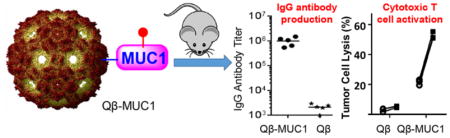
Abstract
Mucin-1 (MUC1) is one of the top ranked tumor associated antigens. In order to generate effective anti-MUC1 immune responses as potential anticancer vaccines, MUC1 peptides and glycopeptides have been covalently conjugated to bacteriophage Qβ. Immunization of mice with these constructs led to highly potent antibody responses with IgG titers over one million, which are among the highest anti-MUC1 IgG titers reported to date. Furthermore, the high IgG antibody levels persisted for more than six months. The constructs also elicited MUC1 specific cytotoxic T cells, which can selectively kill MUC1 positive tumor cells. The unique abilities of Qβ-MUC1 conjugates to powerfully induce both antibody and cytotoxic T cell immunity targeting tumor cells bode well for future translation of the constructs as anticancer vaccines.
Graphical Abstract
INTRODUCTION
Mucin-1 (MUC1), a glycoprotein overexpressed on the surface of a wide range of tumor cells, is an exciting antigenic target for antitumor vaccines.1,2 On normal cells, MUC1 is extensively glycosylated in its extracellular tandem repeat region with large and elongated O-linked glycans, which shield the protein backbone from the immune system. In comparison, tumor associated MUC1 is under-glycosylated with fewer and highly truncated O-glycans such as N-acetyl galactosamine (GalNAc) linked to a serine or threonine residue (Tn antigen).3 The glycosylation patterns structurally distinguish tumor associated MUC1 from that on normal cells.4 In addition, the expression levels of MUC1 on tumor cells can be 100 times higher than those on normal cells and the high MUC1 overexpressions are associated with increased tumor metastasis and shortened patient survival.1,5
There has been significant interest in harnessing MUC1 specific immune responses to combat cancer.1,6,7 Antibodies and cytotoxic T cells (CTLs) against MUC1 have been observed in some cancer patients, although their levels are typically too low to eradicate the growing tumors.5 To elicit strong MUC1 specific immunity, immunization with MUC1 alone is not sufficient. Conjugate vaccine candidates have been produced by linking synthetic MUC1 peptides with various carriers, including immunogenic proteins,8,9 nanoparticles,10,11 polymers,12 immunostimulating glycans,13,14 liposomes,15 synthetic platforms such as peptides, self-assembling systems, and calixrenes.16–18 Studies showed that most of these constructs can induce anti-MUC1 antibodies with typical titers of several thousands in mice. Clinical studies suggest that the levels of MUC1 antibodies can be positively correlated with better prognosis of patients.5 Thus, there is a continual need to develop vaccine constructs to induce higher titers of anti-MUC1 antibodies.
Besides antibodies, CTLs are another arm of adaptive immunity, complementing antitumor antibodies. The tandem repeat region of MUC1 contains multiple CTL epitopes.19 MUC1 specific CTLs can recognize epitopes presented by major histocompatibility class-I (MHC-I) on cell surface and directly kill MUC1 expressing tumor cells.20 Constructs capable of activating both MUC1 specific antibodies and cytotoxic T cells are attractive for cancer immunotherapy.
Virus-like particles (VLPs) are a class of biological nanoparticles formed through self-assembly of multiple monomer units.21 With their highly ordered structures, VLPs such as bacteriophage Qβ have great potentials as antigen carriers.22–24 As glycopeptides are important tumor antigens, we have become interested in evaluating whether Qβ is capable of enhancing anticancer responses against glycopeptides such as MUC1. Herein, we report that by conjugating MUC1 to Qβ, superior titers (over 2,000,000) of MUC1 specific IgG antibodies were elicited in mice, which were among the highest murine IgG titers reported to date. The antibodies lasted more than six months and killed tumor cells through complement mediated cytotoxicity. In addition, MUC1 specific CTLs were also generated following vaccination with cytotoxic activities against tumor associated human MUC1 bearing cells in vitro and in vivo. This is the first time that Qβ has been shown to potently boost immune responses against glycopeptide antigens.
RESULTS AND DISCUSSION
Synthesis of Qβ-MUC1 Conjugates
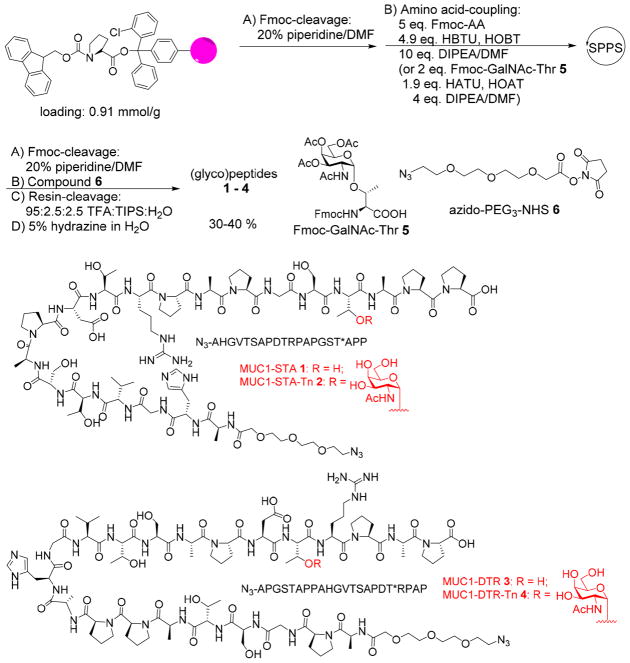
MUC1 has a large extracellular N-terminal domain, consisting of a variable number of tandem repeats of 20 amino acid residues with the sequence of PDTRPAPGSTAPPAHGVTSA.1,25 The five serine and threonine residues within each tandem repeat can be potentially glycosylated. For our vaccine studies, we designed MUC1 (glyco)peptides 1–4, which contain 20–22 amino acid residues as the backbone covering one full length of the tandem repeat region. Peptides 1 and 3 represent two possible sequences of the repeat region designated MUC1-STA and MUC1-DTR (STA and DTR are the three amino acid sequence containing a threonine closest to the C-terminus). To establish possible influence of glycosylation on immune responses, GalNAc was installed on the threonine residue closest to the C-terminus producing MUC1 glycopeptides 2 and 4 designated MUC1-STA-Tn and MUC1-DTR-Tn. We focused on GalNAc modified MUC1 as they are widely expressed in cancer26 and can potentially function as CTL epitopes.27
The synthesis of the MUC1 (glyco)peptides was performed through solid-phase peptide synthesis (SPPS) using Fmoc chemistry (Scheme 1). The coupling of Fmoc-protected amino acids to peptide chains was carried out with (2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/hydroxybenzotriazole (HOBt). For glycopeptide synthesis, Fmoc protected GalNAc-threonine 5 (Fmoc-GalNAc-Thr)10 was used as a building block, which was introduced into the peptide chain mediated by 1-[bis-(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]-pyridinium 3-oxid hexafluorophosphate (HATU)/1-hydroxy-7-azabenzotriazole (HOAt). After assembly of (glyco)peptides, the N-terminal Fmoc group was removed and an azide terminated linker azido-PEG3-NHS 628 was incorporated at the N-terminus. The resulting (glyco)peptides were cleaved from the resins by trifluoroacetic acid (TFA)/triisopropyl silane (TIPS)/H2O, and the O-acetates on the saccharide moiety were removed by 5% hydrazine in H2O. C18 reverse-phase HPLC purification produced the desired MUC1 (glyco)-peptides 1–4 in 30–40% yields.
