Abstract
Peripheral infiltration is defined as the inadvertent delivery of nonvesicant fluid or medication into surrounding tissue that has the potential to harm the patient. Vesicant fluid that has leaked into the tissue space is called extravasation. At present, there is no agreement in the literature on the best practice for managing these injuries in pediatric patients. The purpose of this study was to identify occurrences of peripheral infiltration injuries and examine treatment modalities used to treat pediatric patients who suffered such an injury.
Keywords: dressing, extravasation, healing, infiltration, interventions, intravenous, neonate, pediatric, physical therapy, vesicant
BACKGROUND AND OBJECTIVES
Intravenous (IV) infusion, a process for administering medication and fluid for various medical conditions, is often performed on pediatric patients in the hospital.1 All patients may be at risk for suffering an infiltration injury, but the pediatric population, which includes neonates, infants, and children, are especially at risk because of their small and weaker blood vessels, immature skin, lack of subdermal fat, and constant movement.1–3 Infiltration is defined as an inadvertent delivery of nonvesicant fluid or medication into surrounding tissue with the potential to harm the patient.4(S150) The resulting harm may include blistering of the skin, necrosis, pain, and infection.5 If a vesicant fluid or medication has been infused into the surrounding tissue space, it is called extravasation.4(S149) Vesicant fluids can be composed of, but not limited to, calcium, potassium, parenteral nutrition, acyclovir, and a variety of other substances.5,6 These infiltrations of vesicant and nonvesicant fluids can cause considerable damage to tissue and, in extreme cases, may result in compartment syndrome.5 Other factors that contribute to the severity of an infiltration injury include the osmolality of the infusing agent, duration of the exposure, pH of the solution, possible chemical irritation, and the mechanical pressure that has leaked into the tissue space, causing structural damage.7
The pediatric population is at greater risk than adults for infiltration because of many factors. Risk factors in the neonate population include a marked decrease in cell-layer thickness of the skin compared to that of an adult, a fragile vascular system because of decreased vessel diameter and delicate blood vessels, decreased adhesion between the dermis and epidermis, and highly flexible and distensible subcutaneous tissue.8 While these injuries also occur in older children, in general they are less severe because older children have the ability to voice distress before damage becomes extensive, unlike nonverbal or intubated neonates or infants.9 Because pediatric patients lack skin maturity, fluid leakage occurring at an infiltration site exerts pressure on the subdermal plexus, causing blood vessel deformity, which may result in ischemia.9 These mechanical forces may lead to severe injury in pediatric patients, even in an instance when infusion agents, such as isotonic IV fluids (eg, 0.9% sodium chloride), are being delivered. For the purposes of this article, all wounds resulting from damage that occurred as the result of vesicant and nonvesicant fluid infusions that were reviewed will be described as a peripheral infiltration injury (Figures 1 and 2).
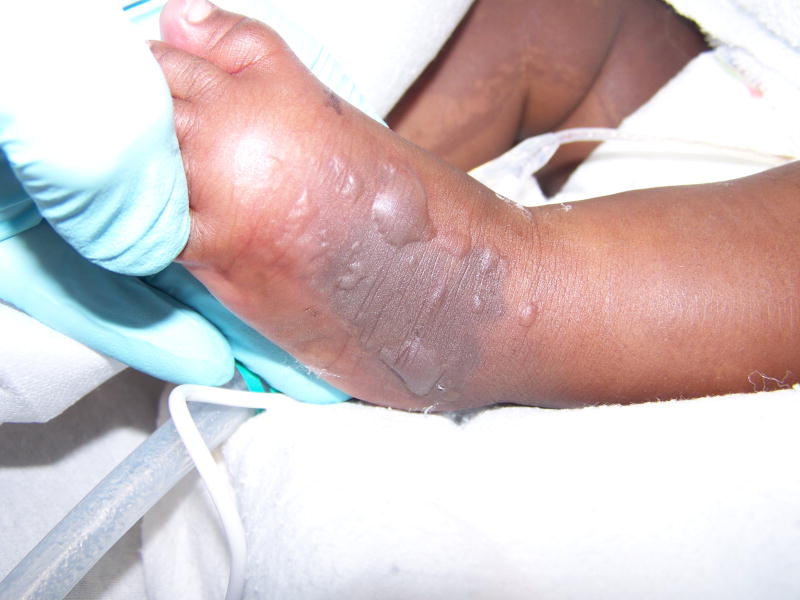
Figure 1.
