Abstract
The redundant mechanisms involved in blood coagulation are crucial for rapid hemostasis. Yet they also create challenges in blood processing in medical devices and lab-on-a-chip systems. In this work, we investigate the effects of both shear stress and hypothermic blood storage on thrombus formation in microfluidic processing. For fresh blood, thrombosis occurs only at high shear, and the glycoprotein IIb/IIIa inhibitor tirofiban is highly effective in preventing thrombus formation. Blood storage generally activates platelets and primes them towards thrombosis via multiple mechanisms. Thrombus formation of stored blood at low shear can be adequately inhibited by glycoprotein IIb/IIIa inhibitors. At high shear, von Willebrand factor-mediated thrombosis contributes significantly and requires additional treatments with thiol-containing antioxidants—such as N acetylcysteine and reduced glutathione—that interfere with von Willebrand factor polymerization. We further demonstrate the effectiveness of these anti-thrombotic strategies in microfluidic devices made of cyclic olefin copolymer, a popular material used in the healthcare industry. This work identifies effective anti-thrombotic strategies that are applicable in a wide range of blood- and organ-on-a-chip applications.
Graphical Abstract

Introduction
Microfluidic blood-on-a-chip technology is an exciting area encompassing applications such as blood cell fractionation1–3, blood cleansing4, point-of-care diagnostics5–7, and the detection of rare events such as parasites8, bacteria9, and circulating tumor cells (CTCs)10. However, thrombus formation inevitably occurs during in vitro blood manipulation despite the use of anticoagulants. Clot formation in microfluidic devices is particularly challenging due to the highly sensitive biophysical mechanisms that trigger the coagulation response. Under low shear conditions (<30 dyn/cm2), coagulation is primarily initiated by activated platelets upon contact with foreign materials or extracellular matrix proteins11. Under higher shear conditions (>100 dyn/cm2), thrombosis can also be initiated under mechanisms independent of platelet-activation12–14. In these events, plasma von Willebrand factor (vWF) unfold under shear, exposing binding sites that allow them to polymerize as well as initiating the coagulation cascade15. Further complicating these issues are the profound consequences of platelet activation as a result of blood storage16. Although low-temperature storage minimizes the functional degradation of platelets17–20—commonly known as storage lesions21—such conditions greatly exacerbate thrombosis by inducing spontaneous platelet aggregation22 and potentiating shear-induced binding to vWF23.
Various groups have reported thrombus formation in microfluidic devices. Zheng et al.24 found fibrous structures with attached platelets in a deterministic lateral displacement (DLD) device designed to separate leukocytes from red blood cells. The authors observed these fibrous structures even with blood that was diluted up to 50 times, and that blood storage at room temperature for 2 days caused ~15 times more device blockage at the same dilution level. Inglis et al.25 processed undiluted whole blood and observed large (20–200 μm) clot-like structures that blocked the device, and they reported a significant increase in flow resistance for blood that has been stored in room temperature for more than 24 hours. Because a pre-filter was used to remove aggregates prior to blood loading, the clots were likely caused by the microfluidic processing per se, highlighting the biophysical basis of thrombosis and challenges of processing large volumes of whole blood. More recently, D’Silva et al.26 investigated the effects of ion chelation and thrombin inhibition on clot formation. However, the authors did not specify the age and storage conditions of the blood, except that they were shipped at 4 °C and used within 36 hours of blood draw. Taken together, given the overwhelming challenges of blood storage and lack of sufficient knowledge regarding biophysical effects on clot formation, researchers often resort to empirical experimentation to determine acceptable blood processing conditions. This knowledge gap imposes an especially heavy burden on the latest advances in large-volume, high-shear blood processing in which the flow rates can reach 20~200 mL per hour. These levels of throughput are necessitated by the rarity of target cells27,28, or by the sheer cell number or volume required for therapeutic efficacy, for instance in leukapheresis, ex vivo cell expansion, or extracorporeal blood cleansing4,29–31.
To address this gap, we developed a microfluidic system to investigate the effects of both shear stress and blood storage on blood processing and explore anti-thrombotic strategies that mitigate thrombus formation. For blood storage, we focus on hypothermic temperature (4 °C) as opposed to room or ambient temperatures, because cooling has well-known benefits in biopreservation and will be broadly applicable to diverse biomedical technologies. Furthermore, by maintaining blood cell viability, hypothermic preservation minimizes the confounding factors that lead to microfluidic clogging due to cell degradation and extracellular DNA32. To examine blood clogging, we use a microfluidic filter design and quantify platelet accumulation in real-time using fluorescence microscopy. Our results indicate that glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors such as tirofiban (tiro) and eptifibatide are highly effective in inhibiting thrombus formation, albeit insufficient for high-shear processing of sensitized (i.e., stored) blood. In such conditions, antioxidants such as N acetylcysteine (NAC) and reduced glutathione (GSH) appear promising in preventing vWF-mediated thrombosis. This work suggests strategies that counter thrombosis and will find broad applications in microfluidic blood processing.