Scheme 1.
Solid-Phase Synthesis of MUC1 (Glyco)peptides
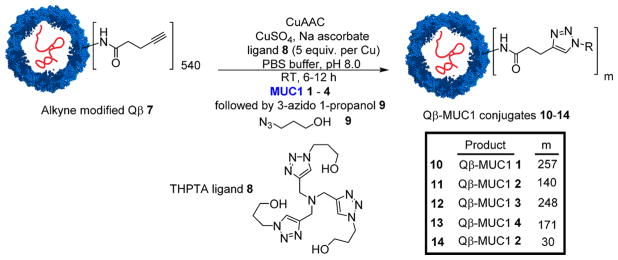
The ligation of MUC1 onto Qβ-VLP was performed with the copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction optimized for bioconjugations.29 Azide modified MUC1 peptides 1–4 were coupled with alkyne functionalized Qβ 724 promoted by a Cu catalyst with tris (3-hydroxypropyltriazolylmethyl)amine (THPTA) ligand 8 (Scheme 2). The average numbers of (glyco)peptides introduced onto Qβ were 257, 140, 248, and 171 (with an estimated distribution of ±15%) for conjugates 10–13, respectively (SI Figures S1–S4). The unreacted alkyne groups on Qβ capsids were capped using a large excess of 3-azido 1-propanol 9 by a second CuAAC reaction. By reducing the reagent concentration and reaction time during conjugation of MUC1 glycopeptide 2 with Qβ-alkyne 7, Qβ-MUC1 14 was also synthesized bearing on average 30 copies of MUC1 glycopeptide 2 per capsid for analysis of antigen density effects.
Scheme 2.
Synthesis of Qβ-MUC1 Conjugates
Qβ-MUC1 Conjugates Can Generate Robust Titers of anti-MUC1 IgG Antibodies and High Density of MUC1 is Critical for High Levels of IgG
With Qβ-MUC1 conjugates in hand, their abilities to induce immune responses were investigated. Groups of C57BL6 mice were immunized with the conjugates three times biweekly (i.e., injections on days 0, 14, and 28) at equal total MUC1 concentrations per injection. Serum samples were taken 1 week after the final boost (day 35) and antibody titers and subtypes were determined by enzyme linked immunosorbent assay (ELISA) against the specific MUC1 glycopeptide structure used for immunization.
The first parameter we investigated is the effect of local antigen density on antibody responses by comparing Qβ-MUC1 constructs 11 and 14 (Figure 1). The anti-MUC1 antibody responses elicited by Qβ-MUC1 11 were predominantly IgGs. The mean total IgG titers produced were 1,013,300, which was 500 times higher than titers from control mice immunized with Qβ only. Interestingly, despite receiving the same total amounts of MUC1, mice immunized with the Qβ-MUC1 14 gave average IgG antibody titers only 20% of those receiving Qβ-MUC1 11 with higher local density of MUC1. Furthermore, the intragroup variations of IgG titers by 11 were smaller than those induced by 14.
Figure 1.
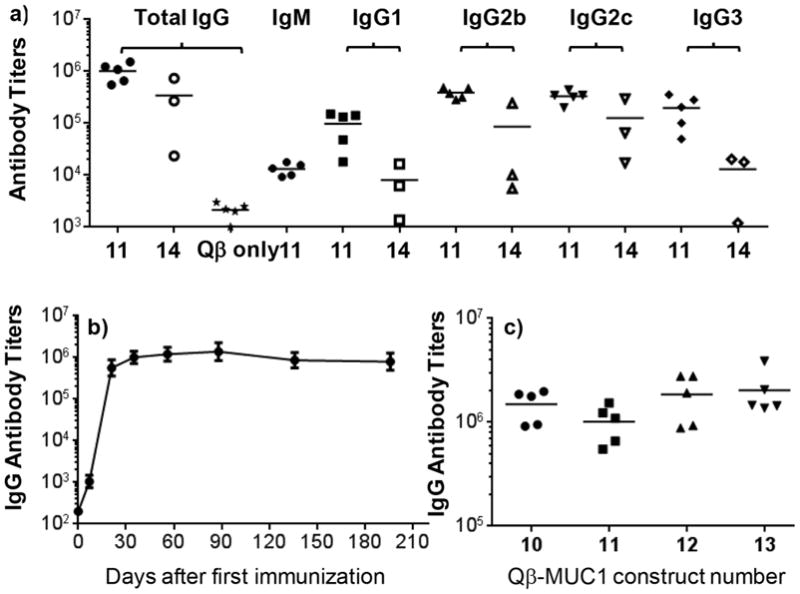
(a) Titers of anti-MUC1 total IgG and IgG subtypes from mice immunized with Qβ-MUC1 constructS 11 and 14, as well as Qβ only as the control. The average IgG titers induced by 11 were much higher than those by construct 14 containing low MUC1 density or Qβ only. (b) High anti-MUC1 IgG antibody titers induced by 11 lasted more than 200 days. (c) Anti-MUC1 IgG titers from mice immunized with Qβ-MUC1 constructs 10–13, respectively. All ELISA measurements were performed against the plated specific MUC1 glycopeptide used for immunization.