Grade 4 peripheral infiltration injury on the dorsum of an infant’s left foot.
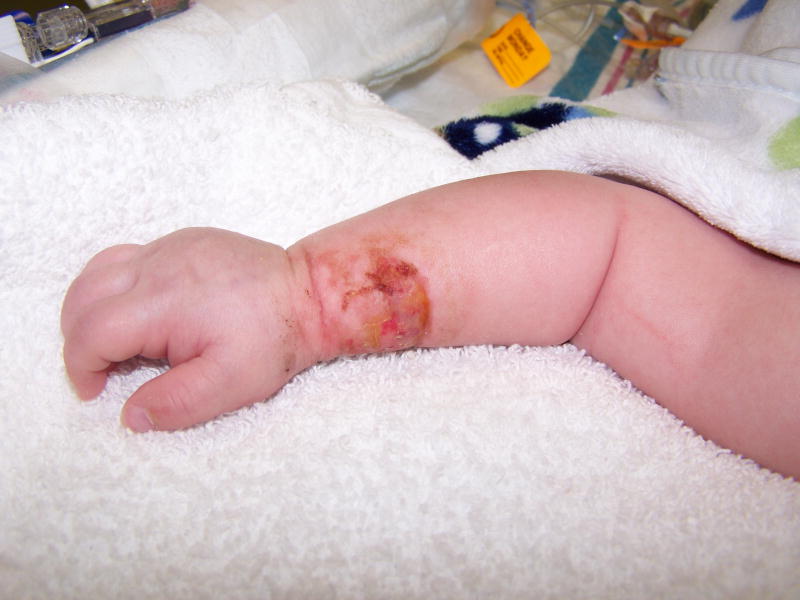
Figure 2.
Grade 4 peripheral infiltration injury on the right wrist of an infant.
Reported peripheral infiltration injury rates in pediatrics vary widely among children, infants, and neonates. The highest significant risk factor for developing an infiltration injury is based on age, with pediatric patients having the highest risk.10 This factor combined with other associated independent factors, such as the site of the injury, the type of fluid infused, the inability of the patient to communicate pain at the site, and smaller vessel vasculature, makes treatment challenging.2,9,10 Peripheral infiltration injury rates among pediatric patients receiving IV infusions can range from 10% to 30% as reported by Treadwell,11 or may be as high as 58% as reported by Gault.6 Higher injury rates are described in the neonate population with a reported range of up to 55% by Treadwell11 and a reported rate as high as 70% by Irving12 (Figures 3 and 4).
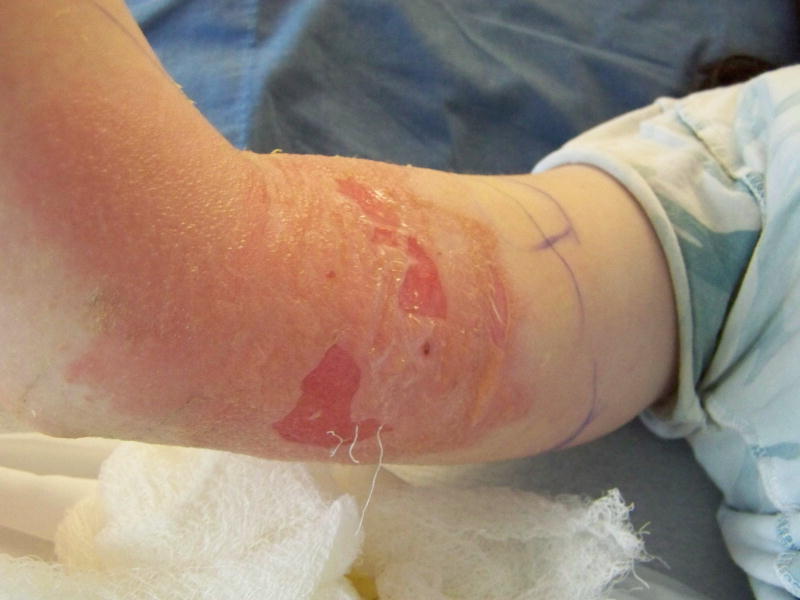
Figure 3.