Results
Design and operation of microfluidic device
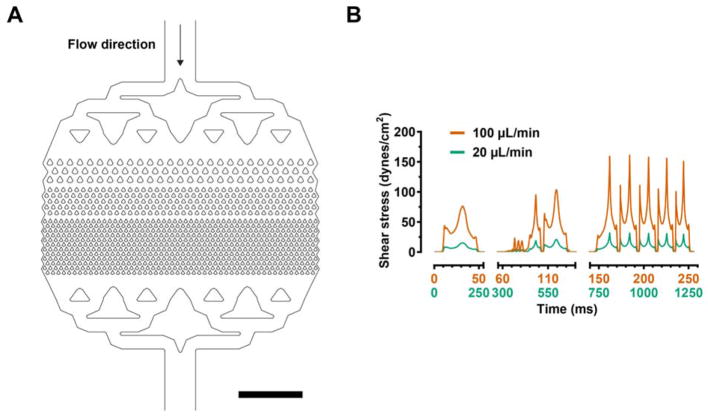
We fabricated a microfluidic device in polydimethylsiloxane (PDMS) using standard soft lithography techniques. The device consists of kite-shaped features with a minimum gap size of 30 μm (Fig. 1A), effectively functioning as a microfluidic filter that traps large aggregates and clots. We operated the device at two flow rates, 20 and 100 μL/min, using undiluted whole blood. Numerical modeling of fluid flow indicates that the maximum shear stresses are ~30 and ~150 dyn/cm2, for the 20 and 100 μL/min flow rate conditions, respectively. These flow rates were chosen to investigate thrombus formation that (1) is predominantly dependent on platelet activation under the low shear condition (<30 dyn/cm2); or (2) can also be initiated by high shear (>100 dyn/cm2) without pre-activation of platelets12–14.
Figure 1.
(A) Computer-Aided Design (CAD) representation of the microfluidic filter device and (B) the fluid shear stress computed from the CFD model at the imposed bulk flow rates (20 and 100 μL/min). The blood flows through 3 stages of kite-shaped pillars with progressively narrower gap sizes (30 μm at the narrowest stage). Each plotted shear stress curve was calculated along a streamline that originated exactly one micron from the pillar side-wall in its narrowest gap, at the mid-height of the channel. One such streamline was seeded in the middle of the first, second, and third levels of the filter in (A). The surface normal for shear stress computation was then defined as the normal to the streamline with zero span-wise component due to symmetry at the midplane. The sections on the time axis correspond to the time needed for the fluid particle to travel across each stage. Scale bar represents 1 mm.
Thrombus formation during processing of fresh blood
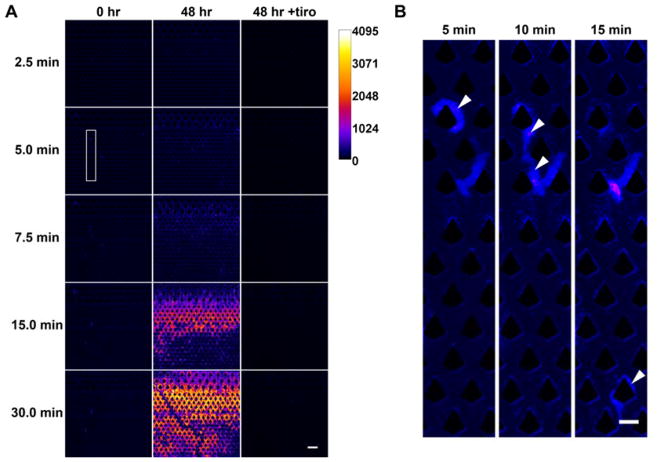
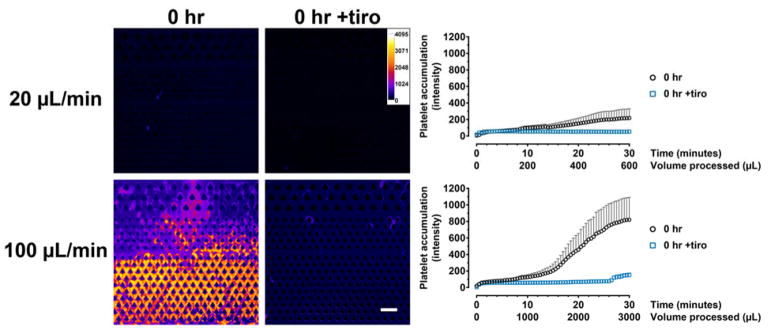
To quantify thrombus formation, we acquired time-lapse fluorescence images of the microfluidic device during blood processing (Fig. 2A) to visualize the accumulation of platelets. Blood that was freshly obtained (i.e., within 4 hours of blood draw and never exposed to low temperatures) did not result in significant clot formation when processed under low flow (20 μL/min). Time-lapse images indicate transient adhesion of platelets which were subsequently dislodged under flow (Fig. 2B). By contrast, under high flow (100 μL/min), clots rapidly formed in the device and grew steadily over time (Fig. 3). The GPIIb/IIIa inhibitor tirofiban, which is a clinically used anti-platelet agent33, effectively inhibited thrombosis in both low- and high-shear conditions (Fig. 3; p < 0.0001 for tiro vs. untreated control for both low and high flow). These results identify GPIIb/IIIa inhibition as an effective strategy in inhibiting thrombosis in particular for high-shear processing of fresh blood.
Figure 2.
Time-lapse assay for quantifying platelet accumulation. Whole blood was labeled with DiOC6 prior to microfluidic processing and fluorescence images were acquired in 30-second intervals. The fluorescence intensity was pseudocolored for easy visualization. (A) Fresh (0 hr) blood could be processed without platelet accumulation under low shear (20 μL/min) but cold-stored (48 hr) blood resulted in stable aggregates. The presence of tirofiban (tiro) completely inhibited platelet accumulation. (B) Close-up images (with enhanced contrast) of the boxed region in (A) demonstrating that adhered platelets (from fresh blood) can be easily dislodged under flow. The arrowheads point to an aggregate (5 min) that was displaced and carried downstream over time (10 min and 15 min). Scale bar in (A) and (B) represents 100 μm and 50 μm, respectively.