Subtyping of IgG titers indicated that all major subtypes of IgGs including IgG1, IgG2b, and IgG2c were elicited (Figure 1a). Construct 11 also produced a large amount of IgG3 antibody, a subtype of IgG antibodies in mice that is traditionally induced by carbohydrate antigens.30
We next examined the kinetics of MUC1 antibody generation as well as antibody persistence. IgG antibody responses to construct 11 approached the peak value 21 days after the first immunization. The super high IgG titers maintained for more than 6 months highlighting the power of Qβ as a carrier for glycopeptide for inducing long lasting immune responses (Figure 1b).
In order to establish the generality of Qβ as a MUC1 carrier, other Qβ-MUC1 constructs were tested following the same immunization protocol as for 11. As shown in Figure 1c, all these Qβ-MUC1 vaccines elicited consistent and high anti-MUC1 IgG titers comparable to that of Qβ-MUC1 11 with the average IgG titers from mice immunized with Qβ-MUC1 13 exceeding 2,000,000. As a negative control, groups of C57BL6 mice were immunized with MUC1 glycopeptide admixed Qβ without the covalent conjugation. As shown in SI Figure S6, the anti-MUC1 IgG antibody titers induced were below 400, thus suggesting covalent conjugation of MUC1 glycopeptide with Qβ is critical for production of high antibody titers.
Microarray Analysis of the Antibodies Induced by Qβ-MUC1 Conjugates 10–13
To better understand the binding specificity, the post-immune sera from Qβ-MUC1 10–13 vaccination were screened against a (glyco)peptide microarray with various MUC1 glycopeptides in addition to mucin-5 glycopeptides, mucins from porcine stomach and bovine submaxillary glands, as well as several other glycoproteins (SI Figure S7).4 On the microarray, there are 72 MUC1 glycopeptides each with one MUC1 tandem repeat PAHGVT*SAPDT*RPAPGST*A (* denotes the potential glycosylation sites). The glycan structures are diverse, which include Tn, Thomsen-Friedenreich (T) antigen, as well as a number of core 1, core 2, and core 3 oligosaccharides (SI Figure S7). The microarray slides were incubated with individual mouse serum and unbound antibodies were removed by thorough washing. A fluorescently labeled secondary antibody was then added to the microarray to quantify the relative amounts of serum antibody bound to individual array components.
As shown in SI Figure S7, the antibodies induced by Qβ-MUC1 conjugates 10–13 exhibited broad and strong recognition to almost all MUC1 glycopeptides carrying Tn, T, core1, core2, core3, or mix glycans. This suggests a broad repertoire of anti-MUC1 IgG antibodies were elicited through vaccination, which bodes well for anticancer vaccine development as glycosylations of MUC1 proteins on tumor cell surface are generally heterogeneous. The antibodies were specific to MUC1 as there was little binding to mucin-5 glycopeptides or to any other proteins.
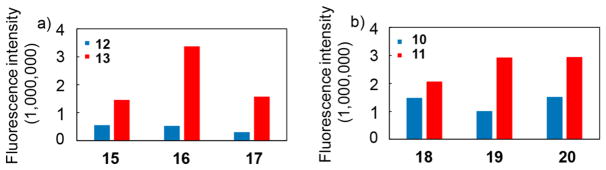
Close examination of microarray binding profiles revealed interesting binding trend. MUC1 glycopeptides 15–17 PAHGVTSAPDT*RPAPGSTA differed only in the glycan structure attached to the threonine in the middle of the peptide chain (* represents the location of glycosylation: Tn for 15, T for 16, and a core 2 hexasaccharide C2T2Hex for 17; for glycan structures, see SI Figure S7). Despite the larger size of T antigen and the core 2 hexasaccharide compared to Tn, all three glycopeptides were recognized well (Figure 2a). Immunogen Qβ-MUC1 13 contained Tn in the DTR region of its MUC1 and the antibodies induced by Qβ-MUC1 13 exhibited much stronger binding to the glycopeptides 15–17 compared to Qβ-MUC1 12 lacking any glycans. The same phenomena were observed of antibodies induced by 11 (glycosylation in the STA region) vs 10 against glycopeptides 18–20 PAHGVTSAPDTRPAPGST*A (* represents the location of glycosylation: Tn for 18, T for 19, and a core 2 hexasaccharide for 20; Figure 2b).
Figure 2.
MUC1 glycopeptide microarray screening showed that IgG antibodies generated could recognize MUC1 glycopeptides bearing a wide range of glycan structures suggesting that a broad repertoire of antibodies was produced. Immunization with glycosylated MUC1 antigen (13 and 11) led to stronger binding to glycopeptides compared to the nonglycosylated counterparts (12 and 10).