Grade 4 peripheral infiltration injury on the right upper extremity of a child.
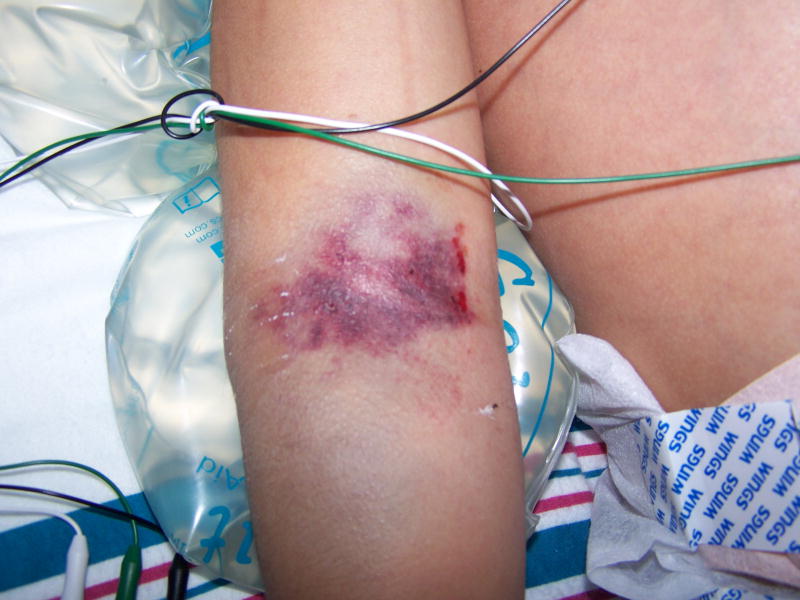
Figure 4.
Grade 4 peripheral infiltration injury on a child’s right antecubital region.
Treatment approaches used to treat peripheral infiltration injuries include elevation of the extremity, managing with a temperature modality (hot/cold), medications, and/or the application of various wound care products.4(S98–S101),13 While the common goal is to manage tissue damage after a peripheral infiltration injury, the best treatment remains controversial.7 Currently, there is no research on comparing outcomes of various treatment modalities for damaged, necrotic tissue in hospitalized children, infants, and neonates.13 To begin investigating this question, a retrospective study was conducted to examine the occurrences, courses of treatment, and outcomes related to peripheral infiltration injuries. This study was conducted at Arkansas Children’s Hospital (ACH). The Magnet-designated, nonprofit, 370-bed facility is the only pediatric hospital in the state with a level-1 trauma center, a burn facility, as well as critical care areas, which include an intensive care unit, a cardiac intensive care unit, and a neonatal intensive care unit (Figure 5).
Figure 5.
