Abstract
Background
Maternal pre-pregnancy obesity, in term-born children, is associated with an increased risk of attention problems, however this relationship has not been explored among children born extremely preterm.
Aim
To estimate the risk of attention problems at age 10 years in children born very preterm to overweight (i.e., body mass index (BMI) 25-29 kg/m2) and obese (i.e., BMI ≥ 30 kg/m2) women relative to the risk among children born to women who were neither overweight nor obese (i.e. BMI < 25 kg/m2).
Study design
Multi-center prospective cohort study.
Methods
A total of 764 children born before the 28th week of gestation and whose mother’s pre-pregnancy height and pre-pregnancy weight were obtained at birth had an IQ ≥ 70 at age 10 years when parents and teachers completed Child Symptom Inventory-4 questionnaires that included items about the presence of ADHD.
Results
Compared to children whose mother’s pre-pregnancy weight was in the normal range (BMI <25 kg/m2), children were at increased risk of parent-identified ADHD behaviors if their mother was overweight (odds ratio (OR) = 1.9; 95% confidence interval (CI): 1.1, 3.3), or obese (OR = 2.3; 95% CI: 1.4, 3.9). They were not at increased risk of teacher-identified ADHD characteristics if their mother was overweight before her pregnancy (OR = 1.0; 95% CI: 0.6, 1.8), or obese (OR = 1.0; 95% CI: 0.6, 1.6).
Conclusion
Maternal overweight and obesity are associated with increased risk of parent-identified ADHD characteristics at 10 years of age in children born extremely preterm.
Introduction
More than one-third of women entering pregnancy in 2013-2014 in the United States were obese [1]. The offspring of obese mothers also appear to be at increased risk of developmental delays [2, 3], learning problems in reading and math skills [4], schizophrenia [5], autism [6] and attention problems (AP) [7-9]. More specifically, children born very preterm (<32 weeks) and/or with a very low birth weight (<1,500 g) score less favorably on parent and teacher ratings of attention problems compared to term-born controls [10-13]. In addition, the lower the gestational age, the higher the risk for attention problems [14, 15]. Furthermore, attention problems are more common in school-aged children born preterm than in children born near term [16-21].
Although both maternal obesity and prematurity are risk factors for attention problems in term-born children, to our knowledge, no report has assessed the relationship between high maternal pre-pregnancy body mass index (BMI) and attention problems among children born extremely preterm. Using data obtained from the ELGAN (Extremely Low Gestational Age Newborns) Study [22], we evaluated the hypothesis that compared to extremely preterm children born to women who had a normal pre-pregnancy weight (i.e., BMI < 25 kg/m2), those born to women who were overweight (i.e., BMI ≥ 25, < 30 kg/m2) or obese (i.e., BMI ≥ 30 kg/m2) before the pregnancy are more likely to have Attention Deficit Hyperactivity Disorder (ADHD) behaviors at age ten years. When the children were 10 years old, the ELGAN Study asked parents to complete the Child Symptom Inventory-4 (CSI-4). The child’s teacher was asked to do the same, thereby providing information about a second (the school) environment.
Methods
The ELGAN study
The ELGAN study is a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants [22]. Enrollees into this study included 1506 infants born before the 28th week of gestation during the years 2002-2004 and 1200 survived to 2 years. Of the 1198 known to be alive at age 10 years, 966 were targeted for recruitment. The enrollment and consent processes near the time of birth and at age 10 years were approved by the individual institutional review boards.
Demographic and pregnancy variables
After delivery, a trained research nurse interviewed each mother in her native language using a structured data collection form, and obtained information about characteristics of the mother and pregnancy, as well as exposures during pregnancy.
Maternal body mass index (BMI)
Each mother was asked to provide her height and pre-pregnancy weight shortly before, or shortly after delivery when they were interviewed, usually by a research nurse. These data were used to calculate her BMI. The United States government classifies BMIs as follows: < 18.5 kg/m2 is underweight, 18.5–24.9 kg/m2 is normal, 25.0–29.9 kg/m2 is overweight, 30.0–34.9 kg/m2 is obese, 35.0–39.9 kg/m2 is very obese, and ≥ 40 kg/m2 is extreme obesity [23]. We collapsed these BMI groups into < 25, 25-29.9, and ≥ 30 kg/m2.
Infant characteristics
Neonatal data were collected from the newborn’s medical record. The gestational age estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), last menstrual period without fetal ultrasound (7%), and gestational age recorded in the log of the neonatal intensive care unit (1%). The birth weight z-score represents the number of standard deviations the infant’s birth weight was above or below the median weight of infants at the same gestational age in referent samples not delivered for preeclampsia or fetal indications [24, 25].
