Synopsis
Neurologic complications of cancer may involve both the central nervous system (CNS) and peripheral nervous system (PNS) manifesting as brain, leptomeningeal, intramedullary, intradural, epidural, plexus, and skull base metastases. Excluding brain involvement, neurologic complications affecting these other sites are relatively infrequent, but collectively they affect more than 25% of patients with metastatic cancer causing significant morbidity and mortality. Early diagnosis and intervention optimize quality of life and improve survival.
Keywords: Neurological complications, cancer, CNS, PNS, epidural cord compression, leptomeningeal, skull base, nerve plexuses
Introduction
Metastatic cancer may involve both the central nervous system (CNS) and peripheral nervous system (PNS). Metastatic complications of the CNS and PNS are relatively rare, but collectively they affect around 25% of patients with cancer. The incidence and site of metastatic involvement within the CNS and PNS differ based on the primary histology (Table 1). Most patients diagnosed with metastatic complications involving the CNS and PNS have widespread systemic disease, and some have more than one nervous system site involved. These metastatic complications usually occur in the late stages of disease, but rarely they are the presenting manifestation of malignancy. The number of patients suffering from metastases to the nervous system is likely to increase with prolonged control of systemic disease. Most importantly, metastases to the CNS or PNS can result in significant morbidity as well as mortality. This chapter will focus on leptomeningeal, intramedullary, intradural, epidural, plexus, and skull base metastases. Brain metastases are discussed in Chapter 10.
Table 1.
Metastatic Complications of Cancers and Common Primaries
| Metastatic Complication | Common Primary Malignancies | Overall Incidence |
|---|---|---|
| Leptomeningeal | Breast, lung (small cell), non-Hodgkins lymphoma, leukemias, stomach, melanoma1,6 | 4–15%6 |
| Intramedullary | Lung (small cell), breast32 | 0.9–2.1%32 |
| Intracranial | Dural Breast, lung > head/neck, leukemia, lymphoma, multiple myeloma36 | 9–10%36 |
| Epidural Spinal Cord Compression | Breast, lung, prostate, lymphoma41 | 5%41 |
| Nerve Plexuses | ||
| Brachial | Lung, breast > lymphoma, sarcoma, melanoma54 | 0.43%57 |
| Lumbosacral | Colorectal, sarcoma, breast, lymphoma, genitourinary56 | 0.71%57 |
| Skull Base | Lung, breast, prostate, lymphoma, and head/neck59,61 | 4%59 |
Leptomeningeal Metastases (LM)
Clinical Presentation
The most common presentation in patients with LM include symptoms and signs involving several sites along the neuroaxis. The symptoms and signs of LM are considered according to their regional anatomic localization: brain (cerebral), cranial nerves (CN), and spinal cord (Table 2).
Table 2.
Clinical Presentation of Leptomeningeal Metastases.
| Anatomical Location | Symptoms/Signs |
|---|---|
| Brain (Cerebral) | Headache, lethargy, confusion |
| Cranial Nerves | Vision changes, diplopia, facial numbness/weakness, hearing loss |
| Spinal Cord | Pain (back/neck, but can be radicular), focal weakness, numbness, bladder/bowel symptoms |
Pathophysiology
Mechanisms for the invasion of cancer cells to the leptomeninges include arterial and venous hematogenous dissemination, as well as direct extension of metastatic tumor from the brain, spinal cord, or cranial or peripheral nerves.1 Direct seeding of the cerebrospinal fluid (CSF) can occur in as many as 36% of patients after resection of a posterior fossa brain metastasis.2 LM can cause elevated intracranial pressure (ICP) with or without hydrocephalus.1 Symptoms of elevated ICP can be prominent and are often confused with direct LM involvement. Hydrocephalus develops due to obstruction of CSF outflow by leptomeningeal tumor resulting in impaired CSF absorption.1 However, elevated ICP can also occur in the absence of hydrocephalus when the ventricular system is unable to dilate due to diffuse subarachnoid tumor. As neuro-imaging can be disarmingly normal in this situation, leptomeningeal metastases is commonly missed, leading to severe headache and intractable nausea and vomiting. Increased baseline ICP prevents the brain from adjusting to transient rises in pressure that occur normally with positional changes in cerebral blood volume and vascular resistance. When these plateau waves occur in the setting of elevated ICP, they lead to decreased cerebral blood flow causing the acute onset of transient neurologic symptoms including headache, loss of consciousness, and even focal findings such as weakness or paresthesias that are almost always precipitated by a change in body position.1 They are often confused with seizures, and a delay in proper diagnosis can be fatal.
Diagnosis
The gold standard for diagnosing LM is the identification of malignant cells in the CSF.3 However, CSF cytologic examination has a high rate of false negatives, and sensitivity of CSF cytology does not approach 90% until 3 LPs have been performed.3 Obtaining a large volume of CSF and rapid processing can improve the yield.3 Cisternal tap has been shown to result in positive cytology in cases where lumbar tap was negative.4 In addition to cytology, CSF flow cytometry should be performed in patients with lymphoma or leukemia, as it can be 2–3 times more sensitive at detecting LM.5 The CSF profile often demonstrates elevated protein, pleocytosis in about one-half of patients, and hypoglycorrhachia in a minority.6 An elevated opening pressure is present in at least 50% of patients.6 Biochemical and molecular markers (Table 3) can also be obtained in the CSF and compared to serum concentrations to aid in the diagnosis of LM in specific settings.1,6 More recently, circulating tumor cells (CTC) for epithelial primaries have been identified in the CSF and have a 95% sensitivity, which is far superior to CSF cytology.7 Furthermore, CTCs have been shown to be useful in monitoring response to treatment in breast cancer.8 The best method for collecting CTCs from CSF is an ongoing area of research, so this approach is not yet routinely used in clinical settings.
Table 3.
Cerebrospinal Fluid Evaluation Based on Suspected Malignancy.