IgG Antibodies Induced by Qβ-MUC1 Conjugates Are Capable of Binding MUC1 Expressing Tumor Cells and Selectively Killing Tumor Cells through Complement Mediated Cytotoxicity
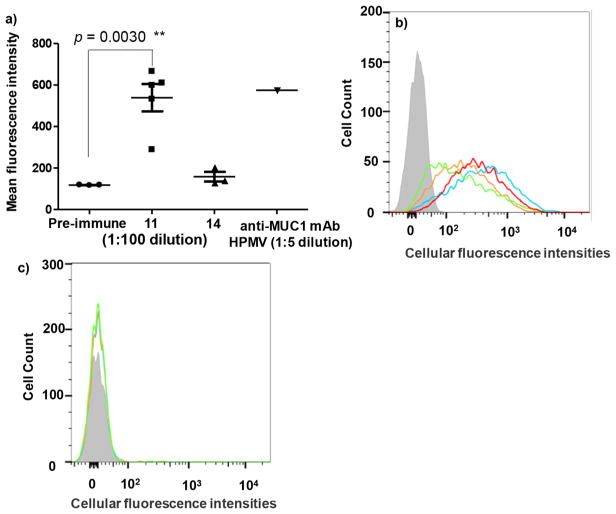
For an effective vaccine, it is critical that antibodies generated can recognize the antigen expressed in its native environment, i.e., on tumor cells. To establish this, we performed flow cytometry studies using MUC1 transfected mouse lymphoma cell RMA-MUC1. RMA-MUC1 cells express human MUC1 on the cell surface as confirmed by cellular binding with a commercially available anti-MUC1 mAb HPMV at 1:5 dilution (Figure 3a).
Figure 3.
Flow cytometry analysis of the specific recognition of tumor cells by anti-MUC1 IgG antibodies. (a) Mean fluorescence intensities of binding of RMA-MUC1 cells by pre-immune sera and sera from mice immunized with vaccine constructs 11 and 14 respectively (1:100 dilution); MUC1 expression on RMA-MUC1 was confirmed by anti-MUC1 mAb HPMV (1:5 dilution). Immunization with 11 induced antibodies capable of binding RMA-MUC1 much stronger than those from 14 immunized mice. (b) Binding of RMA-MUC1 cells by sera from mice immunized with 10 (blue curve), 11 (orange curve), 12 (green curve), and 13 (red curve) at 1:50 dilution. The gray filled trace was from pre-immune serum. All post-immune sera showed strong binding to RMA-MUC1 cells. (c) Little binding to RMA cells lacking MUC1 was observed with anti-MUC1 sera at 1:50 dilution.
To test the recognition of RMA-MUC1 cells by the post-immune sera, RMA-MUC1 cells were incubated with the sera. After washing off unbound antibodies, cells were treated with a fluorescently labeled anti-IgG secondary antibody. The sera from pre-immunized mice gave little binding to RMA-MUC1 cells (Figure 3a). In contrast, the post-immune sera exhibited good recognition of RMA-MUC1 cells even at 1:100 dilution (Figure 3a). Consistent with the ELISA results on the impact of MUC1 density on Qβ, stronger binding was observed from mouse sera following immunization with vaccine construct 11 vs 14 (low MUC1 density) (Figure 3a). The binding of antibodies induced by 11 to tumor cells was MUC1 dependent, as the post-immune sera did not exhibit significant recognition of RMA cells lacking the MUC1 transgene demonstrating the specificities of the antibodies (Figure 3b vs c).
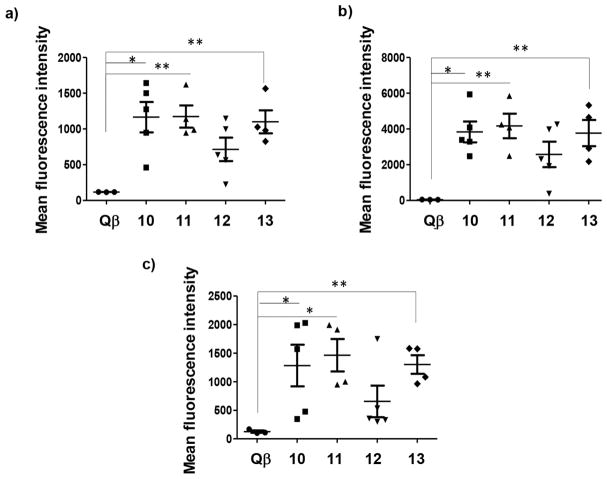
To test the generality of tumor cell recognition, besides RMA-MUC1 cells, binding to mouse melanoma B16-MUC1 cells as well as breast cancer MCF-7 cells were measured with the post-immune sera. Mice immunized with any of the three Qβ-MUC1 constructs 10, 11, and 13 produced antibodies capable of strong recognition of all MUC1 expressing tumor cells tested (Figure 4). Antibodies induced by construct 12 with nonglycosylated peptide 3 showed weaker binding to MUC1 expressing tumor cells, suggesting that Tn glycosylation of MUC1 glycopeptide in PDT*R domain contributes to the generation of antibodies for stronger tumor cell binding.
Figure 4.
Flow cytometry analysis showed that immunization with Qβ-MUC1 conjugates 10–13 induced IgG antibodies capable of binding with a panel of MUC1 expressing tumor cells stronger than sera from mice immunized with Qβ only. Mean fluorescence intensities of binding to (a) RMA-MUC1 cells; (b) B16-MUC1 cells; and (c) MCF-7 cells. Binding was tested with 1:20 dilutions of the post-immune sera. * p < 0.05, ** p < 0.01, *** p < 0.001. The p values were determined through a two-tailed t-test using GraphPad Prism.
With the strong recognition of MUC1 expressing tumor cells, the abilities of the post-immune sera to kill the tumor cells were measured. Incubation of MUC1 expressing tumor cells with the post-immune sera and rabbit complement led to significantly higher percentages of tumor cell death compared to cells treated with control sera (Figure 5 and SI Figure S5). Post-immune sera could kill tumor cells efficiently and in a MUC1-dependent manner.
Figure 5.
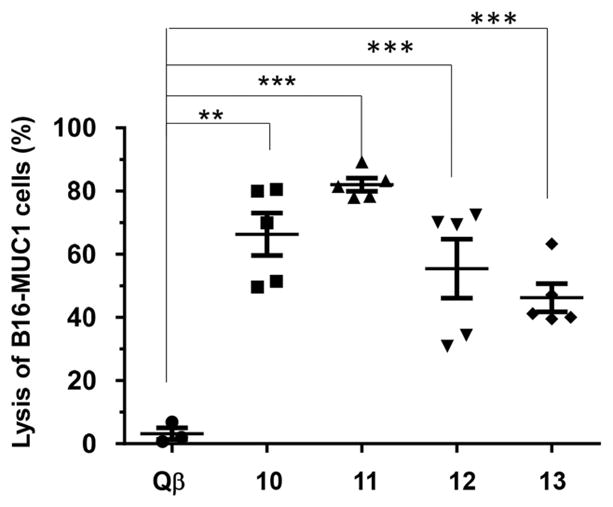
Complement dependent cytotoxicity to B16-MUC1 cells induced by sera from mice immunized with Qβ control, Qβ-MUC1 10–13, respectively (**p < 0.01; ***p < 0.001). The p values were determined through a two-tailed t-test using GraphPad Prism.