ADHD assessment
While the child was being assessed for neurocognitive function and academic achievement, the parent or caregiver completed questionnaires regarding the child’s medical and neurological status and behavior, including the Child Symptom Inventory-4 Parent Checklist (CSI-4) [26, 27]. The child’s current teacher was also asked to complete the Child Symptom Inventory-4 Teacher Checklist. Teachers and parents did not make any ADD DSM-IV diagnosis. Rather, the CSI-4 program identified children as screening positive for these diagnoses based on the parents’ or teachers’ acknowledging selected characteristics of the child that aligned with DSM-IV symptoms.
Both the parent checklist and the teacher version include the same 18 items specific for ADHD symptoms (9 for the inattentive domain and 9 for the hyperactive/impulsive domain) that are each rated on a scale from 0 (never) to 3 (very often). For this study, a child was classified as screening positive for each ADHD type if s/he was reported to have 6 or more of the 9 “symptoms” either sometimes or very often. Parents and teachers differ considerably in whom they consider to have ADHD symptoms [28, 29]. Consequently, we evaluated parent-identified ADHD symptoms separately from teacher-identified ADHD symptoms.
Data analysis
We began data analysis by seeking to identify potential sources of bias associated with which children were and were not assessed (Supplement Table A). We then evaluated the null hypothesis that children whose mothers were overweight or obese were no more likely than their peers to have CSI-4-defined ADHD behaviors, first as identified by the parent, and then as identified by the teacher. In Table 2 and Supplement Tables C and D we tried to identify potential confounders of the relationship between maternal pre-pregnancy BMI and the child’s screening positive for ADHD. In essence, we were looking for characteristics that varied with BMI and ADHD symptoms identified by parent/teacher. The potential confounders identified this way and from a review from the literature included mother’s identification as Black, maternal age, mother’s years of education and government-provided insurance. These were then included in random-effects multivariable logistic regression models of parent-identified ADHD characteristics, and separately for teacher-identified ADHD characteristics, allowing us to calculate odds ratios and 95% confidence intervals (Table 3). Fertility treatment, a strong correlate of socio-economic class, was not included in these analyses because these models already had four other correlates of low social class, non-white race, low maternal age at the time of delivery, less than a high school education, and eligibility for public (government provided) medical care insurance. The random-effects models adjusted for the correlation among children from multifetal pregnancies.
Table 2.
The percent of children who had the characteristic at the top of the column who also had the characteristic listed on the left. These are column percents.
| Maternal characteristics | Maternal pre-pregnancy BMI (kg/m2) | ADHD symptoms identified by | ||||||
|---|---|---|---|---|---|---|---|---|
| Parent | Teacher | |||||||
| < 25 | 25,<30 | ≥ 30 | Yes | No | Yes | No | ||
| Racial identity | White | 72 | 55 | 60 | 64 | 67 | 55 | 73 |
| Black | 20 | 30 | 29 | 27 | 23 | 35 | 20 | |
| Other | 7 | 15 | 12 | 9 | 10 | 11 | 8 | |
| Hispanic | Yes | 5 | 15 | 13 | 7 | 9 | 7 | 8 |
| Maternal age | < 21 | 15 | 11 | 7 | 18 | 11 | 16 | 11 |
| 21-35 | 65 | 66 | 73 | 69 | 66 | 65 | 65 | |
| > 35 | 20 | 22 | 20 | 13 | 23 | 18 | 24 | |
| Years of education | < 12 | 36 | 46 | 39 | 48 | 36 | 50 | 37 |
| 12 to < 16 | 21 | 22 | 35 | 19 | 24 | 27 | 22 | |
| ≥ 16 | 44 | 32 | 26 | 32 | 39 | 22 | 40 | |
| Single | Yes | 64 | 64 | 56 | 53 | 65 | 64 | 66 |
| Self supported? | Yes | 68 | 70 | 69 | 69 | 69 | 39 | 39 |
| Public insurance | Yes | 29 | 34 | 38 | 44 | 29 | 43 | 29 |
| Pre-pregnancy Body Mass Index (BMI) |
<25 | 46 | 62 | 54 | 58 | |||
| 25, <30 | 25 | 18 | 21 | 19 | ||||
| ≥ 30 | 30 | 20 | 25 | 23 | ||||
| Fertility assistance | Yes | 27 | 21 | 21 | 19 | 26 | 15 | 25 |
| Maximum column N | 450 | 149 | 165 | 151 | 613 | 121 | 441 | |
ADHDSM = Attention deficit/hyperactivity entity according to criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
Table 3.