| Tests/Markers | Specific Tumor Associations (if applicable) |
|---|---|
| All Cancers/Unknown | |
| Standard: | |
| Cell Count with Differential | |
| Glucose | |
| Protein | |
| Cytology | |
| If Available: | |
| Cell-Free DNA | |
| Solid Tumors | |
| Standard: | |
| CEA | GI/Lung |
| AFP | Germ Cell |
| βHCG | Germ Cell |
| Melanin | Melanoma |
| CA 125 | Ovarian |
| CA 15–3 | Breast |
| 5-HIAA | Carcinoid |
| PSA | Prostate |
| CA 19–9 | Adenocarcinoma |
| If Available: | |
| Circulating Tumor Cells | Epithelial Tumors |
| FISH (interphase cytogenetics) | |
| Protein S-100 | Melanoma |
| HMB45 | Melanoma |
| TTF 1 | Lung/Thyroid |
| MAGE, MART-1, tyrosinase | Melanoma |
| Hematologic Cancers | |
| Standard: | |
| β-2 microglobulin | Lymphoma |
| LDH | Lymphoma |
| Flow Cytometry | |
| IgH Gene Rearrangement | |
Magnetic resonance imaging (MRI) of the brain and spine is the imaging study of choice when evaluating for LM with a sensitivity of 34–71%.9 Definitive imaging characteristics of LM include sulcal enhancement, CN deposits, pial enhancement over the spinal cord, and nodular thickening of the cauda equina9 (Figure 1); these findings can be diagnostic in a patient with known cancer, even if the CSF cytology is negative.10 LM from hematologic primaries are less apparent on MRI than solid tumor primaries.10 Imaging for LM should always include the brain and the complete spine as the disease can be multi-focal. LP should not be delayed for MRI due to concern for inciting pachymeningeal enhancement because this is actually quite rare.11
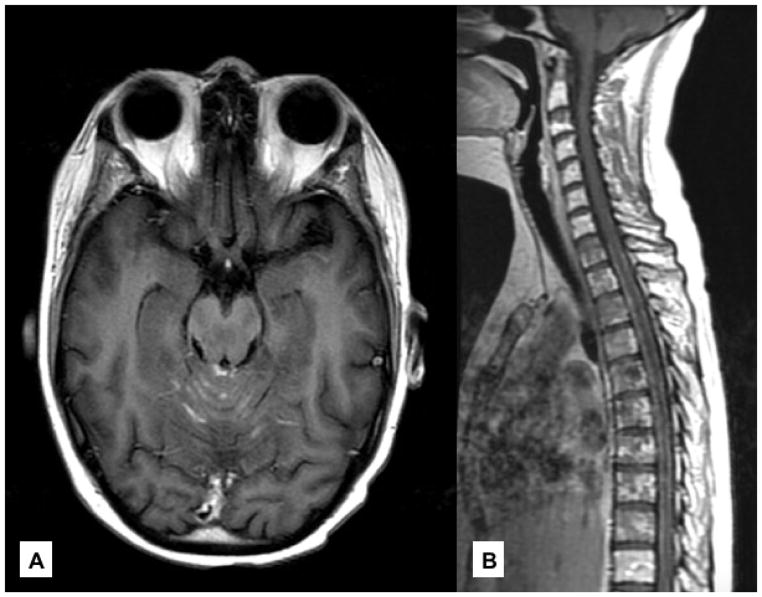
Figure 1. Leptomeningeal Metastases.
(A) Metastatic breast cancer with leptomeningeal disease lining the cerebellar folia on axial, T1 post-contrast imaging. (B) Leptomeningeal disease coating the spinal cord on sagittal, T1 post-contrast imaging.
Treatment
Radiation Therapy
Current practice for treatment of LM with RT is to irradiate symptomatic areas for palliation. It is unclear if treating areas of bulky disease that are asymptomatic prevents the development of symptoms. Craniospinal RT is not employed due to its associated toxicities including bone marrow suppression which inhibits the ability to deliver subsequent chemotherapy. Whole brain RT is typically reserved for patients who develop hydrocephalus, hemispheric symptoms, or multiple cranial neuropathies, although RT to the skull base may suffice to treat isolated cranial neuropathies.1 However, even if RT is symptomatically helpful, it may not prolong survival.12
Chemotherapy
Chemotherapy can be delivered systemically or intrathecally for LM. There are no randomized trials comparing these methods of delivery. Depending on the agent, systemic chemotherapy does not always achieve a therapeutic concentration in the CSF. Intrathecal (IT) chemotherapy allows for the direct administration of drug into the CSF via LP or Ommaya reservoir, although IT chemotherapy does not penetrate bulky disease. Compared to LP, Intra-Ommaya (IO) chemotherapy achieves better distribution throughout the CSF, achieves therapeutic concentrations for a longer duration, and guarantees the drug is delivered into the CSF compartment given that ~10% of injections via LP actually deliver the drug into the epidural space, despite CSF return.1,13 For these reasons, along with the ease of administration and patient tolerability, IO delivery is preferred over LP delivery.
Methotrexate (MTX), thiotepa, and cytarabine are the primary agents given intrathecally. Treatment response to MTX has been seen in 36–75% of patients with LM from breast cancer and some patients have survived more than 1 year with this approach.14,15 The primary toxicity associated with IT-MTX is neutropenia,14 but this can be prevented with oral leucovorin 10 mg twice a day for a few days; leucovorin does not cross the blood-brain barrier and cannot rescue the tumor cells in the CSF. IT combination regimens have failed to show any improvement in response or survival over single agent use15. IT cytarabine is available in a standard formulation and was previously available in a depot formulation (Depocyte)6 which allowed for every 2-week dosing. MTX and cytarabine are most effective for LM from hematologic malignancies. IT thiotepa has similar response rates and toxicities as MTX.6 The main side effects for IT chemotherapy are headache and arachnoiditis which are most severe with Depocyte and require pre- and post-instillation prophylactic glucocorticoids6.
IT chemotherapy requires normal CSF flow dynamics to deliver the drug throughout the CSF space. CSF flow can be studied using a radioisotope instilled into the ventricular system via an Ommaya reservoir or by LP.16 Impaired CSF flow is associated with poor outcome and an increased risk of neurotoxicities.16 Unfortunately, IT chemotherapy has limited impact on the outcome of LM patients, except for those with hematologic malignancies where it can be curative.17
Systemically administered chemotherapies for LM include cytarabine, MTX, and thiotepa. Penetration of the CNS by cytarabine and MTX can be achieved with high dose regimens, and thiotepa is known to cross the blood-brain barrier. However, these are not usually active agents against the most common solid tumors that cause LM, such as lung and breast cancer. There is also some evidence that administration of drugs or regimens optimal for the primary tumor, regardless of their ability to penetrate the blood-CSF barrier, is the best approach. The presence of tumor in the CSF causes some disruption of the blood-CSF barrier allowing penetration of drugs that typically do not cross into the CNS.17
Targeted and Immunotherapies
A significant number of small series have documented response of LM to targeted therapies and immunotherapies. In non-small cell lung cancer (NSCLC), therapies directed at inhibition of ALK fusion proteins and EGFR mutations using alectinib and afatinib, respectively, have produced responses in patients with LM.18,19 A larger study found that patients with EGFR-mutant NSCLC LM had prolonged survival with treatment of tyrosine kinase inhibitors.12 Changing the dose and schedule to facilitate access of some agents into the CSF, such as using a pulsatile high dose schedule for erlotinib, an EGFR inhibitor, can also control CNS disease including LM, even when there is progression on the standard dosing schedule.20 Choice of agent can also be adjusted on the basis of whether resistance mutations have been acquired within the CNS compartment. These mutations may be identified in the CSF using cell-free DNA technology and can guide treatment decisions.21
There are now multiple reports of LM responding to immunotherapy. Patients with NSCLC LM have responded to nivolumab, a monoclonal antibody to programmed cell death-1 (PD-1) that prevents down regulation of the immune system.22 In a series of 39 melanoma patients with LM, 21 received targeted therapy with BRAF inhibitors (vemurafenib/dabrafenib) or immunotherapy with antibodies to CTLA-4 (ipilimumab) with or without radiation, and were found to have increased survival with long term survivors.23
Surgery
Surgical intervention for LM is reserved for those patients who develop elevated ICP (with or without hydrocephalus) and require the placement of a ventriculoperitoneal shunt which can be lifesaving. A shunt prevents the delivery of chemotherapy via an Ommaya reservoir, and should never be turned off to facilitate IO drug delivery.