Figure 3.
Tirofiban effectively inhibits clot formation in fresh blood (0 hr) under both low- (20 μL/min) and high-shear (100 μL/min) flow conditions. Pseudocolored images show the extent of platelet accumulation at the end of the experiment (30 min). The intensity plots represent area-averaged fluorescence intensity (20 μL/min: 0 hr, n = 4; 0 hr +tiro, n = 5. 100 μL/min: 0 hr, n = 6; 0 hr +tiro, n = 5).
Blood storage generally potentiates thrombus formation
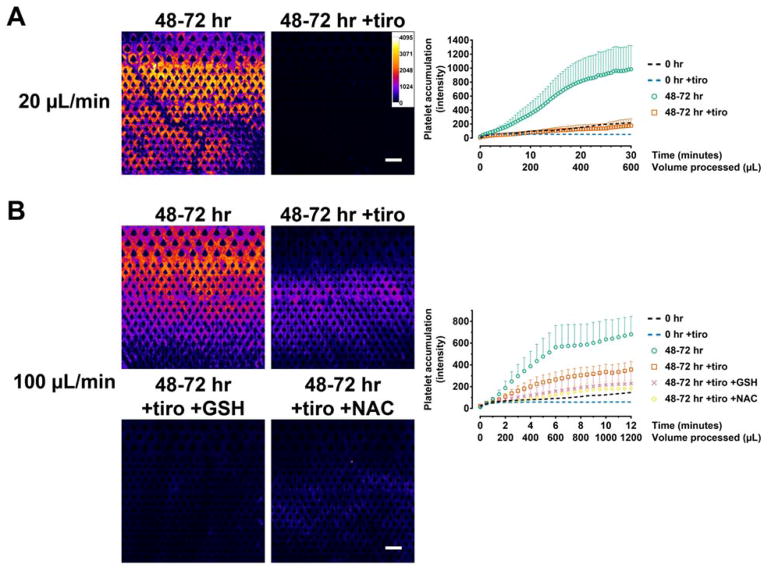
The functional deterioration and activation of platelets associated with storage is collectively referred to as storage lesion21. Although low-temperature (4 °C) storage inhibits certain aspects of lesions—for instance in terms of lactate accumulation and release of inflammatory mediators, and hemostatic function in vitro and in vivo17–20—cooling also induces a host of complex changes which remain incompletely understood. These include spontaneous aggregation22, irreversible disc-to-sphere shape changes, clustering of GPIb, and rapid clearance upon transfusion34 As such, whole blood storage in general (regardless of temperature) presents significant challenges to blood-on-a-chip technologies in which fine microfabricated structures are obstructed by aggregates and clots24–26. We investigated cold storage, which is the preferred method for biostabilization and has clear advantages in blood-on-a-chip applications in terms of cell viability and preventing bacterial growth. We observed profound thrombus formation in the processing of cold-stored blood. Even at low shear, platelets accumulated immediately as flow initiated and the resulting clots grew over time (Fig. 2A and 4A). The addition of tirofiban in blood storage significantly decreased clot formation in this flow condition (Fig. 2A and 4A; p < 0.0001 for tiro vs. untreated), and the final platelet intensity in the device decreased by 82% compared to the untreated condition. Under high flow, clot formation occurred immediately similar to low flow conditions (Fig. 4B). Clot buildup was very rapid such that some devices became completely clogged during the course of the experiment, and therefore we only analyzed the data for up to 12 minutes. Tirofiban was partially effective at inhibiting thrombosis under high flow (p < 0.0001 for tiro vs. untreated control), but it only reduced thrombus intensity by 52% at the end of the experiment (Fig. 4B). Together, these results indicate that cold storage generally potentiates thrombus formation at both low- and high-shear conditions, and that alternative mechanisms independent of GPIIb/IIIa likely exist in high-shear processing of stored blood.
Figure 4.
Anti-thrombotic strategies for sensitized (cold-stored) whole blood. (A) Hypothermic blood storage results in platelet activation that generally potentiates clot formation. Stored blood forms clots rapidly even under low shear. The presence of tirofiban in blood storage effectively inhibited clot formation. n = 5 each for 48–72 hr and 48–72 hr +tiro. (B) Under high shear, tirofiban reduced clot formation but the amount of clot was still significant. The addition of GSH and NAC prior to blood processing further reduced clot formation. 48–72 hr, n = 6; 48–72 hr +tiro, n = 17; 48–72 hr +tiro +GSH, n = 9; 48–72 hr +tiro +NAC, n = 9. Data from fresh blood (0 hr data from Figure 3) are included as dotted lines as a baseline reference but is not used for statistical comparisons. Scale bars represent 100 μm.
Mechanisms of cold-induced activation
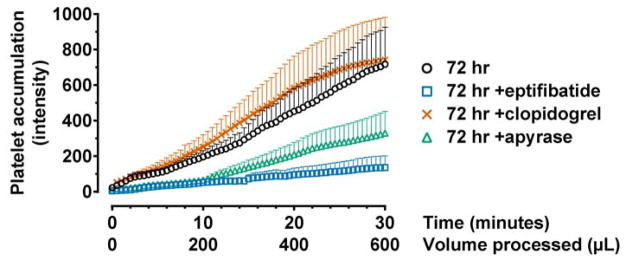
To further understand the activation mechanisms of cold storage, we explored additional anti-platelet agents including eptifibatide, another GPIIb/IIIa inhibitor; clopidogrel, which inhibits the G-protein-coupled ADP receptor, P2Y12; and apyrase, an ADP scavenger. When processed at low shear, eptifibatide effectively inhibited thrombus formation (81% reduction of final intensity; Fig. 5; p < 0.0001 compared to untreated). Apyrase also resulted in significant thrombus reduction (Fig. 5; p < 0.0001 compared to untreated) but its effect was inferior to that of eptifibatide (p = 0.0007). Clopidogrel did not result in any reduction (Fig. 5). These results confirm the indispensable role of GPIIb/IIIa in cold-induced activation and suggest that ADP participates at least partly in this activation response.