Odds ratios (point estimates and 95% confidence intervals) for parent or teacher identified ADHD at the top of the column associated with the risk factor listed on the left in models of that contain all the other variables that have an odds ratio in that column. Both models account for correlation between children from the same pregnancy.
| Variables | ADHD symptoms | ||
|---|---|---|---|
| Parent-identified | Teacher-identified | ||
| Maternal pre-pregnancy BMI (kg/m2) | 25,<30 | 1.9 (1.1, 3.3) | 1.0 (0.6, 1.8) |
| ≥ 30 | 2.3 (1.4, 3.9) | 1.0 (0.6, 1.6) | |
| Non-white race | Yes | ----- | 1.8 (1.1, 2.9) |
| Maternal age, years | <21 | 2.4 (1.1, 5.4) | 1.1 (0.5, 2.4) |
| 21-35 | 1.7 (0.97, 3.0) | 1.0 (0.6, 1.8) | |
| Years of education | <12 | 0.9 (0.6, 1.6) | 1.9 (1.04, 3.5) |
| 12, <16 | 0.6 (0.4, 1.1) | 1.9 (1.03, 3.4) | |
| Public insurance | Yes | 1.5 (0.9, 2.5) | 1.0 (0.6, 1.9) |
Results
Sample description (Table 1) and Characteristics of children eligible for recruitment (Supplement Table A)
Table 1.
Flowchart of sample description
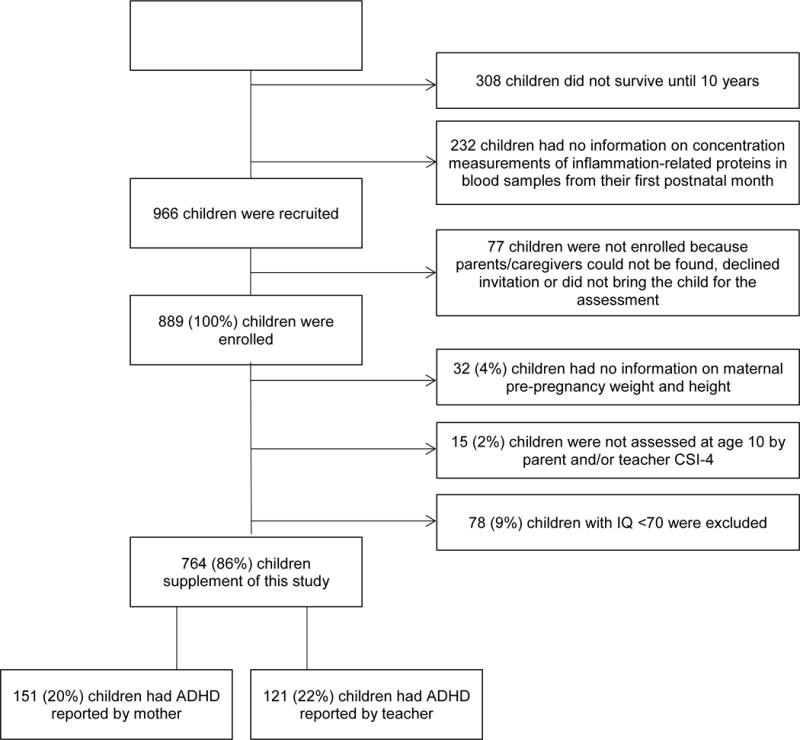
|
Of the 966 identified for recruitment, 889 were re-enrolled at age 10 years. Of the 889, 764 had an IQ ≥ 70, a CSI-4 was completed by a parent and/or a teacher, and 10 years previously, we had collected mother’s pre-pregnancy weight and height. They comprise the cohort for this report. A total of 151 children (20%) were reported to have ADHD symptoms by the mother, while a teacher identified 121 (22%).
Maternal and infant characteristics associated with CSI-4 availability (Supplement Table B)
Of the 77 eligible children not re-enrolled at age 10, 3 would not have been eligible for inclusion in this report because their mother’s BMI had not been obtained at birth. To identify potential sources of bias, we compared the remaining 74 children to the 764 for whom a CSI-4 was available. The children for whom a CSI-4 was available were more likely than the children not enrolled to have a mother who identified as White and non-Hispanic, was older at the time of delivery, had more formal education, was married at the time of delivery, was not eligible for government-provided medical care insurance, and received fertility (conception) assistance. The infants were more likely to be female, shared the uterus with a sibling, and gestationally older, and heavier at birth.
Maternal characteristics (Table 2)
In describing the contents of Supplement Tables C and D, we focus on those variables that are potential confounders of relationships between maternal BMI and offspring attention problems.
Overweight and obese women were more likely than others to identify as Black and the children of Black mothers were more likely than others to have teacher-identified ADHD behavioral characteristics. Women who delivered before age 21 years were under-represented among the obese, but their children were more likely than others to be identified as having ADHD behaviors by both them and the teacher. Women who had some post-college education were less likely than others to be overweight or obese and their children were less likely than others to be identified as having ADHD characteristics.