Prognosis
Untreated patients with LM have rapid progression of disease and die within 4–6 weeks.24 With the initiation of treatment for LM, patients can see small improvements in survival that vary depending on histology, but the majority have continued neurologic deterioration that leads to death.25 Patients with leukemia can obtain complete eradication of LM and have a median overall survival (mOS) of 11.3 months.25 Those with breast cancer who respond to IT chemotherapy have a mOS greater than one year.15 Patients with melanoma have a mOS of 4.7 months, and lung cancer a mOS of only 1.8 months.25 Survival at 1-year is seen in 48% of patients with lymphoreticular and breast cancers, 26% in melanoma, and 18% in lung cancer.25
Favorable prognostic factors for response to IT treatment include controlled systemic disease at diagnosis, low initial CSF protein (likely a surrogate for good CSF flow), and concomitant systemic chemotherapy.14 Poor prognostic factors include having a lung or melanoma primary, 12 months or less from diagnosis of primary to LM, KPS ≤70, age ≥50, lack of cytologic response in CSF, and lack of concurrent systemic chemotherapy.17 In a few studies, the use of systemic chemotherapy has been associated with increased OS on multivariable analysis.17,25
Intramedullary Metastases
Pathophysiology
Intramedullary metastases (IMM) primarily arise by direct hematogenous dissemination or by direct extension from LM in the subarachnoid space.1 IMM tends to be evenly distributed along the cervical, thoracic, and lumbar spine26, although some studies have shown the thoracic or lumbar spine to be more involved.27–29 When accounting for the length of spinal segments, the lumbar spine is disproportionally involved in IMM.30 Up to 33% of patients with IMM have multifocal intramedullary disease.27
Clinical Presentation
At least one-half of patients have brain metastases26,29,31,32, and it is common for LM to be present31,32. The most common presenting symptoms are pain, sensory changes, and weakness.32 Pain can be localized or radicular30,32, and weakness can be bilateral or unilateral32. Although bladder and bowel dysfunction may occur later, several studies show that more than 50% of patients had bladder/bowel dysfunction at presentation.30,32 A sensory level and spasticity are common.30 Brown Sequard syndrome (characterized by ipsilateral weakness and loss of vibration/proprioception as well as contralateral loss of pain/temperature sensation) can be the presenting manifestation of IMM.32 The clinical presentation of patients with IMM is characterized by a rapid neurologic decline resulting in paraparesis or paraplegia.29 Symptoms can be present anywhere from 7 to 63 days prior to the diagnosis of IMM.28,29
Diagnosis
The diagnosis of IMM is made by spine MRI with contrast (Figure 2). On MRI, IMM may be nodular or ring enhancing1,27,32; hemorrhage and intratumoral cystic changes are rare33. In a patient with known cancer, differentiating between IMM, LM, radiation myelopathy, and paraneoplastic myelopathy based on imaging can be difficult. However, radiation myelopathy and necrotizing myelopathy are usually painless and insidious, whereas pain occurs early in IMM and is accompanied by rapid evolution of symptoms.34 CSF studies are not helpful in differentiating intramedullary lesions, unless LM are confirmed in the CSF.
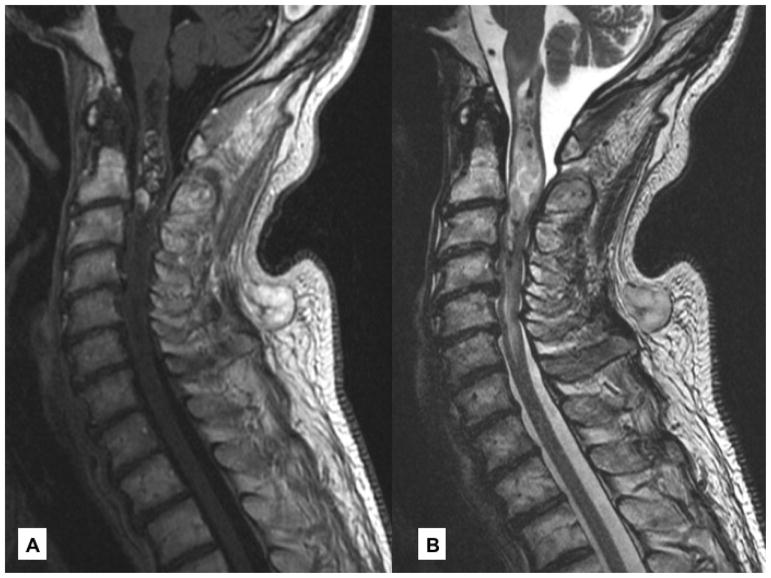
Figure 2. Intramedullary Metastases.
(A) Sagittal, T1 post-contrast imaging reveals an enhancing intramedullary metastasis at C2/C3 in a patient with adenoid cystic carcinoma of the submandibular gland. (B) Surrounding vasogenic edema is best appreciated on sagittal, T2.
Treatment/Prognosis
Treatment options include steroids, RT, surgical resection, chemotherapy, or a combination of these approaches.30,32,34 RT is effective at ameliorating symptoms.34 Several studies have explored surgery and micro-surgical approaches followed by RT with improvement in OS and function26,31 but was limited to patients with a high functional status. Some advocate for surgical resection in patients with a solitary IMM from a radio-resistant primary with well controlled systemic disease, although this is an uncommon situation.26,31
The mOS of patients with IMM ranges from 12 to 31 weeks.28,32 Some breast cancer patients fare better29,32, but other reports suggest that lung or breast primary was associated with inferior survival35. On spinal MRI, multiple IMM, involvement of 3 segments or greater, and visualization of any systemic involvement were associated with decreased survival.35 The presence of LM was not associated with inferior survival.35
Intracranial Dural Metastases
Pathophysiology
Intracranial dural metastases (IDM) are calvarial metastases with direct extension to the epidural and subdural spaces or direct involvement of the subdural space from hematogenous spread.1 Direct extension from a skull metastasis explains the predilection of those cancers which commonly spread to bone to affect the dura.36
Clinical Presentation
IDM usually present with bulky dural disease causing mass effect, edema, and compression of underlying parenchyma; rarely it can lead to a subdural hematoma (SDH) or effusion which may require cytologic evaluation for a definitive diagnosis.1 Alteration in mental status, visual complaints, hemiparesis, and seizures are seen frequently. Eleven percent of patients may be asymptomatic with IDM identified on imaging done for other purposes.36
Diagnosis
IDM is diagnosed by brain MRI with contrast, and the lesions enhance homogeneously with an associated dural tail37 (Figure 3). The main differential is a meningioma, but IDM often have more irregular borders and underlying edema than a typical meningioma; this can be a challenging differential diagnosis, especially in women with breast cancer who have an increased incidence of meningioma.38 Dynamic susceptibility contrast-MR/perfusion weighted imaging can assist as relative cerebral blood volume is low in most IDMs but elevated in meningiomas.37 Other imaging characteristics seen with IDMs include skull metastases, bony erosions, vasogenic edema, brain invasion, venous sinus compression or occlusion, and a SDH/effusion. A single lesion is most common, but diffuse dural involvement can also be seen.36 Those with a SDH/effusion may also exhibit brisk enhancement, and there are frequently nodules along the dura or marked underlying edema to suggest this is not a benign process.