Figure 5.
Mechanisms of storage-induced activation. Blood was stored with the indicated anti-platelet agents for 72 hours and processed at low shear. Eptifibatide, another clinically used GPIIb/IIIa inhibitor, effectively inhibited clot formation. Apyrase, an ADP scavenger, also resulted in clot reduction but was not as effective as eptifibatide. Clopidogrel, a P2Y12 inhibitor, did not reduce clot formation. 72 hr, n = 7; 72 hr +eptifibatide, n = 5; 72 hr +clopidogrel, n = 7; 72 hr +apyrase, n = 7.
High-shear processing of stored blood activates von Willebrand factor-mediated thrombosis
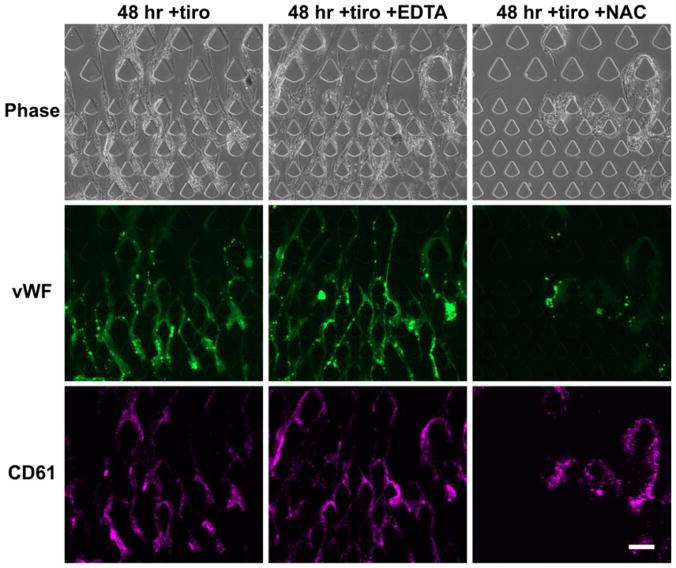
In addition to inducing spontaneous platelet aggregation and activation, cooling also increases the sensitivity of platelets to shear-induced aggregation and vWF binding23. We immunostained the microfluidic device after high-shear processing of stored blood and confirmed that long vWF fibers are present (Fig. 6), suggesting that vWF-mediated thrombosis may explain the lack of efficacy of tirofiban. Furthermore, treatment with the ion chelator EDTA did not reduce the formation of vWF fibers (Fig. 6); instead, these fibers appeared longer and more continuous, consistent with previous findings that divalent ions are required for the degradation of vWF polymers by ADAMTS1335,36—the principal vWF-degrading enzyme in plasma. Taken together, these results highlight the redundant thrombosis mechanisms mediated by vWF.
Figure 6.
High-shear processing of stored blood results in vWF-mediated thrombosis. Immunofluorescence staining identified vWF fibers that co-localize with platelet accumulation. Treatment with EDTA, which chelates diavlent ions required in the coagulation cascade, did not inhibit vWF fiber formation or platelet accumulation. Instead, the vWF fibers appeared longer and more continuous. The addition of NAC during blood processing effectively inhibited vWF fiber formation. Scale bar represents 100 μm.
It has been reported that the antioxidant NAC could reduce the disulfide bonds required for vWF polymerization, and thereby reduce vWF multimers and inhibit vWF-mediated thrombosis37. We found that treating stored blood with NAC effectively decreased vWF fiber accumulation (Fig. 6) and thrombosis (Fig. 4B; p < 0.0001 compared to untreated and tiro). The final thrombus intensity decreased by 73% compared to the untreated condition and 49% compared to tirofiban only (Fig. 4B). We reasoned that another thiol-containing antioxidant, GSH, may exert anti-thrombotic function similar to NAC. At equimolar concentration, GSH treatment exerted anti-thrombotic effects with comparable potency (Fig. 4B; p < 0.0001 compared to untreated, p = 0.0004 compared to tiro), decreasing the final thrombus intensity by 67% compared to the untreated condition (Fig. 4B). In summary, these data show that high-shear processing of stored blood is particularly vulnerable to vWF-mediated thrombosis, and that anti-vWF agents such as NAC and GSH are effective counter strategies.