Obese women and to a smaller extent, overweight women, were more likely than women of normal pre-pregnancy weight to report that they were eligible for government-provided insurance. The children of these insurance-eligible women were more likely than others to be identified as having ADHD characteristics by both parent and teacher.
The last potential confounder in this table is another socioeconomic characteristic, fertility (conception) assistance. Obese and overweight women were less likely than their normal weight peers to report fertility assistance and the children whose mothers received fertility assistance were less likely than others to have parent- or teacher-identified ADHD behaviors.
Delivery characteristics (Supplement Table C) and Infant characteristics (Supplement Table D)
None of the characteristics in either table appears to be a potential confounder.
Multivariable analyses (Table 3)
We created multivariable models of the risk of parent- or teacher-identified ADHD that included mother’s pre-pregnancy overweight and obesity, and five indicators of her socioeconomic status, racial identification (but only for teacher-identified ADHD, not for parent-identified ADHD), age at the time of delivery, years of formal education, and eligibility for government-provided insurance. Table 3 is offered to show the odds ratios of ADHD symptoms associated with maternal pre-pregnancy overweight and obesity, and with potential confounders. The odds ratios of potential confounders should be as just that, and not as measures of causal effects [30].
In the model for parent-reported ADHD behaviors, the risk was increased if the mother’s pre-pregnancy BMI was in the overweight range (odds ratio (OR) = 1.9; 95% confidence interval (CI): 1.1, 3.3), or in the obese range (OR = 2.3; 95% CI: 1.4, 3.9). Only mother’s low age at the delivery contributed statistically significant information about increased risk (OR = 2.4; 95% CI: 1.1, 5.4).
In the model for teacher-identified ADHD characteristics, the risk was not increased if the mother was overweight before her pregnancy (OR = 1.0; 95% CI: 0.6, 1.8), or obese (OR = 1.0; 95% CI: 0.6, 1.6). Of the five socioeconomic indicators in the original model, only fertility assistance dropped out. Of the remaining four, two were associated with increased risk: mother’s identification as not white (OR = 1.8; 95% CI: 1.1, 2.9), and mother’s formal education (< 12 years: OR = 1.9; 95% CI: 1.04, 3.5, and 12, < 16 years: OR = 1.9; 95% CI: 1.03, 3.4).
Discussion
Our main findings are that mother’s pre-pregnancy overweight and obesity status are associated with increased risk of parent-identified ADHD characteristics, but not at increased risk of teacher-identified ADHD behaviors.
Previous studies of maternal obesity and attention problems
Of the studies that evaluated the relationship between maternal obesity and ADHD in childhood, none was limited to children born preterm. One prospective cohort study of school-age children found that children of mothers with pre-pregnancy overweight or obesity were more likely than others to have high ADHD symptom scores [7]. The small number of children in some groups (e.g., n=33 in the lean and large weight-gain group) limited the power of the findings. Three studies reported that children aged 6, 7 and 9 years, respectively, of obese mothers had significantly greater ADHD symptom severity than children of normal weight or overweight mothers [31-33]. Pre-pregnancy obesity was also associated with increased risk of offspring ADHD in children from 9 to 18 years old [34]. One study reported that maternal overweight was not associated with attention/hyperactivity problems [35].
Definition of ADHD behaviors
Studies evaluating the association between maternal BMI and attention problems used different rating scales for ADHD behaviors, making it difficult to compare the findings of these studies. The CSI-4, which was used in the present study to evaluate parent’s or teacher’s report on ADHD symptoms in the child, has not been adequately compared to other ADHD assessment instruments [36]. We acknowledge that the CSI-4 is not routinely used to assess the existence of ADHD characteristics [37], but certainly contains the symptoms that portray the DSM diagnosis of ADHD. The CSI-4 identifies children as screening positive for the inattentive and/or hyperactive/impulsive categories of DSM-IV-related ADHD. Because of poor agreement between parent and teacher about these domains, we chose to view all ADHD behaviors as “an important unitary component to ADHD symptoms” [38]. This decision is supported by concerns about the validity and stability of subtypes [29, 39-42].
Preterm birth and attention problems in children
Attention problems are more common in school-aged children born preterm than in children born near term [16-21]. Prematurity is a risk factor for ADHD. Indeed, the lower the gestational age at birth, the higher the risk of an attention deficit hyperactivity disorder (ADHD) [14, 15]. The CSI-4 is not a diagnostic instrument. Rather, it screens for symptoms likely to meet DSM-5 criteria. Although parents and teachers frequently did not agree on which children screened positive for ADHD, [43] parents and teachers differed minimally in the prevalence of screening children in our sample as positive for ADHD (20% and 22%, respectively).