Immunization with Qβ-MUC1 Can Induce MUC1 Specific CTLs in Vitro and in Vivo
MUC1 is known to contain several CTL epitopes within its tandem repeat regions.27,31 As one Qβ capsid can deliver hundreds of copies of MUC1, we tested whether Qβ-MUC1 constructs can elicit MUC1 specific CTL responses in immunized mice.
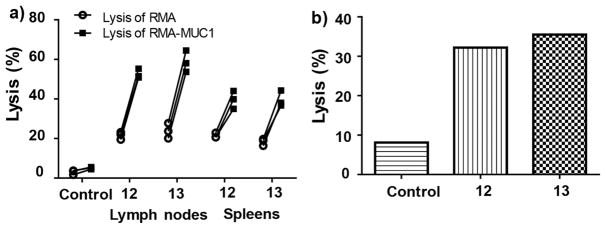
MUC1 specific cytolytic activities were first measured using an in vitro CTL assay. The spleens and lymph nodes were harvested from mice immunized with constructs 12 and 13 as well as from control mice immunized with Qβ only. Splenocytes and lymph node cells were isolated and incubated with RMA-MUC1 and RMA cells, respectively, and the viabilities of the tumor cells were measured. As shown in Figure 6a, cells from mice receiving Qβ only did not lead to significant death of either RMA or RMA-MUC1 cells indicating Qβ by itself was not effective in generating antitumor CTL responses. In comparison, lymph node cells from Qβ-MUC1 immunized mice led to significantly higher lysis of RMA-MUC1 cells than RMA cells, suggesting that MUC1 dependent CTL activities were generated by Qβ-MUC1 (Figure 6a). Similar phenomena have been observed with spleen cells from the immunized mice.
Figure 6.
MUC1-specific CTL activities have been elicited through immunization with Qβ -MUC1 constructs. The CTL activities were analyzed (a) in vitro and (b) in vivo. Lymphocytes were harvested from lymph nodes and spleen of mice immunized with Qβ (control) or conjugates 12, 13, and analyzed for their cytotoxic activities against RMA and RMA-MUC1 cells by flow cytometry. (b) CFSE labeled syngeneic splenocytes pulsed with MUC1 (CFSEhigh) or not (CFSElow) were injected intravenously into mice immunized with Qβ (control) or Qβ-MUC1 construct 12 or 13. After 24 h, mice were sacrificed and lymph nodes were harvested. Analysis by flow cytometry showed significantly higher lysis of MUC1 pulsed target cells. Control groups for both panels were mice immunized with Qβ only.
An in vivo cytotoxicity assay for CTLs was carried out. Splenocytes from naïve mice were harvested and labeled with two different concentrations of carboxyfluorescein succinimidyl ester (CFSE).32 The CFSEhigh cells were pulsed with a mixture of MUC1 (glyco)peptides 3 or 4, mixed with the same number of nonpulsed CFSElow cells and intravenously injected into mice immunized with Qβ-MUC1 constructs 12 and 13. As shown in Figure 6b, CFSEhigh cells were lysed much more than the CFSElow cells that were not incubated with MUC1, suggesting Qβ-MUC1 vaccinations led to activation and expansion of CTLs specific against MUC1.
Discussion
Tumor associated carbohydrate antigens are appealing targets for the development of anticancer vaccines.33 Virus-like particles such as Qβ have become a powerful class of carriers for vaccine development during the past 20 years.22,23 The Qβ VLP consist of 180 copies of a monomeric capsid protein assembled in an icosahedral manner with a diameter of 28 nm.34 As a result, antigens can be displayed on the external surfaces of Qβ in a highly organized manner, which can cross-link B cell receptors effectively resulting in potent B cell activation for antibody secretion. Qβ has been shown to be able to boost antibody responses against antigens such as carbohydrates,35–37 antigenic determinant from glycolipids,38 proteins,39 and small molecular haptens such as nicotine.40 This is the first time that tumor associated glycopeptides have been conjugated with Qβ for anticancer vaccine development. Super high titers (over 1 million) of anti-MUC1 IgG as well as all subtypes of IgG antibodies have been generated, which are much higher than titers (typically several thousands) elicited by other carriers.8–18
The density of antigen in an immunogen is an important factor for B cell potentiation.41 Dintzis and co-workers have shown that haptens with spacing between 5 and 10 nm exhibited the strongest activation of B cells.42 Significant deviation from this range reduces antibody production. Similar phenomena were reported by Kiessling43 and Plough44 groups as well as our own studies using polymers.45 The external surface of each Qβ monomer unit has three lysines (K2, K13, and K16), which together with the free amine at the N-terminus gives four potential sites for conjugation.34 The alkyne functionalized Qβ bears 540 copies of alkyne out of the maximum 720 potential sites.24 Based on analysis of the crystal structure of Qβ, functionalization of neighboring lysines by MUC1 would place the antigens within the optimal distances for B cell activation.34 Comparison of Qβ-MUC1 14 (30 copies of MUC1 per capsid) vs Qβ-MUC1 11 (high valency 140 copies of MUC1 per capsid) showed that while both constructs elicited significant amounts of anti-MUC1 IgG antibodies, superior responses were obtained from construct 11 with higher valency and local density of MUC1 antigen. This is presumably because the distances between MUC1 glycopeptides in construct 14 are too large for effective cross-linking of B cell receptors. The ability to present antigens in high local density is a significant advantage of VLPs such as Qβ as B cell antigen carrier.