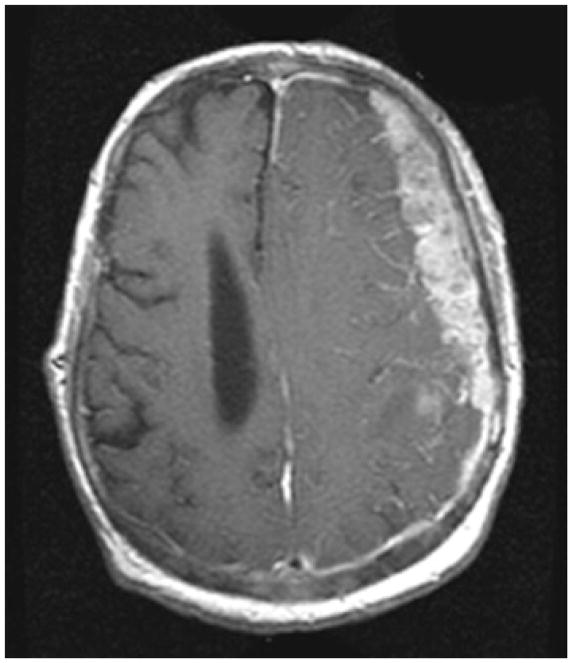
Figure 3. Dural Metastases.
Enhancing, dural metastasis along the left hemisphere in a patient with metastatic prostate cancer. Note the irregular border of the lesion abutting brain and the marked mass effect.
Treatment and Prognosis
There is no standard treatment for IDM, but surgical resection, RT, chemotherapy, and combinations of these 3 modalities are utilized depending upon the clinical situation. RT has been associated with improved OS while metastatic lung cancer and low KPS were associated with worse outcomes.36 The mOS of patients with IDM is 9.5 months with a progression-free survival of 3.7 months36. Chemotherapy may play an important role as dural disease is outside the blood-brain barrier.
Epidural Spinal Cord Compression
Pathophysiology
Epidural spinal cord compression (ESCC) is caused by cancer that affects the epidural space by extension from the vertebral bodies or other bony spinal elements, infiltration through a foramen, or direct hematogenous dissemination to the epidural space.1 Epidural disease due to direct extension from vertebral metastases occurs in 85–90% of ESCC.1 The posterior vertebral body is the most common location of metastatic involvement leading to anterior cord compression.39 In addition, metastatic involvement of the vertebral body can lead to vertebral collapse with spinal instability and potential herniation of bone, metastatic disease, and/or disc causing cord compression.1 Paravertebral metastases can invade the epidural space via the intervertebral foramina, but this mechanism accounts for only 10–15% of ESCC, and is usually due to lymphoma or neuroblastoma. Compression typically occurs along the lateral cord and the bone is normal; thus, MRI is the only imaging modality that can visualize this ESCC.1 Direct metastases to the epidural space via hematogenous spread is very rare and seen exclusively in leukemia and lymphoma.1
Clinical Presentation
ESCC is a neurologic emergency due to the possibility of sudden paraparesis which can occur unexpectedly, is difficult to reverse, and is likely due to a venous infarction of the spinal cord. Patients with ESCC experience back pain, weakness, sensory changes, and autonomic dysfunction (Table 4), but significant diagnostic delays are frequent. In one study, the median times from onset of radicular pain, weakness, sensory, and bladder problems to diagnosis were 40, 21, 13, and 3 days, respectively.40 More striking is that in patients without a diagnosis of malignancy, the diagnosis was delayed by an additional 4 weeks.41
Table 4.
Presenting Complaints and Physical Exam Findings in Patients with Epidural Spinal Cord Compression.
| Presenting Complaint | Frequency |
|---|---|
| Back Pain | 61–96%66,67 |
| - Local or radicular pain | |
| - Exacerbated when supine, cough, sneeze, movement or Valsalva | |
| - Wakes patients at night, sleep in a seated position to alleviate pain | |
| Weakness | 2–37%66,67 |
| Sensory Changes | 0%67 |
| - not a common complaint, but more commonly found on exam | |
| Bowel and Bladder Incontinence | 0–2%66,67 |
| Gait Disturbance/Ataxia | 2%67 |
|
| |
| Physical Exam Finding | Frequency |
|
| |
| Weakness | 87–96%67,68 |
| Sensory Levels and Deficits | 78–90%67,68 |
| Autonomic Dysfunction | 57–69%67,68 |
| Ataxia | 14%67 |
Diagnosis
The differential diagnosis for ESCC includes epidural abscess/hematoma, a primary epidural tumor, or vertebral collapse from any etiology, such as osteoporosis. MRI is the definitive imaging modality due to its high sensitivity and specificity at detecting metastatic ESCC, and it can detect multiple sites of ESCC1 (Figure 4). Multiple sites of epidural spinal metastases are seen in about 30% of patients, necessitating whole spine imaging in all patients.42 The most common location for cord compression is the thoracic spine followed by the lumbosacral, and then the cervical level.40,42 CT myelogram is useful in cases where MRI is contraindicated.
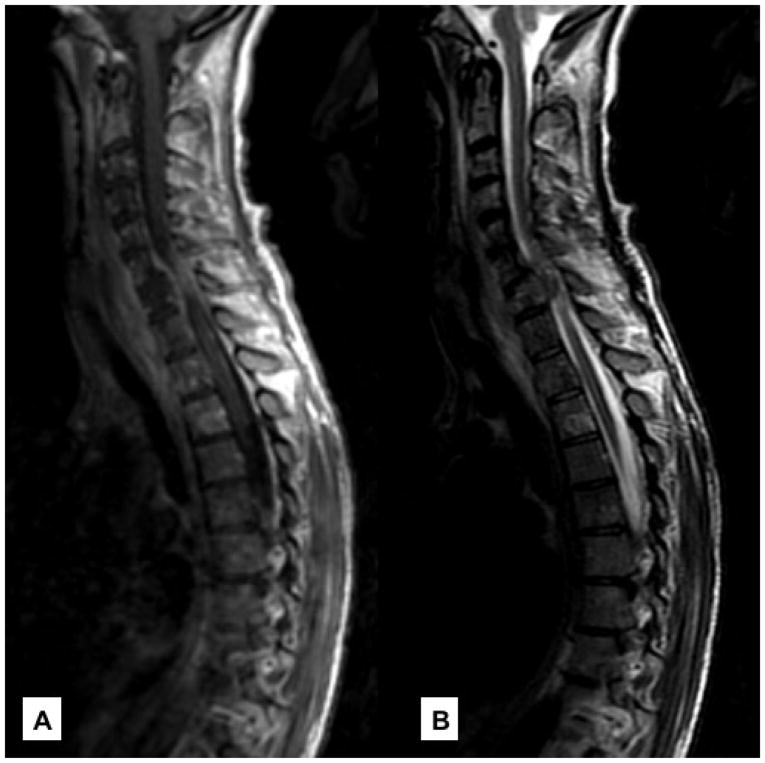
Figure 4. Metastatic Epidural Spinal Cord Compression.
(A) Sagittal, T1 post-contrast image showing extensive epidural disease arising from the vertebral body causing spinal cord compression at C6/C7 in a patient with metastatic breast cancer. (B) Cord compression is better visualized on T2.
Treatment
Treatment for ESCC is palliative and includes relieving pain, preserving or improving neurological function, stabilizing the spine, and addressing the tumor.