Discrepancy between parent-identified and teacher-identified ADHD behaviors
We found that the children of overweight or obese mothers were at increased risk of parent-identified ADHD characteristics, but not at increased risk of teacher-identified ADHD behaviors. Although the rates of identification were approximately the same, the same children were not being identified by parents and teachers. This discrepancy might reflect, in part, the tendency of parents of ELGANs to view their children as vulnerable [44], and to have experienced psychological distress and express its consequences later [45]. This discrepancy might also reflect the teachers’ having observed many more children, and consequently having broader perspective than parents. In addition, teachers’ ADHD scores can show instability over time [46]. Another possible explanation for finding discrepancies between parent and teacher outcomes, include diverse settings in which parents and teachers observe children [47]. The teacher might see the child only when the child is medicated, or within the relatively structured environment of the classroom setting, whereas the parent has seen the child when not medicated and within the relatively unstructured home setting.
Socioeconomic relationships
Mother’s young age at time of delivery contributed statistically significant socio-economic information about increased risk on parent-identified ADHD. Mother’s identification as not white and mother’s limited formal education were potential confounders on teacher-identified ADHD. Young age at time of delivery [48, 49], low maternal education [8, 49-51], non-white maternal ethnicity [20], all indicators of low socio-economic status, appear to be associated with attention problems in children born prematurely. Others have also reported that indicators/correlates of low socioeconomic status, such as maternal smoking [35], Hispanic ethnicity [51, 52], and family monthly income [35, 48], housing tenure [48], and marital status [48] are associated with attention problems. The possibility of unmeasured confounding in this present study remains.
Maternal obesity can convey additional risk information
Maternal pre-pregnancy obesity is associated with inflammation in the offspring [53, 54] and epigenetic phenomena [55, 56]. Epigenetic processes have been invoked to explain the relationships between impaired development and social status [57], immaturity [58], and inflammation [59].
Observers
The American Academy of Pediatrics (AAP) clinical practice ADHD guideline recommends that reports of a child’s behaviors be collected from a classroom teacher and from a parent [60]. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) also requires “several inattentive or hyperactive-impulsive symptoms … in two or more settings [61].”
In the ELGAN Study the agreement between parent and teacher report was low. Thus, we decided to evaluate parent-identified ADHD behaviors separately from teacher-identified ADHD behaviors [43]. Our finding a relationship between both maternal pre-pregnancy overweight and maternal pre-pregnancy obesity with a child’s risk of parent-identified of ADHD behaviors, but not with teacher-identified ADHD behaviors adds to the literature about how teachers differ from parents in the behaviors they identify under the rubric of attention-related symptoms [62]. We have additional findings that the antecedents of parent-identified ADHD behaviors sometimes differ from the antecedents of teacher-identified ADHD behaviors [63].
Strengths and limitations
Among our study’s strengths are its prospective design, the large numbers of children, the enrollment of infants based on gestational age rather than birth weight [64], the low attrition rate, and the innovation of examining these features in the ELGAN population. Perhaps the major limitation of our study is the reliance on the gravida’s report of her pre-pregnancy weight. Women have a tendency to under-report their weight [65]. Consequently, our reliance on the mother’s report might have resulted in misclassification, and possible underestimation of the relationship between mother’s pre-pregnancy obesity and her child’s tendency to have ADHD symptoms. The limitations of the CSI-4 are acknowledged [66, 67].
Conclusion
Compared to the children of women with normal pre-pregnancy weight, those born to overweight and obese mothers appear to be at increased risk of parent-identified ADHD behaviors.
Supplementary Material
Highlights.
Children born very preterm (<32 weeks) have been described via parent and teacher ratings as showing significantly more attention problems when compared to children born near term.
Although both maternal obesity and prematurity have been shown to be risk factors for attention problems in children, to our knowledge, no report has assessed the relationship between high maternal pre-pregnancy body mass index (BMI) and attention problems 10 years later among children born extremely preterm.
Compared to the children of women with normal pre-pregnancy weight, those born to overweight and obese mothers appear to be at increased risk of parent-identified ADHD behaviors, but not of teacher-identified ADHD behaviors.