Applicability in microfluidic devices made of other materials
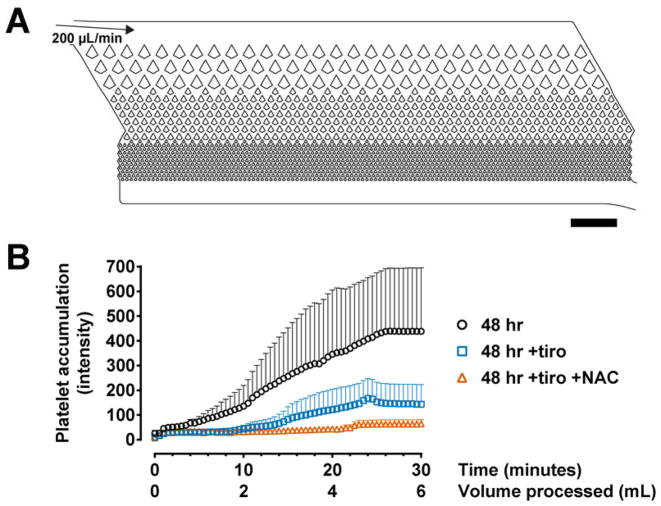
While PDMS soft lithography remains the most common method in microfluidic device fabrication, much progress is being made in using other materials to accommodate manufacturing demands in the diagnostic and biopharmaceutical industries. We are interested to understand whether our anti-thrombotic strategies are effective in devices made of such materials. We used a new generation of the CTC-iChip which is under development for the high-throughput detection of rare cells28; this device is made of cyclic olefin copolymer (COC) and can be mass-produced by injection molding. We processed 48-hour stored whole blood at 200 μL/min and quantified platelet accumulation in the microfluidic filter array (Fig. 7A), which functions as a pre-filter prior to downstream cell-sorting mechanisms. Similar to PDMS devices, untreated whole blood resulted in massive clot formation which is effectively inhibited by tirofiban (Fig. 7B; p < 0.0001). Additional treatment with NAC further reduced clot formation, such that 6 mL of stored blood can be processed in 30 minutes without appreciable increases in platelet accumulation (Fig. 7B; p = 0.0421 compared to tiro only). Together, these data suggest the general applicability of GPIIb/IIIa inhibitors and antioxidants for blood processing in plastic microdevices.
Figure 7.
Anti-thrombotic strategies are effective in microfluidic devices made of materials other than PDMS. (A) CAD representation of the microfluidic filter in the CTC-iChip manufactured using injection-molded COC plastic. The channel depth is 52 μm and the narrowest constrictions have a gap size of 30 μm. (B) Treatments with tirofiban and NAC produced similar anti-thrombotic effects at high shear as observed in PDMS devices. 48 hr, n = 6; 48 hr +tiro, n = 7; 48 hr +tiro +NAC, n = 7. Scale bar represents 1 mm.
Discussion
This paper provides insights into several practical aspects in microfluidic blood processing. For fresh blood, high shear stress results in thrombus formation which is effectively prevented by GPIIb/IIIa inhibitors. Blood storage significantly primes platelets for thrombus formation in low- and high-shear conditions. For low-shear processing, GPIIb/IIIa inhibition is an effective anti-thrombotic strategy. At high shear stress however, vWF-mediated mechanisms contribute significantly to thrombus formation, requiring additional anti-vWF treatments such as NAC and GSH to enable processing.
Coagulation26, together with other considerations in blood stability32,38,39, remains a challenging issue in microfluidic processing. These challenges stem primarily from the biological complexity of this tissue, both in terms of its cellular components and the redundant mechanisms in coagulation. We have previously shown that aging of blood specimens results in cellular degradation (e.g., DNA from neutrophil extracellular traps) and morphological changes (e.g., echinocytosis of red blood cells), both of which significantly interfere with microfluidic blood sorting mechanisms32,38,39. The blood storage condition that we selected for this study have been validated to adequately preserve viability and morphology of diverse hematologic cell types32, and therefore serves as an excellent starting point to investigate anti-thrombotic strategies. To this end, publications that describe the consequences of blood aging are largely anecdotal, and information on shear stress are often not provided24,25. The recent work by D’Silva et al.26 has begun to shed light on clotting mechanisms in microfluidic processing. They found that platelet-rich plasma and leukocytes were the major blood components that contribute to clot formation. They also concluded that thrombin inhibition and ion chelation were effective strategies to reduce clogging. Unfortunately, interpretation of their results is not straightforward, because the blood was shipped overnight at 4 °C, and no other information on blood storage (e.g., temperature) was available except that “experiments were performed within 36 hours of the draw time”26. It is therefore unclear to what extent their findings were confounded by factors such as the duration of blood cooling and cellular degradation as a result of storage. Our study therefore provides much-needed insights that take into account both the effects of shear stress and storage in clot formation.
Based on the classical description of the coagulation cascade, activation of platelets regardless of the initiating agonists leads to the “final common pathway” in which thrombin converts plasma fibrinogen into hemostatic fibrin clots. Glycoprotein IIb/IIIa, the most abundant receptor on platelets, mediates fibrinogen binding and is indispensable for the stable aggregation of activated platelets under physiologically relevant shear stresses (up to ~100 dyn/cm2)14,40. Our results on two GPIIb/IIIa inhibitors, together with that by D’Silva et al.26 demonstrating the participation of thrombin, strongly support that cold storage also leads to the classical final common pathway in coagulation. Furthermore, our data suggest that ADP plays a role in this activation process upstream of thrombin. It is established that ADP is released from the dense granules of activated platelets and serves to amplify platelet response via the ADP receptors P2Y1 and P2Y1241. The P2Y12 inhibitor, clopidogrel, is however ineffective in vitro because this pro-drug requires hepatic metabolism for activity42.
Von Willebrand factor-mediated thrombosis is shear-dependent. At low shear (<30 dyn/cm2), vWF remains inactive. When shear increases beyond ~80 dyn/cm2, vWF polymerization occurs and begin to induce platelet aggregation and activation12. Of note, GPIIb/IIIa- and vWF-mediated mechanisms are not mutually exclusive—they are both functional at this moderate shear range14. As shear further increases, the relative contribution of vWF to thrombus formation increases dramatically, ultimately taking over GPIIb/IIIa as the predominant mechanism at very high, pathological shear levels (~300 dyn/cm2)14,43. GPIIb/IIIa inhibition is generally effective in preventing thrombosis except for the high-shear processing of cold-stored blood. This can be explained by the priming effect of cooling which enhances platelet-vWF binding23. Taken together, our results show that cold storage lowers the shear stress threshold for vWF activation which is an important consideration in microfluidic processing.