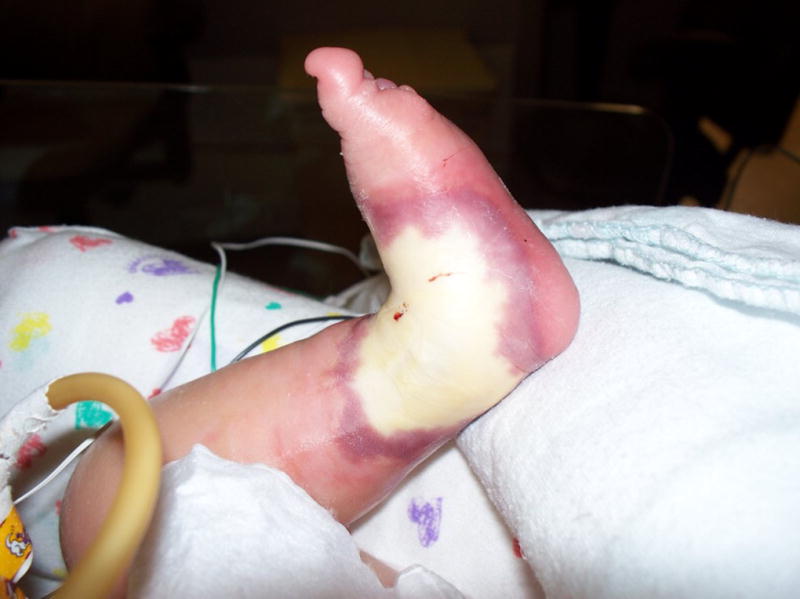
Acute peripheral infiltration injury on the left foot of an infant undergoing an infusion of lipids.
At ACH, nurses, physicians, and physical therapists are consulted to treat and manage moderate (grade 3) to severe (grade 4) peripheral infiltration injuries after acute insult occurs at the insertion site. The physicians supervising the patient care areas that include the burn, intensive care, and cardiovascular intensive care units establish the plan of care and treatment approaches for individual patients on each unit. Since peripheral infiltration injuries occur throughout the critical care areas, different physician or physical therapy teams can be consulted for individual patients. Physical therapy is consulted for more serious skin insults, because of the educational training received in the clinical doctorate program and the strong collaborative relationship it has with the nursing department. ACH has a burn trauma center which serves the region and nearby states. Burn physicians are consulted for more serious peripheral infiltration injuries secondary to on-site availability, as well as their knowledge of treating severe skin injury.
Grade 3 and 4 peripheral infiltration injuries typically have pain at the site, a level of swelling, blanching of the area, a decreased level of capillary refill, decreased or absent pulses, and, in the case of grade 4 injuries, an area of necrosis or skin blistering (Table 1). Acute treatments include removal of the vascular access device (VAD), elevation of the limb, use of a warm or cold compress, and the injection of an antidote to relieve tissue damage. At present, there is no agreement in the literature on the best practice for managing these injuries.14 The purpose of this retrospective study was to identify occurrences of peripheral infiltration injuries during 2014 and 2015 in all units at ACH and to examine the modalities used to treat these pediatric patients. The outcomes examined were the documented rate of time to granulation tissue and time to complete healing. An additional purpose of the study was to examine differences in treatments and products used by health care professionals treating the injuries.
TABLE 1.
Peripheral Infiltration Gradea
| Characteristics | |
|---|---|
| Grade |
Key points
|
| 0 |
|
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
Infiltration of blood products, irritants, and/or vesicants regardless of appearance
|
Pediatric PIV Infiltration Scale. Adapted from PIV Infiltration Scale courtesy of Children’s Medical Center, Dallas, Texas. Used with permission.
METHODS
Approval for the study was obtained from the institutional review boards of the University of Arkansas for Medical Sciences (UAMS) and ACH. A review of 158 ACH cases between January 2014 and December 2015 was performed to assess the prevalence, grade of peripheral infiltrate, and treatment and to examine the time to granulation tissue, as well as time to complete healing. A total of 147 cases met the inclusion criteria, which included treatment of children from birth to age 18 years (Table 2) who had suffered a grade 3 or grade 4 peripheral infiltration injury (Table 3). Cases were excluded if treatment occurred for injuries related to peripherally inserted central catheters, infusion ports, or umbilical catheters. Cases outside the specified age parameters also were excluded. Identified grade 1 and grade 2 peripheral infiltration injuries were not included in the review.