Steroids
High dose steroids are the first treatment in the acute management of malignant ESCC.43 Glucocorticoids reduce local edema at the site of compression and can improve neurological function. Although the subject of several randomized trials, there continues to be no standard dosing regimen, but in clinical practice an initial dose from 10–100 mg of dexamethasone is used, followed by a maintenance dose of 16–96 mg per day with a rapid taper following treatment.43
Surgery
Surgery should be directed to the site of the disease with the goal of extirpating the tumor mass. Thus, anterior and antero-lateral approaches are the optimal approaches given that most disease arises from the vertebral body. A randomized trial comparing tumor resection plus RT versus RT alone for management of ESCC found that patients who underwent surgical resection and RT were more likely to regain ambulation and retain it for a longer duration of time compared to RT alone. These patients were also more likely to maintain continence, have reduced pain and steroid use, and have improved OS.44 Therefore, surgery should be considered in patients with ESCC who have a reasonable overall prognosis.44 In addition, other considerations may prompt a surgical approach as the first step such as spinal instability necessitating stabilization, deterioration during RT, need to establish a pathologic diagnosis, radioresistant tumors, and recurrence where additional RT is not an option.39
Radiation Therapy
RT should be given to all patients who are non-surgical candidates with ESCC and should be considered as first line therapy in patients with highly radiosensitive tumors such as myeloma, lymphoma, Ewing sarcoma, neuroblastoma, or seminoma.39 RT, particularly short course RT, is also advocated in patients with a poor prognosis as it is non-invasive and completed rapidly in an outpatient setting.45 RT should also follow surgery.
Currently, conventional RT is often replaced by stereotactic body radiation therapy (SBRT), especially in the post-operative setting. Conventional RT requires a relatively large treatment field encompassing one to two vertebral bodies and includes normal spinal cord. However, SBRT allows for the delivery of conformal high dose RT in a single dose or hypofractionated doses, which allows for the safe and effective delivery of RT without spinal cord toxicity and minimal systemic toxicities.46 Local recurrence rates of less than 5% at 1 year have been reported in patients who underwent surgery followed by high-dose hypofractionated SBRT, irrespective of tumor radiosensitivity46, leading to advocating for this approach in consensus guidelines47. SBRT also limits bone marrow toxicity compared to conventional RT, providing the opportunity for multi-modality treatment with chemotherapy.
The success of SBRT in the treatment of ESCC has led to the implementation of a surgical procedure termed “separation surgery” which is followed by SBRT. This procedure allows for decompression and stabilization, but focuses on providing a separation between the spinal cord and the tumor, as opposed to achieving a gross total resection.46 This allows for SBRT to be delivered safely and avoids the risks associated with a gross total resection while providing long term disease control.46
Systemic therapies
Systemic therapies such as chemotherapy, hormonal therapy, and immunotherapy have a limited role in the treatment of ESCC. Most treatments directed at ESCC including surgery and RT are focal, and patients often need chemotherapy, hormonal therapy, or immunotherapy for active systemic disease. However, in certain situations where ESCC is due to highly chemosensitive primaries, such as lymphoma or seminoma, it may be treated with systemic treatment, provided the patient has few or no neurologic signs.46 There may also be a role for combining RT with immunotherapy which may result in a more robust immune response leading to better clinical outcomes.48
Emerging Therapies
A phase I study in patients with progressive epidural disease despite surgery and RT found that spinal intra-arterial chemotherapy (melphalan) was safe and feasible, and effective at stabilizing epidural disease.49 MR-guided spinal laser interstitial thermal therapy is also being explored as a less invasive alternative to surgery for ESCC; it achieved significant decompression months after the procedure in a select patient population.50 Percutaneous kyphoplasty does not treat ESCC, but it is safe and effective for spinal metastases51, and may be useful in preventing ESCC. It is also useful for vertebral body compression fractures that can occur with long-term follow up after SBRT.
Prognosis
In patients with ESCC the most important factor that predicts ambulatory status following treatment is the patient’s ambulatory function prior to treatment.44 Some patients who are non-ambulatory at presentation can regain ambulation, particularly if surgery is the first intervention. In regards to survival, visceral metastases, other bony metastases, tumor type, motor function prior to and after treatment, rapidity of motor symptoms, interval between diagnosis and development of ESCC, sphincter dysfunction, number of epidural metastases, and number of vertebral bodies involved can all influence survival in patients with ESCC. In general, patients with lung cancer fare poorly, while breast, prostate, and myeloma/lymphoma patients can have prolonged survival.1 Most of the factors influencing survival in patients with ESCC reflect the aggressive nature of the primary tumor, as the majority of patients with ESCC die from their systemic disease.1 The mOS in patients with ESCC is 2.9 – 10 months52,53 with significantly lower survival times in non-ambulatory patients.
Nerve Plexuses
Brachial Plexus
Metastasis to the brachial plexus occurs primarily from lymphatic spread, but direct invasion can occur as well.1,54 It is thought that the spread via lymphatics from the lung and breast to the lateral axillary lymph nodes leads to preferential involvement of the lower trunk (C8 and T1) due to their proximity. Compression or invasion via Pancoast tumors (apical lung tumors) are common and also lead to lower plexus involvement.55 The upper trunk is usually involved only when tumor extends to involve the whole plexus.54,55
Patients with neoplastic brachial plexopathy often start with severe pain (75%) followed by sensory abnormalities and weakness of the affected arm.54 The pain usually begins in the shoulder girdle, radiates to the elbow, down the medial forearm, and into the fourth and fifth digits.54 Patients typically complain of hand weakness.1 Horner syndrome is seen in approximately 50% of patients due to the close proximity of the sympathetic ganglion to the frequently involved T1 nerve root.54 Horner syndrome often suggests there has been extension of the tumor into the cervical-thoracic epidural space which requires this area be imaged along with the plexus itself. Weakness, atrophy, and sensory changes primarily localize to the C7, C8, and T1 nerve roots.54
Lumbosacral Plexus
Mechanisms of metastatic lumbosacral plexopathy include direct extension by an abdominopelvic primary (accounting for 70%), soft or bony tissue metastases causing compression, extra-abdominal metastasis directly to the plexus, lymph node or muscle involvement with compression, and tumor extension to the plexus along nerves.1,56 The lower plexus (L5-S3) is involved in approximately one-half of cases followed by 30% involving the upper plexus (L1–L4), and 20% affecting the entire plexus.56
As seen in brachial plexopathies, lumbosacral plexopathies also begin with pain, followed by numbness/paresthesias, and weakness within weeks to months.56 Pain typically has an achy or pressure-like quality that is more commonly local or radicular, but can be referred; incontinence is rare.56 Examination demonstrates asymmetric weakness that can progress to focal paralysis.56 Reflexes are lost and asymmetric early in the course.56 A positive straight leg test is common and gait difficulty correlates with the level of lumbosacral plexus involvement.56
Diagnosis
MRI identifies plexus metastases and should include the cervical spine when a Horner syndrome is present57 (Figure 5). CT is excellent for detecting plexus involvement when MRI is not possible. PET and PET-CT may be helpful when other imaging modalities are negative but clinical suspicion for a plexopathy is high57, especially with direct invasion of nerves as seen in neurolymphomatosis. Electromyography and nerve conduction studies (EMG/NCS) can localize a peripheral nerve lesion.56 In cases where history, exam, imaging, and EMG/NCS fail to provide a diagnosis, surgical exploration can be useful.