The subject of vWF-mediated thrombosis is an area of on-going investigation due to its clinical relevance in thrombotic thrombocytopenic purpura (TTP), a life-threatening condition triggered by the accumulation of ultra-large vWF multimers in the circulation. TTP is caused by a decreased activity of the enzyme ADAMTS13, a metalloproteinase that cleaves vWF multimers in the circulation44. Recently, Chen et al. found that NAC reduced vWF multimers both in vitro and in vivo by interfering with disulfide bonding37. Clinical studies are now underway to determine the efficacy and safety of NAC as a treatment for TTP45,46. We found that NAC and another antioxidant, GSH, exhibited similar anti-thrombotic effects for high-shear processing of cold-stored blood. Therefore, thiol-containing antioxidants are attractive anti-vWF agents that may be useful for the microfluidic processing of sensitized blood under challenging shear conditions. Further research may explore whether recombinant ADAMTS1347 or clot-lysing agents such as plasmin48 may provide additional benefits.
A major advantage of our anti-thrombotic agents is their proven biocompatibility. GPIIb/IIIa inhibitors are used in cardiovascular medicine33 and are non-cytotoxic in whole blood storage32. Similarly, high doses of NAC (hundreds of mg per kg) are used clinically for the treatment of acetaminophen overdose49–51. These additives should be relatively safe for therapeutic blood product manufacturing or extracorporeal devices in which large volumes of processed blood (or cells) are intended to be infused (or returned) to patients. Nonetheless, their full effects on blood or immune cells for particular biomedical applications remain to be understood. Lastly, our results obtained using COC devices suggest the general efficacy of our anti-thrombotic strategies in a range of materials. Additional considerations especially for point-of-care diagnostics include the pre-coating of these agents on-chip and optimization of their release profiles.
In conclusion, we have identified the mechanisms that predispose blood to thrombosis in microfluidic processing and investigated the corresponding anti-thrombotic strategies. Our findings are of value to the scientific community in a broad range of microfluidic technologies in blood- and organ-on-a-chip applications. Furthermore, our microfluidic device may be useful for the functional screening of anti-vWF treatments for TTP, which continues to be a major challenge in the clinic52,53.
Materials and Methods
Blood specimens and storage
Blood samples were purchased from Research Blood Components (Brighton, MA) or acquired from healthy donors in Massachusetts General Hospital (MGH). Informed consent was obtained from all donors and all experiments were performed in compliance with the relevant laws and guidelines approved by the MGH Institutional Review Board (protocol DF/HCC 05-300). Blood was drawn into Acid Citrate Dextrose-A tubes (BD Vacutainer; 8.5 mL) and used or stored within four hours. For blood storage (48–72 hours), the sample was transferred to 15 mL conical tubes and stored undisturbed and protected from light at 4 °C. Where indicated, the following were added to blood before storage: tirofiban (Sigma), 0.5 μg/mL; eptifibatide (Tocris), 50 μg/mL; clopidogrel (Sigma), 20 μg/mL; and apyrase (grade VII; Sigma), 2 U/mL. After storage, blood was equilibrated to room temperature in a water bath before further handling for experiments.
Design of the PDMS microfluidic filter
The complete three-stage filter design is depicted in Fig. 1A from a top view (52 μm channel depth). In stage 1, there are three rows of round-corner posts (either 26 or 27 posts per row as they alternate); these large posts are each 86 μm wide with a narrowest constriction (gap) of 60 μm between neighboring posts. In stage 2, there are five rows of round-corner posts (40–41 per row) with a post width and gap of 58 μm and 40 μm respectively. The final stage of the filter has 10 rows of 41–42 sharp corner posts (65 μm wide with 30 μm gaps). This resulted in a total filter width of 4 mm in each of the three stages. To ensure equal spread of the blood flow prior to clogging, a network of branching channels was included upstream and downstream of the filter region, enabling connection by a single input and output port.
Microfluidic blood processing and time-lapse fluorescence assay
PDMS microfluidic devices were produced using standard soft lithography techniques and bonded to glass substrates. CTC-iChips are produced by a third party28 using medical grade cyclic olefin copolymer (COC). The devices were primed with 70% ethanol followed by PBS containing 0.2% (w/v) Pluronic F-68 before blood processing. For time-lapse experiments, whole blood was incubated with DiOC6 (1 μM; Life Technologies) for 10 minutes to label platelets, before it was loaded into a syringes (BD) and processed in the device using a syringe pump (Harvard Apparatus PHD 2000) at the indicated flow rates. Where indicated, GSH (30 mM; Sigma), NAC (30 mM; Sigma), or EDTA (4 mM; Ambion) were added to blood immediately prior to blood processing. Fluorescence images were acquired at 30-second intervals with a QImaging Retiga 2000R camera using a Nikon Plan Fluor 4×/0.13 objective on a Nikon Eclipse 90i microscope. The area-averaged fluorescence intensity of each image was measured (using NIS Elements ver. 3.22) in a rectangular region-of-interest (ROI) that includes all microfluidic pillars in the device within the image. The ROIs for PDMS devices and CTC-iChips measured 2940 μm by 1740 μm and 2900 μm by 2160 μm, respectively. Fluorescence images were pseudocolored using Fiji for easy visualization.