TABLE 2.
Demographic Characteristics of Study Subjects
| Gender | N = 147 |
| Male | 59% (n = 87) |
| Female | 41% (n = 60) |
| Ethnicity | N = 147 |
| White | 52% (n = 76) |
| Black | 31% (n = 46) |
| Hispanic/Latino | 12% (n = 18) |
| Other | 5% (n = 7) |
TABLE 3.
Site of Injury
| Site of Injury (N = 147) |
Right (n = 78) |
Left (n = 62) |
Other (n = 7) |
|---|---|---|---|
| Antecubital | 23% (n = 18) | 19% (n = 12) | |
| Upper extremity | 9% (n = 7) | 2% (n = 1) | |
| Forearm | 9% (n = 7) | 11% (n = 7) | |
| Wrist | 6% (n = 5) | 15% (n = 9) | |
| Hand | 24% (n = 19) | 29% (n = 18) | |
| Lower extremity | 6% (n = 5) | 5% (n = 3) | |
| Foot | 23% (n =18) | 19% (n = 12) | |
| Other | |||
| Arm | n = 2 | ||
| Foot | n = 2 | ||
| Scalp | n = 2 | ||
| Hand | n = 1 |
ACH is participating in a collaborative with Children’s Hospitals’ Solutions for Patient Safety Network and serves as a pioneer group for reduction in moderate to severe peripheral infiltrations.15 At the time of this study, the number of grade 3 and grade 4 infiltrations were comparable to those of other hospitals with similar patient populations and nursing staffs.
RESULTS
One hundred and forty-seven patients with peripheral infiltration were managed by nursing staff, with initial treatments consisting of removal of the VAD, use of warm compresses, elevation of the limb, use of cold packs, or a combination of these treatments. Nursing management also included an injectable antidote of hyaluronidase or phentolamine for 75 cases in which the VAD was removed. (Tables 4 and 5) None of the patients in the study required surgical intervention for wound healing or had an infection resulting from the wound. In addition, none of the cases reviewed developed compartment syndrome.
TABLE 4.
Nursing Treatments
| Nursing Care Provided at Time of Infiltration (N = 147) |
Percent of Care |
|---|---|
| Infusion catheter removed (n = 75) | 51% |
| ICR + heat (compress/pack) (n = 32) | 22% |
| ICR + elevation (n = 9) | 6% |
| ICR + heat + elevation (n = 28) | 19% |
| ICR + cold (compress/pack) (n =3) | 2% |
| Total | 100% |
Abbreviations: ICR, infusion catheter removed.
TABLE 5.
Injectable Antidote Treatments
| Injectable Treatment After Infusion Catheter Removed (n = 75) |
Percent of Care |
|---|---|
| Hyaluronidase (n = 25) | 33% |
| Phentolamine (n = 1) | 2% |
| ICR only (n = 49) | 65% |
| Total | 100% |
Abbreviation: ICR, infusion catheter removed.
After initial treatment by nursing staff, it was determined that 5 patients ranging in age from 3 days to 14 years old required specialized wound care from physical therapy services or the burn service team for the treatment of severe grade 4 peripheral infiltration burns. The burn service team typically consisted of 1 of a combination of one of the following combinations of an attending physician, a burn resident physician, and/or a burn nurse. The primary dressing used by the burn team for 2 patients was Polysporin antibiotic (Johnson & Johnson: New Brunswick, NJ) with Xeroform (Medtronic: Minneapolis, MN) combination covered with sterile gauze, which was changed daily. Healing ranged between 13 to 25 treatment days, with an average healing time of 19 days.
The primary dressing used by physical therapy services to treat grade 4 infiltration burns was a Mepilex or Mepilex Ag foam dressing (Mölnlycke Health Care: Gothenburg, Sweden). Three patients were treated, with treatment days ranging from 9 to 25 and an average healing time of 17 days. One patient was not included in the healing-time average because the patient had been discharged, and the healing assessment could not be completed (Table 6).