Acknowledgments
Participating institutions and ELGAN Study collaborators who made this report possible
Baystate Medical Center, Springfield MA (Bhavesh Shah, Karen Christianson)
Beth Israel Deaconess Medical Center, Boston MA (Camilia R. Martin, Colleen Hallisey, Caitlin Hurley, Miren Creixell)
Brigham & Women’s Hospital, Boston MA (Linda J. Van Marter) Children’s Hospital, Boston MA (Alan Leviton, Kathleen Lee, Anne McGovern, Elizabeth Allred, Jill Gambardella, Susan Ursprung, Ruth Blomquist)
Massachusetts General Hospital, Boston MA (Robert Insoft, Jennifer G. Wilson, Maureen Pimental)
New England Medical Center, Boston MA (Cynthia Cole, John Fiascone, Janet Madden, Ellen Nylen, Anne Furey)
U Mass Memorial Health Center, Worcester, MA (Francis Bednarek [deceased], Mary Naples, Beth Powers)
Yale-New Haven Hospital, New Haven CT (Richard Ehrenkranz, Joanne Williams, Elaine Romano)
Forsyth Hospital, Baptist Medical Center, Winston-Salem NC (T. Michael O’Shea, Debbie Gordon, Teresa Harold, Gail Hounsell, Debbie Hiatt)
University Health Systems of Eastern Carolina, Greenville NC (Stephen Engelke, Sherry Moseley, Linda Pare, Donna Smart, Joan Wilson)
North Carolina Children’s Hospital, Chapel Hill NC (Carl Bose, Gennie Bose, Janice Wereszczak)
DeVos Children’s Hospital, Grand Rapids MI (Mariel Portenga, Dinah Sutton)
Sparrow Hospital, Lansing MI (Padmani Karna, Carolyn Solomon)
University of Chicago Hospital, Chicago IL (Michael D. Schreiber, Grace Yoon)
William Beaumont Hospital, Royal Oak MI (Daniel Batton, Beth Kring)
Statement of financial support:
This study was supported by The National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2) and the National Institute of Child Health and Human Development (5P30HD018655-34).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
None of the authors has any financial issue or conflict of interest to disclose.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basatemur E, Gardiner J, Williams C, Melhuish E, Barnes J, Sutcliffe A. Maternal prepregnancy BMI and child cognition: a longitudinal cohort study. Pediatrics. 2013;131(1):56–63. doi: 10.1542/peds.2012-0788. [DOI] [PubMed] [Google Scholar]
- 3.Craig WY, Palomaki GE, Neveux LM, Haddow JE. Maternal body mass index during pregnancy and offspring neurocognitive development. Obstet Med. 2013;(6):20–5. doi: 10.1177/1753495X12472643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanda R, Salsberry PJ, Reagan PB, Fang MZ. The impact of prepregnancy obesity on children’s cognitive test scores. Matern Child Health J. 2013;17(2):222–9. doi: 10.1007/s10995-012-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai M, Minabe Y, Takagai S, Ogai M, Matsumoto H, Mori N, et al. Poor maternal care and high maternal body mass index in pregnancy as a risk factor for schizophrenia in offspring. Acta Psychiatr Scand. 2004;110(4):257–63. doi: 10.1111/j.1600-0447.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- 6.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes. 2008;32(3):550–7. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry. 2010;51(2):134–43. doi: 10.1111/j.1469-7610.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 9.Mina TH, Lahti M, Drake AJ, Raikkonen K, Minnis H, Denison FC, et al. Prenatal exposure to very severe maternal obesity is associated with adverse neuropsychiatric outcomes in children. Psychol Med. 2017;47(2):353–62. doi: 10.1017/S0033291716002452. [DOI] [PubMed] [Google Scholar]
- 10.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 11.Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, et al. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. Journal of Developmental and Behavioral Pediatrics. 2012;33(3):202. doi: 10.1097/DBP.0b013e3182475287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson S, Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin Fetal Neonatal Med. 2014;19(2):97–104. doi: 10.1016/j.siny.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Vanderbilt D, Gleason MM. Mental health concerns of the premature infant through the lifespan. Child Adolesc Psychiatr Clin N Am. 2010;19(2):211–28. vii–viii. doi: 10.1016/j.chc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 14.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry. 2013;70(11):1231–40. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, et al. Preterm birth and poor fetal growth as risk factors of attention-deficit/hyperactivity disorder. Pediatrics. 2015;136(3):e599–e608. doi: 10.1542/peds.2015-1043. [DOI] [PubMed] [Google Scholar]
- 16.Stjernqvist K, Svenningsen NW. Ten-year follow-up of children born before 29 gestational weeks: health, cognitive development, behaviour and school achievement. Acta Paediatr. 1999;88(5):557–62. doi: 10.1080/08035259950169594. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi A, Hagglof B, Sedin G, Gothefors L, Serenius F. Mental health and social competencies of 10- to 12-year-old children born at 23 to 25 weeks of gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2007;120(1):118–33. doi: 10.1542/peds.2006-2988. [DOI] [PubMed] [Google Scholar]
- 18.Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485–92. doi: 10.1542/peds.2008-1216. [DOI] [PubMed] [Google Scholar]