Immunofluorescence staining of vWF fibers and CD61
Stored blood was processed, without DiOC6 labeling, in the PDMS device for 10 minutes at 100 μL/min. The device was then flushed with 0.3% BSA at 50 μL/min for 5 minutes, stained with FITC-conjugated vWF antibody (1:100; Abcam) and Alexa Fluor 647-conjugated CD61 antibody (1:200; clone VI-PL2; BioLegend) at 30 μL/min for 30 minutes, and rinsed again with PBS for 10 minutes. Phase-contrast and fluorescence images were acquired at 40× using an EVOS FL Cell Imaging System (Life Technologies).
Numerical modeling of blood flow
To estimate the flow profile and shear stresses within the filter, a computational fluid dynamics (CFD) model was created using ANSYS Fluent 13. For the high-shear case, the model achieved a 100 μL/min total flow rate using an inlet pressure boundary condition that was 1000 Pa higher than the pressure specified at the outlet boundary, assuming an isotropic constant viscosity of 0.003 Pa·s for the blood. To model the integrated filter, a smallest repeating unit was employed: 12 posts in stage 1, 18 posts in stage 2, and 18.5 posts in stage 3 were modeled, and half the height. This resulted in a mesh of 5.85 million wedge cells. To enable the greatest mesh resolution in the third filter stage which contains sharp post corners, a pressure drop of 600 Pa was applied to a minimal unit of the filter: 1 post width at half height and all ten rows. This resulted in a mesh of 1.85 million wedge cells. In each case, iterations proceeded until the residuals dropped below ~10−15.
To generate the shear stress plots versus time (Fig. 1B), streamlines were seeded at a distance of one micron from the near wall in the narrowest gaps of each layer of the filter (three layers are visible in Fig. 1A), at the channel midplane. Velocity and velocity gradients were then exported along the streamlines to compute the shear stresses along these three paths. Time along the streamline was then computed according to kinematics, and shear stress was computed for the set of surfaces tangent to the streamline and orthogonal to the plane of Fig. 1A. The gaps between filter layers in Figure 1B result from separate seeding of streamlines in each layer of the filter, to ensure that the streamlines start a prescribed distance from the pillar wall in the narrowest gap of each level of the filter.
Statistical analysis
Comparisons of fluorescence intensities in the time-lapse assay used two-way ANOVA followed by Tukey’s posttest (GraphPad Prism 7). Error bars represent SEM.
Acknowledgments
We thank Octavio Hurtado, Kendall Williams, and John Walsh for experimental assistance, and healthy volunteers who donated blood specimens. This work was supported by funding from the US National Institutes of Health (NIH) P41 BioMEMS Resource Center (EB002503; M.T.) and NIH National Institute of Biomedical Imaging and Bioengineering (EB012493; M.T.). S.N.T. was supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- 1.Toner M, Irimia D. Blood-on-a-chip. Annu Rev Biomed Eng. 2005;7:77–103. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis JA, et al. Deterministic hydrodynamics: taking blood apart. Proc Natl Acad Sci U S A. 2006;103:14779–84. doi: 10.1073/pnas.0605967103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu ZTF, Aw Yong KM, Fu J. Microfluidic blood cell sorting: now and beyond. Small. 2014;10:1687–703. doi: 10.1002/smll.201302907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JH, et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat Med. 2014;20:1211–6. doi: 10.1038/nm.3640. [DOI] [PubMed] [Google Scholar]
- 5.Cheng X, et al. A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects. Lab Chip. 2007;7:170–8. doi: 10.1039/b612966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins NN, et al. Microfluidic CD4+ and CD8+ T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci Transl Med. 2013;5:214ra170. doi: 10.1126/scitranslmed.3006870. [DOI] [PubMed] [Google Scholar]
- 7.Hassan U, et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat Commun. 2017;8:15949. doi: 10.1038/ncomms15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holm SH, Beech JP, Barrett MP, Tegenfeldt JO. Separation of parasites from human blood using deterministic lateral displacement. Lab Chip. 2011;11:1326–32. doi: 10.1039/c0lc00560f. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, Willing B, Bjerketorp J, Jansson JK, Hjort K. Soft inertial microfluidics for high throughput separation of bacteria from human blood cells. Lab Chip. 2009;9:1193–9. doi: 10.1039/b817611f. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10:374–394. doi: 10.1016/j.molonc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–92. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–2645. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-Independent Platelet Adhesion and Aggregation under Elevated Shear Stress Running title: Platelet adhesion and aggregation in flowing blood. Blood. 2006;108:1903–1911. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell MJ, et al. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109:566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 15.Casa LDC, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg. 2015;61:1068–1080. doi: 10.1016/j.jvs.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 16.Maurer-Spurej E, et al. Room temperature activates human blood platelets. Lab Invest. 2001;81:581–92. doi: 10.1038/labinvest.3780267. [DOI] [PubMed] [Google Scholar]
- 17.Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of Platelet Concentrates Stored at 22 C and 4 C. Transfusion. 1973;13:61–68. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- 18.Rock G, Figueredo A. Metabolic changes during platelet storage. Transfusion. 1976;16:571–9. doi: 10.1046/j.1537-2995.1976.16677060241.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi JW, Pai SH. Influence of storage temperature on the responsiveness of human platelets to agonists. Ann Clin Lab Sci. 2003;33:79–85. [PubMed] [Google Scholar]