TABLE 6.
Case Study Comparisons
| Case | Service Provider |
Location | Peripheral Infiltration Injury Grade |
Patient Age |
Infusion Substance |
Treatment/Product Used |
Days to Heal |
|---|---|---|---|---|---|---|---|
| Case 1 | Burn team | Dorsum right hand | 4 | 14 years | Cytoxan | Polysporin-Xeroform | 13 |
| Case 2 | Burn team | Left foot | 4 | 6 months | D5 0.45% NS plus 20 mEq KCl | Polysporin-Xeroform | 25 |
| Case 3 | Physical therapy | Right foot | 4 | 3 days | Lipids and TPN | Mepilex Ag | 9 |
| Case 4 | Physical therapy | Right arm (AC) | 4 | 1 month | Lipids and TPN | Mepilex | 25 |
| Case 5 | Physical therapy | Right wrist | 4 | 4 months | D5 0.45% NS plus 20 mEq KCl | Mepilex | Discharge home–unknown |
Abbreviations: AC, antecubital; TPN, total parenteral nutrition; KCl, potassium chloride; NS, normal saline.
CONCLUSIONS
Currently, the initial treatment approach, which is managed by nursing, has been effective for the majority of grade 3 and 4 peripheral infiltration injuries. These initial treatments were consistent with the findings of Thigpen7 and Treadwell,11 and the Infusion Nurses Society’s Infusion Therapy Standards of Practice,4(S98–S101) which describe immediate discontinuation of the infusion, possible use of a modality, and treatment with an injectable antidote.7,11,14 Early identification of the injury and prompt removal of the IV catheter appear to reduce the potential for injury and may reduce the need for involvement by non-nursing disciplines. In this retrospective study, there was no use of unique medications that differed from the medications/fluids that have been described previously in the literature and that resulted in infiltration injury. Consistent with the findings of Treadwell and multiple authors, substances frequently associated with a higher risk for causing infiltrate injuries in pediatric patients were also involved in the reviewed cases.5–7,11 For the cases requiring consultation with physical therapists or physicians, there was no standard treatment protocol that was implemented across disciplines or critical care areas, although all treatment approaches resulted in full wound healing without initial treatment changes. Because a small sample size required treatment beyond nursing management (n = 5), comparisons of specific treatments and products used for peripheral infiltration injuries in pediatric patients were not performed. The management of all the cases reviewed resulted in full healing of the wound sites without surgical intervention or the development of compartment syndrome.
CLINCIAL RELEVANCE.
Prevention is the most important element in the treatment of infiltration injuries. Identification of risk factors for infiltration in the pediatric population is paramount to patient safety and for reducing the opportunity for adverse events to occur. When other disciplines, physical therapy and medicine, were consulted about severe grade 4 infiltrates, there was no established treatment regimen that was consistently performed. More research is needed to compare outcomes of various treatment modalities to determine best practice for the treatment of peripheral infiltration injury requiring additional consultation.
Acknowledgments
Drs Yates and Lowe received a grant from the National Institute of General Medical Sciences, IDeA Program Award P30 GM 110702.
Biographies
Brian Odom, MS, PT, CWS, is an assistant professor in the physical therapy program at Harding University, where he teaches integumentary, cardiopulmonary, and clinical reasoning. Board-certified in wound care, he practices at Arkansas Children’s Hospital. His emphasis in wound care focuses on acute trauma, pressure ulcers, and wounds in intensive care units. He is also a PhD student at the University of Central Arkansas.
Charlotte Yates, PhD, PT, PCS, is an associate professor at the University of Central Arkansas, where she teaches neuroscience, pediatrics, and integumentary. She is a research faculty member at the Center for Translational Neuroscience. A board-certified clinical specialist in pediatrics, Dr Yates practices at Arkansas Children’s Hospital. Her emphasis on wound care is acute trauma.