- 19.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(5):453–63.e1. [PubMed] [Google Scholar]
- 20.Johnson S, Kochhar P, Hennessy E, Marlow N, Wolke D, Hollis C. Antecedents of Attention-Deficit/Hyperactivity Disorder Symptoms in Children Born Extremely Preterm. J Dev Behav Pediatr. 2016;37(4):285–97. doi: 10.1097/DBP.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bora S, Pritchard VE, Chen Z, Inder TE, Woodward LJ. Neonatal cerebral morphometry and later risk of persistent inattention/hyperactivity in children born very preterm. J Child Psychol Psychiatry. 2014;55(7):828–38. doi: 10.1111/jcpp.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NIH. Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks: National Institute of Health. [Available from: http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/bmi_dis.htm]
- 24.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 25.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46(5):566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc Psychol. 2002;31(4):513–24. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- 27.Gadow KD, Sprafkin J. Child Symptom Inventory–4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- 28.Narad ME, Garner AA, Peugh JL, Tamm L, Antonini TN, Kingery KM, et al. Parent-teacher agreement on ADHD symptoms across development. Psychol Assess. 2015;27(1):239–48. doi: 10.1037/a0037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valo S, Tannock R. Diagnostic instability of DSM-IV ADHD subtypes: effects of informant source, instrumentation, and methods for combining symptom reports. J Clin Child Adolesc Psychol. 2010;39(6):749–60. doi: 10.1080/15374416.2010.517172. [DOI] [PubMed] [Google Scholar]
- 30.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–8. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS One. 2012;7(6):e37758. doi: 10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, Lind JN. Maternal Prepregnancy Body Mass Index and Child Psychosocial Development at 6 Years of Age. Pediatrics. 2015 doi: 10.1542/peds.2014-3058. peds. 2014-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, et al. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG. 2016;123(13):2094–103. doi: 10.1111/1471-0528.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q, Sjolander A, Langstrom N, Rodriguez A, Serlachius E, D’Onofrio BM, et al. Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. Int J Epidemiol. 2014;43(1):83–90. doi: 10.1093/ije/dyt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brion MJ, Zeegers M, Jaddoe V, Verhulst F, Tiemeier H, Lawlor DA, et al. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics. 2011;127(1):202–11. doi: 10.1542/peds.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collett BR, Ohan JL, Myers KM. Ten-year review of rating scales. V: scales assessing attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(9):1015–37. doi: 10.1097/01.CHI.0000070245.24125.B6. [DOI] [PubMed] [Google Scholar]
- 37.Chang LY, Wang MY, Tsai PS. Diagnostic Accuracy of Rating Scales for Attention-Deficit/Hyperactivity Disorder: A Meta-analysis. Pediatrics. 2016;137(3):e20152749. doi: 10.1542/peds.2015-2749. [DOI] [PubMed] [Google Scholar]
- 38.Toplak ME, Pitch A, Flora DB, Iwenofu L, Ghelani K, Jain U, et al. The unity and diversity of inattention and hyperactivity/impulsivity in ADHD: evidence for a general factor with separable dimensions. J Abnorm Child Psychol. 2009;37(8):1137–50. doi: 10.1007/s10802-009-9336-y. [DOI] [PubMed] [Google Scholar]
- 39.Bernfeld J. ADHD and factor analysis: are there really three distinct subtypes of ADHD? Appl Neuropsychol Child. 2012;1(2):100–4. doi: 10.1080/21622965.2012.699421. [DOI] [PubMed] [Google Scholar]
- 40.Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62(8):896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- 41.Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srebnicki T, Kolakowski A, Wolanczyk T. Adolescent outcome of child ADHD in primary care setting: stability of diagnosis. J Atten Disord. 2013;17(8):655–9. doi: 10.1177/1087054712437583. [DOI] [PubMed] [Google Scholar]
- 43.Leviton A, Hunter SJ, Scott MN, Hooper SR, Joseph RM, O’Shea TM, et al. Observer variability and agreement identifying attention deficit hyperactivity disorder in 10-year old children born very preterm. Acta Paediatr. 2017;106(8):1317–22. doi: 10.1111/apa.13869. [DOI] [PubMed] [Google Scholar]
- 44.Tallandini MA, Morsan V, Gronchi G, Macagno F. Systematic and Meta-Analytic Review: Triggering Agents of Parental Perception of Child’s Vulnerability in Instances of Preterm Birth. J Pediatr Psychol. 2015;40(6):545–53. doi: 10.1093/jpepsy/jsv010. [DOI] [PubMed] [Google Scholar]
- 45.Holditch-Davis D, Santos H, Levy J, White-Traut R, O’Shea TM, Geraldo V, et al. Patterns of psychological distress in mothers of preterm infants. Infant Behav Dev. 2015;41:154–63. doi: 10.1016/j.infbeh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabiner DL, Murray DW, Rosen L, Hardy K, Skinner A, Underwood M. Instability in teacher ratings of children’s inattentive symptoms: implications for the assessment of ADHD. J Dev Behav Pediatr. 2010;31(3):175–80. doi: 10.1097/DBP.0b013e3181d5a2d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray DW, Kollins SH, Hardy KK, Abikoff HB, Swanson JM, Cunningham C, et al. Parent versus teacher ratings of attention-deficit/hyperactivity disorder symptoms in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) Journal of Child and Adolescent Psychopharmacology. 2007;17(5):605–19. doi: 10.1089/cap.2007.0060. [DOI] [PubMed] [Google Scholar]