CuAAC reaction is a popular reaction for conjugating carbohydrate antigens to carriers for vaccine studies,35,46,47 but it is possible for the resulting triazole linker to be immunogenic. In our studies of Tn based vaccine design, constructs of Tn and Qβ conjugated with a triazole linker elicited antitriazole antibodies.36 The desired anti-Tn IgG responses were significantly suppressed presumably due to sequestration of vaccine constructs by antitriazole antibodies, preventing an effective boost of anti-Tn responses. The use of larger glycan antigens attached via triazoles have shown low antitriazole immune response compared to antiglycan responses in other studies.35,37 In the current study, CuAAC reaction was utilized to attach MUC1 glycopeptides onto Qβ for synthetic ease as the CuAAC is orthogonal to the side chain functional groups present in MUC1. Compared to the small hapten of Tn, the much larger MUC1 may reduce the accessibility of the triazole by antitriazole antibodies. Anti-MUC1 immunities were generated by these Qβ-MUC1 constructs, which recognized and killed MUC1 expressing tumor cells by both complement mediated cytotoxicity and CTLs. It is possible that replacing the triazole with a flexible amide linker to attach MUC1 to Qβ can enhance the anti-MUC1 antibody titers as well as tumor cell binding as observed in Tn studies.36 This will be investigated in the future.
MUC1 is expressed as a glycoprotein on tumor cells with five potential glycosylation sites within one 20-amino-acid residue tandem repeat.1,25 As glycosylation of tumor associated MUC1 is heterogeneous, there are many possible MUC1 sequences for immunogen design. Within the MUC1 backbone, it is known that the most frequent minimal epitopic sequence recognized by IgG and IgM antibodies is RPAPGS, followed by PPAHGVT and PDTRP.48 Glycosylation has been shown to significantly enhance the immunogenicity of MUC1 antigen.49 One MUC1 tandem repeat region contains five potential glycosylation sites and tumor associated glycans include Tn, Tf, STn, and STf. On tumor associated MUC1, glycan structures as well as site occupancy can significantly vary. To generate antibodies targeting MUC1 expressing tumor cells, a variety of MUC1 epitopes have been investigated, which contain glycans on all five glycosylation sites as well as larger glycans such as STn and ST.7 For the current study in wild-type mice, the Tn bearing glycopeptide antigens elicited super high IgG antibody titers, which recognized a wide range of MUC1 structures including those with much larger glycans than Tn. This is possibly due to the powerful immune activation by Qβ. Glycopeptide synthesis is challenging especially if multiple highly complex glycan chains need to be introduced into the immunogen. The relatively simple MUC1 structure adapted in our studies is attractive for future translation.
Antibody and CTL are two arms of adaptive immunity. While many of the MUC1 vaccine studies have focused on humoral responses, comprehensive CTL responses encompassing both antibody production and CTL activation are desirable.15 Besides activating B cells to produce anti-MUC1 IgG antibodies, Qβ-MUC1 constructs also activated MUC1 specific CTLs both in vitro and in vivo. This is most likely due to the uptake of Qβ-MUC1 by antigen presenting cells such as dendritic cells. Intracellular protease digestion of Qβ-MUC1 can release MUC1, which can be cross-presented by MHC class I on the surface of these antigen presenting cells for activation of matched CTLs. Although CTLs do not require multivalent display of CTL epitopes on carrier surface as do B cells, the high density of epitopes on Qβ enables one Qβ particle to deliver over one hundred copies of CTL peptides into a cell, which can effectively increase intracellular concentration of the (glyco)peptide antigen in antigen presenting cells for CTL activation. It is known to be difficult for antigen presenting cells to process and present MUC1 glycopeptides bearing glycans larger than Tn on MHCs due to the steric hindrance posted by the glycan moiety.31 Thus, it is advantageous to use MUC1-Tn as antigen as it can be processed for CTL generation, while at the same time helping to generate antibodies capable of recognizing native glycoproteins on tumor cells.
In conclusion, the Qβ-MUC1 vaccines not only elicited potent and long-lasting anti-MUC1 IgG responses, but also induced robust MUC1-specific cytotoxic T cell responses. Given the prevalence and importance of MUC1 in tumor progression or metastasis, Qβ represents a promising carrier for developing anti-MUC1 vaccine against cancer.
MATERIALS AND METHODS
General Experimental Procedures and Methods for Synthesis
All chemicals were reagent grade and were used as received from the manufacturer, unless otherwise noted. Centrifugal filter units of 10,000 and 100,000 molecular weight cutoff (MWCO) were purchased from EMD Millipore. For protein liquid chromatography, GE ÄKTA Explorer (Amersham Pharmacia) on a Superose-6 column was used. Microfluidic capillary gel electrophoresis was performed with Bioanalyzer 2100 Protein 80 microfluidics chip (Agilent Technologies). For MALDI-TOF MS analysis, each viral sample (10 μL, 1 mg mL−1) was denatured and cleaned using Cleanup C18 Pipette Tips (Agilent Technologies). The mixture (0.6 μL) and matrix solution (0.6 μL, saturated sinapic acid in 50% acetonitrile, 0.1% trifluoroacetic acid) was spotted on a MALDI plate, air-dried, and analyzed by MALDI-TOF mass spectrometry (AB SCIEX Voyager DE Pro MALDI-TOF). Protein concentration was measured using the Coomassie Plus Protein Reagent (Bradford Assay, Pierce) with bovine serum albumin (BSA) as the standard.
RMA cells, RMA-MUC1 cells, and MCF-7 cells were kindly provided by Prof. Olivera J. Finn (University of Pittsburgh). B16-MUC1 cells were kindly provided by Prof. Sandra J. Gendler (Mayo Clinic). RMA cells, RMA-MUC1 cells, and B16-MUC1 cells were cultured in DMEM supplemented with 10% FBS, Penicillin (100 U mL−1)/Streptomycin (100 μg mL−1), 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.3 mg mL−1 G418. MCF-7 cells were cultured in Eagle’s minimum essential medium with L-glutamine (2 mM), nonessential amino acids and sodium pyruvate, bovine insulin (10 μg mL−1), FBS (10%), and Penicillin (100 U mL−1)/Streptomycin (100 μg mL−1).