Leah Lowe, PhD, DPT, PT, PCS, is an assistant professor of physical therapy at the University of Central Arkansas. She teaches in the pediatrics coursework and is the course director for Physical Therapy Research 1–2. She is a board-certified pediatric clinical specialist and practices at Pediatrics Plus, a specialized pediatric health care provider.
References
- 1.Park SM, Jeong IS, Kim KL, Park KJ, Jung MJ, Jun SS. The Effect of Intravenous Infiltration Management Program for Hospitalized Children. J Pediatr Nurs. 2016;31(2):172–178. doi: 10.1016/j.pedn.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Lehr VT, Lulic-Botica M, Lindblad WJ, Kazzi NJ, Aranda JV. Management of infiltration injury in neonates using duoderm hydroactive gel. Am J Perinatol. 2004;21(7):409–414. doi: 10.1055/s-2004-835309. [DOI] [PubMed] [Google Scholar]
- 3.Thomas S, Rowe HN, Keats J, Morgan RJH. The management of extravasation injury in neonates. [Accessed February 22, 2018];World Wide Wounds. http://www.worldwidewounds.com/1997/october/Neonates/Neonate.html. Published October 23, 1997.
- 4.Gorksi L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infus Nurs. 2016;39(suppl 1):S1–S159. [Google Scholar]
- 5.Beall V, Hall B, Mulholland J, Gephart S. Neonatal Extravasation: An Overview and Algorithm for Evidence-based Treatment. Newborn and Infant Nurs Rev. 2013;13(4):189–195. [Google Scholar]
- 6.Gault DT. Extravasation injuries. British J Plast Surg. 1993;46(2):91–96. doi: 10.1016/0007-1226(93)90137-z. [DOI] [PubMed] [Google Scholar]
- 7.Thigpen JL. Peripheral intravenous extravasation: nursing procedure for initial treatment. Neonatal Netw. 2007;26(6):379–384. doi: 10.1891/0730-0832.26.6.379. [DOI] [PubMed] [Google Scholar]
- 8.McCullen KL, Pieper B. A retrospective chart review of risk factors for extravasation among neonates receiving peripheral intravascular fluids. J Wound Ostomy Continence Nurs. 2006;33(2):133–139. doi: 10.1097/00152192-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Amjad I, Murphy T, Nylander-Housholder L, Ranft A. A new approach to management of intravenous infiltration in pediatric patients. J Infus Nurs. 2011;34(4):242–249. doi: 10.1097/NAN.0b013e31821da1b3. [DOI] [PubMed] [Google Scholar]
- 10.Schmit B, Freshwater MF. Pediatric Infiltration Injury and Compartment Syndrome. J Craniofac Surg. 2009;20(4):1021–1024. doi: 10.1097/scs.0b013e3181abb16e. [DOI] [PubMed] [Google Scholar]
- 11.Treadwell T. The Management of Intravenous Infiltration Injuries in Infants and Children. Ostomy Wound Manage. 2012;58(7):40–44. [PubMed] [Google Scholar]
- 12.Irving V. Managing extravasation injuries in preterm neonates. Nurs Times. 2001;97(35):40, 43–46. [PubMed] [Google Scholar]
- 13.Association of Women’s Health, Obstetric and Neonatal Nurses. Evidence-Based Clinical Practice Guideline: Neonatal Skin Care. 3. Washington, DC: Association of Women’s Health, Obstetric and Neonatal Nurses; 2013. [Google Scholar]
- 14.Wilkins CE, Emmerson AJ. Extravasation injuries on regional neonatal units. Arch Dis Child Fetal Neonatal Ed. 2004;89(3):F274–F275. doi: 10.1136/adc.2003.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Children’s Hospitals’ Solutions for Patient Safety National Children’s Network website. [Accessed March 2018];Operational Definitions. http://www.solutionsforpatientsafety.org/wp-content/uploads/sps-operating-definitions.pdf. Published March 2018.