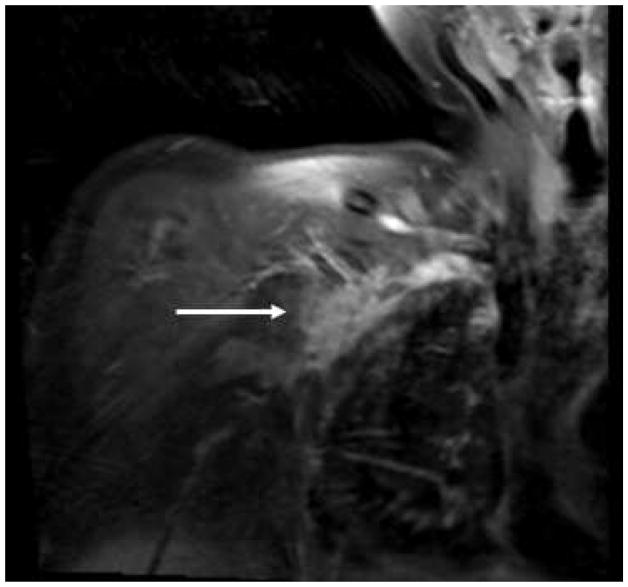
Figure 5.
Brachial Plexus Metastasis: Post-gadolinium MRI demonstrating a right brachial plexus metastasis in a patient with breast cancer.
The most clinically relevant diagnostic dilemma is differentiating a neoplastic plexopathy from radiation plexopathy in a patient who has received prior RT that encompassed the involved plexus (Table 5)54,58. The incidence of radiation plexopathy is approximately 1.2% and can develop months to years after RT.57
Table 5.
Differentiating Neoplastic Plexopathy from Radiation Induced Plexopathy.
| Neoplastic Plexopathy | Radiation Induced Plexopathy |
|---|---|
| Brachial Plexus | |
| Common Cancers | |
| Breast, Lung, Lymphoma, Melanoma, Sarcoma | Prior Radiation Therapy to the Chest, Axilla (Months-Years) |
| Symptoms | |
| Pain (Severe) | Numbness/Paresthesia |
| Signs | |
| Unilateral | |
| Lower Plexus Involvement (C8-T1) | Unilateral |
| Horner Syndrome | Upper (C5-C7) or Entire Plexus Involvement |
| Neck Mass/Supraclavicular Fullness | Lymphedema |
| Diagnostic Evaluations | |
| Paralyzed Hemi-diaphragm on CXR | Myokymic Discharges on EMG/NCS |
| Pancoast Tumor on Imaging | |
|
| |
| Lumbosacral Plexus | |
| Common Cancers | |
| Breast, Colorectal, Genitourinary, Lymphoma, Sarcoma | Prior Radiation Therapy to Pelvis (Months-Years) |
| Symptoms | |
| Pain (Severe) | Weakness in the Lower Extremities |
| Signs | |
| More Commonly Unilateral | More Commonly Bilateral (Asymmetric) |
| Positive Straight Leg Test | Lymphedema |
| Palpable Mass on Rectal Exam | |
| Diagnostic Evaluation | Myokymic Discharges on EMG/NCS |
Treatment
Treatment options for malignant plexopathies include RT, and rarely chemotherapy. Pain management includes analgesics, nerve blocks, cordotomy, and rhizotomy if uncontrolled.54 Despite improvement in pain, most suffer progressive neurological decline.56
Skull Base Metastases
Pathophysiology
The most common mechanism of skull base metastasis is hematogenous spread including both arterial and venous routes.59 However, direct extension and perineural invasion of adjacent cranial nerves can occur from head and neck primaries.60
Clinical Presentation
Patients with skull base metastases present with CN deficits. Seven clinical skull base syndromes1,59,61–63 (Table 6) have been identified due to their stereotyped presentations as a result of compression of CN and vascular structures that are adjacent to the associated basal foramina and sinuses that comprise the skull base.
Table 6.
Syndromes in Skull Base Metastases.
| Location/Syndrome | Presentation |
|---|---|
| Orbital |
|
| Parasellar |
|
| Sella Turcica |
|
| Middle Cranial Fossa |
|
| Jugular Foramen |
|
| Occipital Condyle |
|
| Mandible “Numb-Chin” |
|
Diagnosis
The diagnosis of skull base metastases is made with an MRI60,64 or CT (Figure 6); however, in some patients, MRI and CT are negative despite a high clinical suspicion for skull base metastases. A PET scan may be helpful. A lumbar puncture cannot diagnose skull base metastases, but it is recommended to exclude co-existent LM.
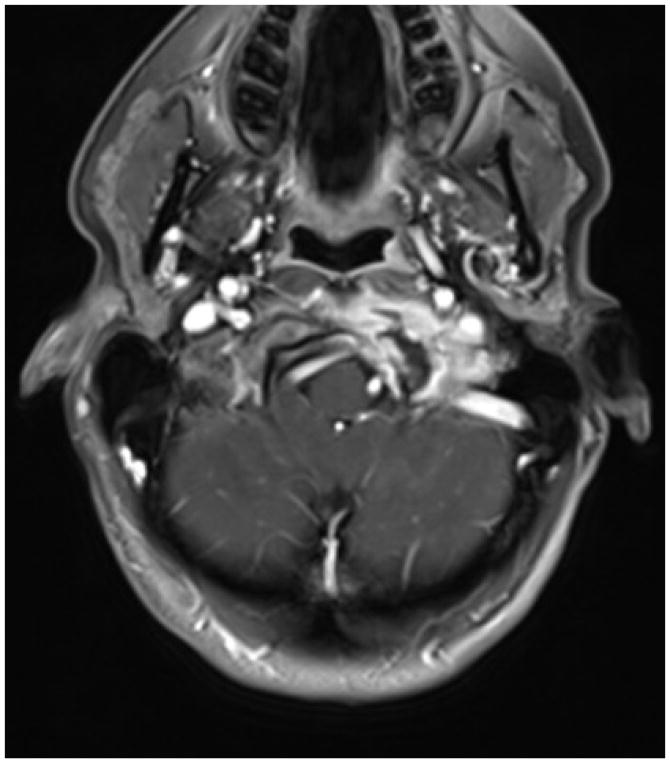
Figure 6.
Skull Base Metastases: Enhancing metastasis involving the left occipital condyle with hypoglossal canal involvement in a patient with breast cancer.
Treatment/Prognosis
Treatment of skull base metastases consists of RT and rarely chemotherapy, but the treatment relies strongly on the histology of the primary.1 Stereotactic radiosurgery has been utilized for both the initial treatment of a skull base metastasis and recurrence.65 Long delays from symptom onset to treatment result in significantly inferior symptomatic improvement. In general, most patients improve following treatment.65
Conclusion
The individual metastatic neurological complications presented in this chapter are infrequent, but together they affect more than 25% of patients with metastatic tumor and can significantly affect a patient’s duration and quality of life. The diagnosis is often challenging as the differential diagnosis is extensive, but localization is possible through history, neurological exam, and modern imaging techniques. For each of them, early diagnosis and intervention are essential to optimize outcomes and have the best opportunity for improved survival.
Key Bullet Points.
Metastates may involve the central and peripheral nervous systems with involvement of the brain, leptomeninges, spinal cord, epidural space, plexus, and skull base.
Excluding the brain parenchyma, metastases to these spaces collectively affect more than 25% of patients with metastatic cancer.