- 20.Reddoch KM, et al. Hemostatic function of apheresis platelets stored at 4°c and 22°c. Shock. 2014;41(Suppl 1):54–61. doi: 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;41:105–13. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Kattlove HE, Alexander B. The effect of cold on platelets. I Cold-induced platelet aggregation. Blood. 1971;38:39–48. [PubMed] [Google Scholar]
- 23.Montgomery RK, Reddoch KM, Evani SJ, Cap AP, Ramasubramanian AK. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion. 2013;53:1520–1530. doi: 10.1111/j.1537-2995.2012.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Siyang, Yung Raylene, Tai Yu-Chong, Kasdan H. Determinstic lateral displacement mems device for continuous blood cell separation; 18th IEEE International Conference on Micro Electro Mechanical Systems, 2005. MEMS 2005; IEEE; 2005. pp. 851–854. [DOI] [Google Scholar]
- 25.Inglis DW, Lord M, Nordon RE. Scaling deterministic lateral displacement arrays for high throughput and dilution-free enrichment of leukocytes. J Micromechanics Microengineering. 2011;21:054024. [Google Scholar]
- 26.D’Silva J, Austin RH, Sturm JC. Inhibition of clot formation in deterministic lateral displacement arrays for processing large volumes of blood for rare cell capture. Lab Chip. 2015;15:2240–2247. doi: 10.1039/c4lc01409j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hur SC, Mach AJ, Di Carlo D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics. 2011;5:22206. doi: 10.1063/1.3576780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fachin F, et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci Rep. 2017;7:10936. doi: 10.1038/s41598-017-11119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mach AJ, Di Carlo D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol Bioeng. 2010;107:302–11. doi: 10.1002/bit.22833. [DOI] [PubMed] [Google Scholar]
- 30.Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH. The bioartificial kidney: current status and future promise. Pediatr Nephrol. 2014;29:343–51. doi: 10.1007/s00467-013-2467-y. [DOI] [PubMed] [Google Scholar]
- 31.Mutlu BR, et al. Non-equilibrium Inertial Separation Array for High-throughput, Large-volume Blood Fractionation. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-10295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong KHK, et al. Whole blood stabilization for the microfluidic isolation and molecular characterization of circulating tumor cells. Nat Commun. 2017;8:1733. doi: 10.1038/s41467-017-01705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N Engl J Med. 1995;332:1553–9. doi: 10.1056/NEJM199506083322306. [DOI] [PubMed] [Google Scholar]
- 34.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2010;42:63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281:850–857. doi: 10.1074/jbc.M504540200. [DOI] [PubMed] [Google Scholar]
- 36.Colace TV, Diamond SL. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol. 2013;33:105–113. doi: 10.1161/ATVBAHA.112.300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, et al. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 2011;121:593–603. doi: 10.1172/JCI41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong KHK, et al. The Role of Physical Stabilization in Whole Blood Preservation. Sci Rep. 2016;6 doi: 10.1038/srep21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandlin RD, et al. Preservative solution that stabilizes erythrocyte morphology and leukocyte viability under ambient conditions. Sci Rep. 2017;7:5658. doi: 10.1038/s41598-017-05978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 41.Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86:222–232. [PubMed] [Google Scholar]
- 42.Savi P, et al. Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol. 1992;44:527–532. doi: 10.1016/0006-2952(92)90445-o. [DOI] [PubMed] [Google Scholar]
- 43.Gogia S, Neelamegham S. Role of fluid shear stress in regulating VWF structure, function and related blood disorders. Biorheology. 2015;52:319–335. doi: 10.3233/BIR-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng XL. ADAMTS13 and von Willebrand Factor in Thrombotic Thrombocytopenic Purpura. Annu Rev Med. 2015;66:211–225. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li GW, et al. Treatment of refractory thrombotic thrombocytopenic purpura with N-Acetylcysteine: A case report. Transfusion. 2014;54:1221–1224. doi: 10.1111/trf.12440. [DOI] [PubMed] [Google Scholar]
- 46.George JN, López JA, Konkle BA. N -Acetylcysteine: an old drug, a new insight, a potentially effective treatment for thrombotic thrombocytopenic purpura. Transfusion. 2014;54:1205–1207. doi: 10.1111/trf.12561. [DOI] [PubMed] [Google Scholar]
- 47.Tersteeg C, et al. Potential for Recombinant ADAMTS13 as an Effective Therapy for Acquired Thrombotic Thrombocytopenic Purpura. Arterioscler Thromb Vasc Biol. 2015;35:2336–2342. doi: 10.1161/ATVBAHA.115.306014. [DOI] [PubMed] [Google Scholar]
- 48.Tersteeg C, et al. Plasmin Cleavage of von Willebrand Factor as an Emergency Bypass for ADAMTS13 Deficiency in Thrombotic Microangiopathy. Circulation. 2014;129:1320–1331. doi: 10.1161/CIRCULATIONAHA.113.006727. [DOI] [PubMed] [Google Scholar]
- 49.Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet (London, England) 1977;2:432–4. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 50.Miller LF, Rumack BH. Clinical safety of high oral doses of acetylcysteine. Semin Oncol. 1983;10:76–85. [PubMed] [Google Scholar]
- 51.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–92. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sayani FA, Abrams CS. How I Treat How I treat refractory thrombotic thrombocytopenic purpura. Blood. 2015;125:3860–3867. doi: 10.1182/blood-2014-11-551580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tersteeg C, et al. Keeping von Willebrand Factor under Control: Alternatives for ADAMTS13. Semin Thromb Hemost. 2016;42:9–17. doi: 10.1055/s-0035-1564838. [DOI] [PubMed] [Google Scholar]