2):41–55. doi: 10.1111/bdi.12341. [DOI] [PubMed] [Google Scholar]
- Bo Q, Mao Z, Li X, Wang Z, Wang C, Ma X. Use of the MATRICS consensus cognitive battery (MCCB) to evaluate cognitive deficits in bipolar disorder: A systematic review and meta-analysis. PloS one. 2017;12(4):e0176212. doi: 10.1371/journal.pone.0176212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Bartholomeusz C, Pantelis C. Meta-analysis of Theory of Mind (ToM) impairment in bipolar disorder. Psychological medicine. 2016;46(2):253–264. doi: 10.1017/S0033291715001993. [DOI] [PubMed] [Google Scholar]
- Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophrenia research. 2005;80(2):213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, Malhotra AK. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychological medicine. 2014;44(14):3083–3096. doi: 10.1017/S0033291714000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia bulletin. 2006;32(suppl_1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12(4):482–486. [Google Scholar]
- Cusi AM, MacQueen GM, McKinnon MC. Patients with bipolar disorder show impaired performance on complex tests of social cognition. Psychiatry research. 2012;200(2):258–264. doi: 10.1016/j.psychres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar disorders. 2012;14(3):217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? The American journal of psychiatry. 1996;153(3):321. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23(1):56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology. 2014;67(3):451–470. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling 2012 [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication monographs. 2009;76(4):408–420. [Google Scholar]
- Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar disorders. 2007;9(1–2):183–196. doi: 10.1111/j.1399-5618.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry research. 2011;188(3):303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Lahera G, Benito A, Montes JM, Fernandez-Liria A, Olbert CM, Penn DL. Social cognition and interaction training (SCIT) for outpatients with bipolar disorder. Journal of affective disorders. 2013;146(1):132–136. doi: 10.1016/j.jad.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. American Journal of Psychiatry. 2013;170(3):334–341. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar disorders. 2007;9(1–2):114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar disorders. 2011;13(4):334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Torrent C, Sanchez-Moreno J, Goikolea JM, Salamero M, Fountoulakis K. Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar disorders. 2007;9(1–2):103–113. doi: 10.1111/j.1399-5618.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Penades R, Vieta E, Colom F, Reinares M, Benabarre A, Salamero M, Gasto C. Executive function in patients with remitted bipolar disorder and schizophrenia and its relationship with functional outcome. Psychotherapy and psychosomatics. 2002;71(1):39–46. doi: 10.1159/000049342. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia research. 2004;71(2):405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS consensus cognitive battery manual. Los Angeles, CA: MATRICS Assessment Inc; 2006. [Google Scholar]
- Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biological psychiatry. 2008;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of affective disorders. 2006;93(1):105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Samamé C. Social cognition throughout the three phases of bipolar disorder: a state-of-the-art overview. Psychiatry research. 2013;210(3):1275–1286. doi: 10.1016/j.psychres.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Samamé C, Martino DJ, Strejilevich SA. Social cognition in euthymic bipolar disorder: systematic review and meta-analytic approach. Acta Psychiatrica Scandinavica. 2012;125(4):266–280. doi: 10.1111/j.1600-0447.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno J, Martinez-Aran A, Tabares-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and disability in bipolar disorder: an extensive review. Psychotherapy and psychosomatics. 2009;78(5):285–297. doi: 10.1159/000228249. [DOI] [PubMed] [Google Scholar]
- Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophrenia bulletin. 2012;39(5):979–992. doi: 10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophrenia bulletin. 2011;37(suppl_2):S41–S54. doi: 10.1093/schbul/sbr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Green MF. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophrenia research. 2007;90(1):316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological methodology. 1982;13:290–312. [Google Scholar]
- Soper D. Interaction (version 1.7.2211) (software) 2013 Retrieved from http://www.danielsoper.com/interaction/default.aspx.
- Tabarés-Seisdedos R, Balanzá-Martínez V, Sánchez-Moreno J, Martinez-Aran A, Salazar-Fraile J, Selva-Vera G, Rubio C, Mata I, Gómez-Beneyto M, Vieta E. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. Journal of affective disorders. 2008;109(3):286–299. doi: 10.1016/j.jad.2007.12.234. [DOI] [PubMed] [Google Scholar]
- Tsitsipa E, Fountoulakis KN. The neurocognitive functioning in bipolar disorder: a systematic review of data. Annals of general psychiatry. 2015;14(1):42. doi: 10.1186/s12991-015-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün BT, editor. Measuring health and disability: Manual for WHO disability assessment schedule WHODAS 2.0. World Health Organization; 2010. [Google Scholar]
- Wilkinson GS. WRAT-3: Wide range achievement test administration manual. Wide Range, Incorporated; 1993. [Google Scholar]
- Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar disorders. 2009;11(2):113–125. doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Torres IJ, Malhi GS, Frangou S, Glahn DC, Bearden CE, Ozerdem A. The International Society for Bipolar Disorders-Battery for Assessment of Neurocognition (ISBD-BANC) Bipolar disorders. 2010;12(4):351–363. doi: 10.1111/j.1399-5618.2010.00830.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.