Metastates to the CNS and PNS can result in significant morbidity and mortality, often causing pain, disability and compromising quality of life.
Early diagnosis and treatment are essential to optimize quality of life and survival.
Acknowledgments
Funding: This research was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Dr. Joe Mendez has no conflict of interest
Dr. Lisa DeAngelis has no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joe S. Mendez, Department of Neurology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, N.Y. 10065, United States
Lisa M. DeAngelis, Department of Neurology, Memorial Sloan Kettering, Cancer Center1275 York Avenue, New York, N.Y. 10065, United States
References
- 1.DeAngelis LM, Posner JB, Posner JB. Neurologic complications of cancer. 2. Oxford ; New York: Oxford University Press; 2009. [Google Scholar]
- 2.Norris LK, Grossman SA, Olivi A. Neoplastic meningitis following surgical resection of isolated cerebellar metastasis: A potentially preventable complication. J Neuro-Oncol. 1997;32(3):215–223. doi: 10.1023/a:1005723801479. [DOI] [PubMed] [Google Scholar]
- 3.Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Rogers LR, Duchesneau PM, Nunez C, et al. Comparison of Cisternal and Lumbar Csf Examination in Leptomeningeal Metastasis. Neurology. 1992;42(6):1239–1241. doi: 10.1212/wnl.42.6.1239. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg JE, Breems DA, Kraan J, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68(20):1674–1679. doi: 10.1212/01.wnl.0000261909.28915.83. [DOI] [PubMed] [Google Scholar]
- 6.Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. J Neurooncol. 2005;75(1):85–99. doi: 10.1007/s11060-004-8101-x. [DOI] [PubMed] [Google Scholar]
- 7.Lin XL, Fleisher M, Omuro AMP, Shagabayeva L, Pentsova E. Prospective validation of cerebrospinal fluid (CSF) circulating tumor cells (CTC) to diagnose leptomeningeal metastasis (LM) from epithelial tumors. J Clin Oncol. 2015;33(15) [Google Scholar]
- 8.Patel AS, Allen JE, Dicker DT, et al. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget. 2011;2(10):752–760. doi: 10.18632/oncotarget.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collie DA, Brush JP, Lammie GA, et al. Imaging features of leptomeningeal metastases. Clin Radiol. 1999;54(11):765–771. doi: 10.1016/s0009-9260(99)91181-9. [DOI] [PubMed] [Google Scholar]
- 10.Freilich RJ, Krol G, Deangelis LM. Neuroimaging and Cerebrospinal-Fluid Cytology in the Diagnosis of Leptomeningeal Metastasis. Ann Neurol. 1995;38(1):51–57. doi: 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 11.Wesley SF, Garcia-Santibanez R, Liang J, Pyburn D. Incidence of meningeal enhancement on brain MRI secondary to lumbar puncture. Neurol-Clin Pract. 2016;6(4):315–320. doi: 10.1212/CPJ.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro WR, Young DF, Mehta BM. Methotrexate - Distribution in Cerebrospinal-Fluid after Intravenous, Ventricular and Lumbar Injections. New Engl J Med. 1975;293(4):161–166. doi: 10.1056/NEJM197507242930402. [DOI] [PubMed] [Google Scholar]
- 14.Fizazi K, Asselain B, Vincent-Salomon A, et al. Meningeal carcinomatosis in patients with breast carcinoma. Clinical features, prognostic factors, and results of a high-dose intrathecal methotrexate regimen. Cancer. 1996;77(7):1315–1323. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1315::AID-CNCR14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Hitchins RN, Bell DR, Woods RL, Levi JA. A Prospective Randomized Trial of Single-Agent Versus Combination Chemotherapy in Meningeal Carcinomatosis. J Clin Oncol. 1987;5(10):1655–1662. doi: 10.1200/JCO.1987.5.10.1655. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998;38(2–3):135–140. doi: 10.1023/a:1005982826121. [DOI] [PubMed] [Google Scholar]
- 17.Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010;136(11):1729–1735. doi: 10.1007/s00432-010-0831-x. [DOI] [PubMed] [Google Scholar]
- 18.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10(2):232–236. doi: 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10(1):156–163. doi: 10.1097/JTO.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13(12):1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pentsova EI, Shah RH, Tang J, et al. Evaluating Cancer of the Central Nervous System Through Next-Generation Sequencing of Cerebrospinal Fluid. J Clin Oncol. 2016;34(20):2404–2415. doi: 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer. 2016;98:114–117. doi: 10.1016/j.lungcan.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Foppen MHG, Brandsma D, Blank CU, van Thienen JV, Haanen JB, Boogerd W. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann Oncol. 2016;27(6):1138–1142. doi: 10.1093/annonc/mdw134. [DOI] [PubMed] [Google Scholar]
- 24.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and Treatment of Leptomeningeal Metastases from Solid Tumors - Experience with 90 Patients. Cancer. 1982;49(4):759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Herrlinger U, Forschler H, Kuker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223(2):167–178. doi: 10.1016/j.jns.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Sung WS, Sung MJ, Chan JH, et al. Intramedullary spinal cord metastases: a 20-year institutional experience with a comprehensive literature review. World Neurosurg. 2013;79(3–4):576–584. doi: 10.1016/j.wneu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Crasto S, Duca S, Davini O, et al. MRI diagnosis of intramedullary metastases from extra-CNS tumors. Eur Radiol. 1997;7(5):732–736. doi: 10.1007/BF02742935. [DOI] [PubMed] [Google Scholar]
- 28.Gasser T, Sandalcioglu IE, El Hamalawi B, van de Nes JA, Stolke D, Wiedemayer H. Surgical treatment of intramedullary spinal cord metastases of systemic cancer: functional outcome and prognosis. J Neurooncol. 2005;73(2):163–168. doi: 10.1007/s11060-004-4275-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee SS, Kim MK, Sym SJ, et al. Intramedullary spinal cord metastases: a single-institution experience. J Neurooncol. 2007;84(1):85–89. doi: 10.1007/s11060-007-9345-z. [DOI] [PubMed] [Google Scholar]
- 30.Edelson RN, Deck MDF, Posner JB. Intramedullary Spinal-Cord Metastases - Clinical and Radiographic Findings in Nine Cases. Neurology. 1972;22(12):1222. doi: 10.1212/wnl.22.12.1222. [DOI] [PubMed] [Google Scholar]
- 31.Dam-Hieu P, Seizeur R, Mineo JF, Metges JP, Meriot P, Simon H. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin Neurol Neurosurg. 2009;111(1):10–17. doi: 10.1016/j.clineuro.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Schiff D, O'Neill BP. Intramedullary spinal cord metastases: clinical features and treatment outcome. Neurology. 1996;47(4):906–912. doi: 10.1212/wnl.47.4.906. [DOI] [PubMed] [Google Scholar]