Supplementary Material
Acknowledgments
We are grateful to the National Cancer Institute, National Institutes of Health (Grant R01CA225101), the Deutsche Forschungsgemeinschaft, DFG (WE 4751/2-1), Fonds der Chemischen Industrie (Liebig fellowship to UW, Li 184/01), Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen and the Bundesministerium für Bildung und Forschung for financial support of our work. We would like to thank O. J. Finn (Univ. of Pittsburgh) and S. J. Gendler (Mayo Clinic) for kindly providing us the cells.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschem-bio.8b00313.
Experimental procedures and characterization including spectra, electrophesis, chromatography, cytotoxicity and ELISA (PDF)
References
- 1.Tang CK, Apostolopoulos V. Strategies used for MUC1 immunotherapy: preclinical studies. Expert Rev Vaccines. 2008;7:951–962. doi: 10.1586/14760584.7.7.951. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 2.Cheever M, Allison J, Ferris A, Finn O, Hastings B, Hecht T, Mellman I, Prindiville S, Viner J, Weiner L, Matrisian L. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA, Taylor-Papadimitriou J, Burchell J, Clausen H. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–1313. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pett C, Cai H, Liu J, Palitzsch B, Schorlemer M, Hartmann S, Stergiou N, Lu M, Kunz H, Schmitt E, Westerlind U. Microarray analysis of antibodies induced with synthetic antitumor vaccines: specificity against diverse mucin core structures. Chem - Eur J. 2017;23:3875–3884. doi: 10.1002/chem.201603921. [DOI] [PubMed] [Google Scholar]
- 5.Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 6.Hossain MK, Wall KA. Immunological evaluation of recent MUC1 glycopeptide cancer vaccines. Vaccines. 2016;4:25. doi: 10.3390/vaccines4030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaidzik N, Westerlind U, Kunz H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem Soc Rev. 2013;42:4421–4442. doi: 10.1039/c3cs35470a. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 8.Stergiou N, Glaffig M, Jonuleit H, Schmitt E, Kunz H. Immunization with a synthetic human MUC1 glycopeptide vaccine against tumor-associated MUC1 breaks tolerance in human MUC1 transgenic mice. Chem Med Chem. 2017;12:1424–1428. doi: 10.1002/cmdc.201700387. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 9.Adluri S, Gilewski T, Zhang S, Ramnath V, Ragupathi G, Livingston PO. Specificity analysis of sera from breast cancer patients vaccinated with MUC1-KLH plus QS-21. Br J Cancer. 1999;79:1806–1812. doi: 10.1038/sj.bjc.6990288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sungsuwan S, Yin Z, Huang X. Lipopeptide coated iron oxide nanoparticles as potential glyco-conjugate based synthetic anti-cancer vaccines. ACS Appl Mater Interfaces. 2015;7:17535–17544. doi: 10.1021/acsami.5b05497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman K, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by poly(D, L-Lactic-co-glycolic acid) microspheres induces type 1 T helper immune response. J Pharm Sci. 1998;87:1421–1427. doi: 10.1021/js980070s. [DOI] [PubMed] [Google Scholar]
- 12.Glaffig M, Palitzsch B, Stergiou N, Schüll C, Strassburger D, Schmitt E, Frey H, Kunz H. Enhanced immunogenicity of multivalent MUC1 glycopeptide antitumour vaccines based on hyperbranched polymers. Org Biomol Chem. 2015;13:10150–10154. doi: 10.1039/c5ob01255d. [DOI] [PubMed] [Google Scholar]
- 13.Karmakar P, Lee K, Sarkar S, Wall KA, Sucheck SJ. Synthesis of a liposomal MUC1 glycopeptide-based immunotherapeutic and evaluation of the effect of L-rhamnose targeting on cellular immune responses. Bioconjugate Chem. 2016;27:110–120. doi: 10.1021/acs.bioconjchem.5b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilaros S, Tsibanis A, Tsikkinis A, Pietersz GA, McKenzie IF, Apostolopoulos V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy. 2013;5:1177–1172. doi: 10.2217/imt.13.126. [DOI] [PubMed] [Google Scholar]
- 15.Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, Boons GJ. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 triparticle vaccine. Proc Natl Acad Sci U S A. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson BL, Day S, Malins LR, Apostolopoulos V, Payne RJ. Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer. Angew Chem, Int Ed. 2011;50:1635–1639. doi: 10.1002/anie.201006115. [DOI] [PubMed] [Google Scholar]
- 17.Geraci C, Consoli GM, Granata G, Galante E, Palmigiano A, Pappalardo M, Di Puma SD, Spadaro A. First self-adjuvant multicomponent potential vaccine candidates by tethering of four or eight MUC1 antigenic immunodominant PDTRP units on a calixarene platform: Synthesis and biological evaluation. Bioconjugate Chem. 2013;24:1710–1720. doi: 10.1021/bc400242y. [DOI] [PubMed] [Google Scholar]
- 18.Liu YF, Sun ZY, Chen PG, Huang ZH, Gao Y, Shi L, Zhao YF, Chen YX, Li YM. Glycopeptide Nanoconjugates Based on Multilayer Self-Assembly as an Antitumor Vaccine. Bioconjugate Chem. 2015;26:1439–1442. doi: 10.1021/acs.bioconjchem.5b00150. [DOI] [PubMed] [Google Scholar]