- 48.Russell AE, Ford T, Russell G. Socioeconomic Associations with ADHD: Findings from a Mediation Analysis. PloS one. 2015;10(6):e0128248. doi: 10.1371/journal.pone.0128248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinonen K, Raikkonen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, et al. Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: a longitudinal study. BMC Pediatr. 2010;10:91. doi: 10.1186/1471-2431-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindstrom K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics. 2011;127(5):858–65. doi: 10.1542/peds.2010-1279. [DOI] [PubMed] [Google Scholar]
- 51.Gray RF, Indurkhya A, McCormick MC. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004;114(3):736–43. doi: 10.1542/peds.2003-1150-L. [DOI] [PubMed] [Google Scholar]
- 52.Russell AE, Ford T, Williams R, Russell G. The Association Between Socioeconomic Disadvantage and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review. Child Psychiatry Hum Dev. 2016;47(3):440–58. doi: 10.1007/s10578-015-0578-3. [DOI] [PubMed] [Google Scholar]
- 53.van der Burg JW, Allred EN, McElrath TF, Fichorova RN, Kuban K, O’Shea TM, et al. Is maternal obesity associated with sustained inflammation in extremely low gestational age newborns? Early Hum Dev. 2013;89(12):949–55. doi: 10.1016/j.earlhumdev.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Thakali KM, Saben J, Faske JB, Lindsey F, Gomez-Acevedo H, Lowery CL, Jr, et al. Maternal pregravid obesity changes gene expression profiles toward greater inflammation and reduced insulin sensitivity in umbilical cord. Pediatr Res. 2014;76(2):202–10. doi: 10.1038/pr.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharp GC, Lawlor DA, Richmond RC, Fraser A, Simpkin A, Suderman M, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2015;44(4):1288–304. doi: 10.1093/ije/dyv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neri C, Edlow AG. Effects of Maternal Obesity on Fetal Programming: Molecular Approaches. Cold Spring Harbor perspectives in medicine. 2016;6(2):a026591. doi: 10.1101/cshperspect.a026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naumova OY, Hein S, Suderman M, Barbot B, Lee M, Raefski A, et al. Epigenetic Patterns Modulate the Connection Between Developmental Dynamics of Parenting and Offspring Psychosocial Adjustment. Child development. 2016;87(1):98–110. doi: 10.1111/cdev.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruickshank MN, Oshlack A, Theda C, Davis PG, Martino D, Sheehan P, et al. Analysis of epigenetic changes in survivors of preterm birth reveals the effect of gestational age and evidence for a long term legacy. Genome medicine. 2013;5(10):96. doi: 10.1186/gm500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claycombe KJ, Brissette CA, Ghribi O. Epigenetics of inflammation, maternal infection, and nutrition. The Journal of nutrition. 2015;145(5):1109S–15S. doi: 10.3945/jn.114.194639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pediatrics AAo. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. American Academy of Pediatrics. Pediatrics. 2000;105(5):1158–70. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 61.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. 2013. Observer variability and agreement identifying attention deficit hyperactivity disorder in 10-year old children born very preterm. DSM-5. [Google Scholar]
- 62.Dirks MA, De Los Reyes A, Briggs Gowan M, Cella D, Wakschlag LS. Annual Research Review: Embracing not erasing contextual variability in children’s behavior–theory and utility in the selection and use of methods and informants in developmental psychopathology. Journal of Child Psychology and Psychiatry. 2012;53(5):558–74. doi: 10.1111/j.1469-7610.2012.02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leviton A, Hooper SR, Hunter SJ, Scott MN, Allred EN, Joseph RM, et al. Antecedents of screening positive for attention deficit hyperactivity disorder in 10-year old children born extremely preterm. doi: 10.1016/j.pediatrneurol.2017.12.010. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–13. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 65.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev. 2017;18(3):350–69. doi: 10.1111/obr.12486. [DOI] [PubMed] [Google Scholar]
- 66.Green JL, Sciberras E, Anderson V, Efron D, Rinehart N. Association between autism symptoms and functioning in children with ADHD. Arch Dis Child. 2016 doi: 10.1136/archdischild-2015-310257. [DOI] [PubMed] [Google Scholar]
- 67.DeVincent CJ, Gadow KD. Relative clinical utility of three Child Symptom Inventory-4 scoring algorithms for differentiating children with autism spectrum disorder vs. attention-deficit hyperactivity disorder. Autism Res. 2009;2(6):312–21. doi: 10.1002/aur.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