- 33.Rykken JB, Diehn FE, Hunt CH, et al. Intramedullary spinal cord metastases: MRI and relevant clinical features from a 13-year institutional case series. AJNR Am J Neuroradiol. 2013;34(10):2043–2049. doi: 10.3174/ajnr.A3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelman MD, Adelstein DJ, Karlins NL. Intramedullary Spinal-Cord Metastasis - Diagnostic and Therapeutic Considerations. Arch Neurol-Chicago. 1987;44(5):526–531. doi: 10.1001/archneur.1987.00520170054022. [DOI] [PubMed] [Google Scholar]
- 35.Diehn FE, Rykken JB, Wald JT, et al. Intramedullary spinal cord metastases: prognostic value of MRI and clinical features from a 13-year institutional case series. AJNR Am J Neuroradiol. 2015;36(3):587–593. doi: 10.3174/ajnr.A4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer. 2009;115(9):1947–1953. doi: 10.1002/cncr.24203. [DOI] [PubMed] [Google Scholar]
- 37.Kremer S, Grand S, Remy C, et al. Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningioma. Neuroradiology. 2004;46(8):642–648. doi: 10.1007/s00234-004-1194-2. [DOI] [PubMed] [Google Scholar]
- 38.Custer BS, Koepsell TD, Mueller BA. The association between breast carcinoma and meningioma in women. Cancer. 2002;94(6):1626–1635. doi: 10.1002/cncr.10410. [DOI] [PubMed] [Google Scholar]
- 39.Siegal T, Siegal T. Current Considerations in the Management of Neoplastic Spinal-Cord Compression. Spine. 1989;14(2):223–228. doi: 10.1097/00007632-198902000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Helweglarsen S, Sorensen PS. Symptoms and Signs in Metastatic Spinal-Cord Compression - a Study of Progression from First Symptom until Diagnosis in 153 Patients. Eur J Cancer. 1994;30a(3):396–398. doi: 10.1016/0959-8049(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 41.Schiff D, ONeill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: Clinical features and diagnostic approach. Neurology. 1997;48(3):1033–1033. doi: 10.1212/wnl.49.2.452. [DOI] [PubMed] [Google Scholar]
- 42.Schiff D, O'Neill BP, Wang CH, O'Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593–1601. [PubMed] [Google Scholar]
- 43.Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 Updated Systematic Review and Clinical Practice Guideline for the Management of Malignant Extradural Spinal Cord Compression. Int J Radiat Oncol. 2012;84(2):312–317. doi: 10.1016/j.ijrobp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 45.Rades D, Dunst J, Schild SE. The first score predicting overall survival in patients with metastatic spinal cord compression. Cancer. 2008;112(1):157–161. doi: 10.1002/cncr.23150. [DOI] [PubMed] [Google Scholar]
- 46.Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following "separation surgery" and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redmond KJ, Lo SS, Soltys SG, et al. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine. 2017;26(3):299–306. doi: 10.3171/2016.8.SPINE16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weichselbaum RR, Liang H, Deng LF, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 49.Patsalides A, Yamada Y, Bilsky M, Lis E, Laufer I, Gobin YP. Spinal intraarterial chemotherapy: interim results of a Phase I clinical trial. J Neurosurg-Spine. 2016;24(2):217–222. doi: 10.3171/2015.5.SPINE14830. [DOI] [PubMed] [Google Scholar]
- 50.Tatsui CE, Lee SH, Amini B, et al. Spinal Laser Interstitial Thermal Therapy: A Novel Alternative to Surgery for Metastatic Epidural Spinal Cord Compression. Neurosurgery. 2016;79(Suppl 1):S73–S82. doi: 10.1227/NEU.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 51.Chen F, Xia YH, Cao WZ, et al. Percutaneous kyphoplasty for the treatment of spinal metastases. Oncol Lett. 2016;11(3):1799–1806. doi: 10.3892/ol.2016.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003;15(4):211–217. doi: 10.1016/s0936-6555(02)00400-4. [DOI] [PubMed] [Google Scholar]
- 53.Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal Metastases from Solid Tumors - Analysis of Factors Affecting Survival. Cancer. 1995;76(8):1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::aid-cncr2820760824>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 54.Kori SH, Foley KM, Posner JB. Brachial-Plexus Lesions in Patients with Cancer - 100 Cases. Neurology. 1981;31(1):45–50. doi: 10.1212/wnl.31.1.45. [DOI] [PubMed] [Google Scholar]
- 55.Gachiani J, Kim DH, Nelson A, Kline D. Management of metastatic tumors invading the peripheral nervous system. Neurosurg Focus. 2007;22(6):E14. [PubMed] [Google Scholar]
- 56.Jaeckle KA, Young DF, Foley KM. The Natural-History of Lumbosacral Plexopathy in Cancer. Neurology. 1985;35(1):8–15. doi: 10.1212/wnl.35.1.8. [DOI] [PubMed] [Google Scholar]
- 57.Jaeckle KA. Neurologic manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol. 2010;30(3):254–262. doi: 10.1055/s-0030-1255219. [DOI] [PubMed] [Google Scholar]
- 58.Thomas JE, Cascino TL, Earle JD. Differential-Diagnosis between Radiation and Tumor Plexopathy of the Pelvis. Neurology. 1985;35(1):1–7. doi: 10.1212/wnl.35.1.1. [DOI] [PubMed] [Google Scholar]
- 59.Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005;75(1):57–61. doi: 10.1007/s11060-004-8098-1. [DOI] [PubMed] [Google Scholar]
- 60.Su CY, Lui CC. Perineural invasion of the trigeminal nerve in patients with nasopharyngeal carcinoma - Imaging and clinical correlations. Cancer. 1996;78(10):2063–2069. doi: 10.1002/(sici)1097-0142(19961115)78:10<2063::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 61.Greenberg HS, Deck MDF, Vikram B, Chu FCH, Posner JB. Metastasis to the Base of the Skull - Clinical Findings in 43 Patients. Neurology. 1981;31(5):530–537. doi: 10.1212/wnl.31.5.530. [DOI] [PubMed] [Google Scholar]
- 62.Lossos A, Siegal T. Numb Chin Syndrome in Cancer-Patients - Etiology, Response to Treatment, and Prognostic-Significance. Neurology. 1992;42(6):1181–1184. doi: 10.1212/wnl.42.6.1181. [DOI] [PubMed] [Google Scholar]
- 63.Max MB, Deck MDF, Rottenberg DA. Pituitary Metastasis - Incidence in Cancer-Patients and Clinical-Differentiation from Pituitary-Adenoma. Neurology. 1981;31(8):998–1002. doi: 10.1212/wnl.31.8.998. [DOI] [PubMed] [Google Scholar]
- 64.Jansen BP, Smitt PAS. Cancer Neurology in Clinical Practice. Springer; 2003. Skull and dural metastases; pp. 87–92. [Google Scholar]
- 65.Miller RC, Foote RL, Coffey RJ, et al. The role of stereotactic radiosurgery in the treatment of malignant skull base tumors. Int J Radiat Oncol. 1997;39(5):977–981. doi: 10.1016/s0360-3016(97)00377-5. [DOI] [PubMed] [Google Scholar]
- 66.Constans JP, Dedivitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal Metastases with Neurological Manifestations - Review of 600 Cases. J Neurosurg. 1983;59(1):111–118. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert RW, Kim JH, Posner JB. Epidural Spinal-Cord Compression from Metastatic Tumor - Diagnosis and Treatment. Ann Neurol. 1978;3(1):40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 68.Bach F, Larsen BH, Rohde K, et al. Metastatic Spinal-Cord Compression - Occurrence, Symptoms, Clinical Presentations and Prognosis in 398 Patients with Spinal-Cord Compression. Acta Neurochir. 1990;107(1–2):37–43. doi: 10.1007/BF01402610. [DOI] [PubMed] [Google Scholar]