- 19.Ninkovic T, Kinarsky L, Engelmann K, Pisarev V, Sherman S, Finn OJ, Hanisch FG. Identification of O-glycosylated decapeptides within the MUC1 repeat domain as potential MHC class I (A2) binding epitopes. Mol Immunol. 2009;47:131–140. doi: 10.1016/j.molimm.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakshminarayanan V, Supekar NT, Wei J, McCurry DB, Dueck AC, Kosiorek HE, Trivedi PP, Bradley JM, Madsen CS, Pathangey LB, Hoelzinger DB, Wolfert MA, Boons GJ, Cohen PA, Gendler SJ. MUC1 vaccines, comprised of glycosylated or non-glycosylated peptides or tumor-derived MUC1, can circumvent immunoediting to control tumor growth in MUC1 transgenic mice. PLoS One. 2016;11:e0145920. doi: 10.1371/journal.pone.0145920. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeltins A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin Immunol. 2017;34:123–132. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 24.Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, BenMohamed L, Gildersleeve JC, Finn MG, Huang X. Boosting immunity to small tumor-associated carbohydrates with bacteriophage Qb capsids. ACS Chem Biol. 2013;8:1253–1262. doi: 10.1021/cb400060x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 26.Beatson R, Maurstad G, Picco G, Arulappu A, Coleman J, Wandell HH, Clausen H, Mandel U, Taylor-Papadimitriou J, Sletmoen M, Burchell JM. The breast cancer-associated glycoforms of MUC1, MUC1-Tn and sialyl-Tn, are expressed in COSMC wild-type cells and bind the C-type lectin MGL. PLoS One. 2015;10:e0125994. doi: 10.1371/journal.pone.0125994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apostolopoulos V, Yuriev E, Ramsland PA, Halton J, Osinski C, Li W, Plebanski M, Paulsen H, McKenzie IF. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci U S A. 2003;100:15029–15034. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin AL, Bernas LM, Rutt BK, Foster PJ, Gillies ER. Enhanced cell uptake of superparamagnetic iron oxide nanoparticles functionalized with dendritic guanidines. Bioconjugate Chem. 2008;19:2375–2384. doi: 10.1021/bc800209u. [DOI] [PubMed] [Google Scholar]
- 29.Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew Chem, Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenspan NS, Cooper LJN. Intermolecular cooperativity - A clue to why mice have IgG3. Immunol Today. 1992;13:164–168. doi: 10.1016/0167-5699(92)90120-V. [DOI] [PubMed] [Google Scholar]
- 31.Vlad AM, Muller S, Cudic M, Paulsen H, Otvos L, Jr, Hanisch FG, Finn OJ. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196:1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004;103:2677–2682. doi: 10.1182/blood-2003-06-2070. [DOI] [PubMed] [Google Scholar]
- 33.Yin Z, Huang X. Recent development in carbohydrate based anticancer vaccines. J Carbohydr Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golmohammadi R, Fridborg K, Bundule M, Valegard K, Liljas L. The crystal structure of bacteriophage Qβ at 3.5Å resolution. Structure. 1996;4:543–554. doi: 10.1016/s0969-2126(96)00060-3. [DOI] [PubMed] [Google Scholar]
- 35.Astronomo RD, Kaltgrad E, Udit AK, Wang SK, Doores KJ, Huang CY, Pantophlet R, Paulson JC, Wong CH, Finn MG, Burton DR. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem Biol. 2010;17:357–370. doi: 10.1016/j.chembiol.2010.03.012. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin Z, Wright WS, McKay C, Baniel C, Kaczanowska K, Bentley P, Gildersleeve JC, Finn MG, BenMohamed L, Huang X. Significant impact of immunogen design on the diversity of antibodies generated by carbohydrate-based anti-cancer vaccine. ACS Chem Biol. 2015;10:2364–2372. doi: 10.1021/acschembio.5b00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moura APV, Santos LCB, Brito CRN, Valencia E, Junqueira C, Filho AAP, Sant’Anna MRV, Gontijo NF, Bartholomeu DC, Fujiwara RT, Gazzinelli RT, McKay CS, Sanhueza CA, Finn MG, Marques AF. Virus-like particle display of the α-Gal carbohydrate for vaccination against Leishmania infection. ACS Cent Sci. 2017;3:1026–1031. doi: 10.1021/acscentsci.7b00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin Z, Dulaney SB, McKay CS, Baniel C, Kaczanowska K, Ramadan S, Finn MG, Huang X. Chemical synthesis of GM2 glycans, bioconjugation with bacteriophage Qb, and the induction of anticancer antibodies. Chem Bio Chem. 2016;17:174–180. doi: 10.1002/cbic.201500499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jegerlehner A, Zabel F, Langer A, Dietmeier K, Jennings GT, Saudan P, Bachmann MF. Bacterially produced recombinant influenza vaccines based on virus-like particles. PLoS One. 2013;8:e78947. doi: 10.1371/journal.pone.0078947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachmann MF, Jennings GT. Therapeutic vaccines for chronic diseases: successes and technical challenges. Philos Trans R Soc, B. 2011;366:2815–2822. doi: 10.1098/rstb.2011.0103. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachmann M, Rohrer U, Kundig T, Burki K, Hengartner H, Zinkernagel R. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 42.Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular masss and hapten valency. J Immunol. 1989;143:1239–1244. [PubMed] [Google Scholar]
- 43.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B celll signaling with defined multivalent ligands. ACS Chem Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 44.Avalos AM, Ploegh HL. Early BCR events and antigen capture, processing, and loading on MHC class II on B cells. Front Immunol. 2014;5:92. doi: 10.3389/fimmu.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin Q, Yin Z, Wu X, Haas KM, Huang X. Valency and density matter: Deciphering impacts of immunogen structures on immune responses against a tumor associated carbohydrate antigen using synthetic glycopolymers. Biomaterials. 2016;101:189–198. doi: 10.1016/j.biomaterials.2016.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horiya S, Bailey J, Guillen-Schlippe YV, Temme JS, Krauss IJ. Directed evolution of multivalent glycopeptides which are tightly recognized by HIV antibody 2G12. J Am Chem Soc. 2014;136:5407–5415. doi: 10.1021/ja500678v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamo R. Advancing homogeneous antimicrobial glycoconjugate vaccines. Acc Chem Res. 2017;50:1270–1279. doi: 10.1021/acs.accounts.7b00106. [DOI] [PubMed] [Google Scholar]
- 48.von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FGM, van Kamp GJ, Schol DJ, Reis CA, Price MR, Livingston PO, Hilgers J. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. Int J Cancer. 2000;86:702–712. doi: 10.1002/(sici)1097-0215(20000601)86:5<702::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 49.von Mensdorff-Pouilly S, Moreno M, Verheijen RHM. Natural and induced humoral responses to MUC1. Cancers. 2011;3:3073–3103. doi: 10.3390/cancers3033073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.