Abstract
Objective
To describe predictors for health-related quality of life, participation, physical activity and cognitive function in patients with intensive care unit (ICU)-acquired muscle weakness 1 year after discharge from rehabilitation.
Design
This is a cohort study.
Participants
We included 150 chronic critically ill individuals with ICU-acquired muscle weakness.
Setting
Postacute ICU and rehabilitation units in Germany.
Measures
We measured health-related quality of life using the EQ-5D, participation using the Reintegration of Normal Living Index, physical activity using the Physical Activity Scale for Individuals With Physical Disabilities, and basal cognitive function using the Montreal Cognitive Assessment (MoCA) at 6 months, and the Clock Drawing Test 6 and 12 months after discharge from postacute treatment. We described the predictors of the results at 12 months.
Results
The best predictors for good health-related quality of life 1 year after discharge were the time until regaining walking ability (OR=0.96, OR per day, 95% CI 0.93 to 0.99) and the mean MoCA score on admission to our postacute ICU and rehabilitation units (OR=1.25,95% CI 1.02 to 1.52). The best predictor for good participation 1 year after discharge was the MoCA sum score on admission to our postacute ICU and rehabilitation units (OR=0.85,95% CI 0.72 to 1.00). The best predictor for good physical activity 1 year after discharge was the Apache sum score on admission to our postacute ICU and rehabilitation units (OR=1.68,95% CI 0.89 to 3.13). The best predictor for normal cognitive function 1 year after discharge was regaining walking function in rehabilitation (OR=8.0,95% CI 0.49 to 13.69).
Conclusion
Recovery of health-related quality of life, participation, physical activity and basal cognitive function was still not complete 12 months after discharge from postacute treatment. We described the predictors for these important outcomes in participants with ICU-acquired muscle weakness 1 year after discharge from rehabilitation.
Trial registration number
DRKS00007181.
Keywords: rehabilitation medicine
Strengths and limitations of this study.
Patient-centred outcomes in the first 12 months of intensive care unit (ICU)-acquired muscle weakness after discharge from postacute ICU and rehabilitation units were documented.
Clinical scores on admission may predict recovery of health-related quality of life, participation, physical activity and cognitive function of individuals with ICU-acquired muscle weakness.
Some of the severely affected ICU patients (eg, patients who were sedated) were excluded from this study.
Electromyography was not used in the study.
Introduction
A prolonged stay at an intensive care unit (ICU) is often associated with long-term impairments of physical and mental health. The Society of Critical Care Medicine recommends a comprehensive treatment for ICU survivors during all phases of recovery.1
One frequent physical impairment after ICU treatment is acquired muscle weakness (ICUAW).2–4 Acquired muscle weakness is characterised by a profound weakness that is greater than normally expected from prolonged bed-rest, and is therefore defined as clinically detected weakness in critically ill patients in whom there is no plausible aetiology other than critical illness.3–5 ICUAW often affects the peripheral as well as the respiratory muscles, limits the activities of daily living, and delays rehabilitation and recovery.6–10
Although full recovery has been reported in approximately 50% of people with ICUAW, progress is related to the severity of the condition (eg, people with severe weakness may take months to improve).5 11 Recent studies showed that ICUAW may have longer term consequences, beyond the acute phase. For example, ICUAW may be an important contributor to poor postintensive care outcomes that include decreased physical, mental and cognitive dysfunctions, which extend beyond the acute hospitalisation and have major impact on the quality of life of ICU survivors.12 Wieske et al13 investigated post-ICU mortality and physical functioning in 80 patients with acquired weakness at 6 months after ICU discharge. They found that ICU-acquired weakness is independently associated with post-ICU mortality and with decreased physical function at 6 months after ICU discharge. In other studies the presence of ICUAW at discharge from ICU was associated with poor long-term outcome (eg, health-related quality of life).14–16 In a 2-year follow-up of 222 survivors of severe critical illness, patients with ICUAW had reduced health-related quality of life compared with patients with normal strength.17
Longitudinal studies have described the recovery of critically ill people with relatively short ICU stay5 13 17–19; however, few studies have been conducted examining critically ill people with ICUAW after long ICU stay (eg, longer than 21 days of ICU treatment).20 Furthermore information is lacking to predict health-related quality of life, participation, physical activity and cognitive function of patients with ICUAW after a long ICU care.
In summary not much is known about participation, physical activity and cognitive function in patients with ICUAW 1 year after discharge from postacute ICU and rehabilitation units (post-ICUaR). Post-ICUaR patients with mechanical respiratory weaning failure and a long ICU stay (ie, more than 14 days of mechanical ventilation) are treated with high-intensity physiotherapy and occupational therapy in order to be weaned off of mechanical ventilation and become independent in daily life activities. Therefore one of the aims of the General Weakness Syndrome Therapy (GymNAST) study was to describe the follow-up results of a patient cohort with ICUAW, and to identify predictors for health-related quality of life, participation, activities and cognitive function in patients with ICUAW after a long ICU and post-ICUaR stay in Germany at 1-year follow-up.21
Methods and analysis
We followed our published protocol for the GymNAST cohort study and describe the results of this preplanned follow-up according to the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology.
We used the following inclusion and exclusion criteria for this cohort:
Inclusion criteria
Participants have chronic critical illness or a contemporary history of chronic critical illness. Chronic critical illness was defined as more than 21 days of ICU treatment including mechanical ventilation and at least 14 additional days with a need for ICU treatment.22 23
Muscle weakness defined as a Medical Research Council (MRC) sum score of less than 48 points.3
A defined reason for ICUAW, such as a clinical diagnosis of critical illness myopathy (CIM) and critical illness polyneuropathy (CIP).24
18 years old or older.
Richmond Agitation Sedation Scale score from −1 to 225 on admission to our post-ICUaR (study onset).
Written informed consent from the participant or legal guardian.
Exclusion criteria
Individuals receiving palliative care.
Comorbidities of the trunk or the lower limbs interfering with upright posture and walking function (eg, amputation or fracture of the lower limb).
Other neuromuscular or neurological disease and/or syndromes causing weakness in patients in the ICU (eg, Guillain-Barré syndrome, myasthenia gravis, porphyria, Lambert-Eaton syndrome, amyotrophic lateral sclerosis, vasculitic neuropathy, cervical myelopathy and botulism).
Severe physical comorbidity before becoming critically ill (eg, muscle weakness due to neurological conditions such as stroke).
All participants included in the GymNAST study were followed after discharge from our post-ICUaR of the Klinik Bavaria Kreischa in Germany (as previously reported).21
Measures and outcomes
According to our protocol,21 our long term follow-up of 6 months and 12 months included the following:
Health-related quality of life, measured with the EQ-5D.26 We used the EQ-5D due to our good experience in a recent cohort study where we used this measurement in severely affected individuals via postal questionnaires.27 In the former multicentre study, we found the EQ-5D was very practical, easy to administer even to severely affected individuals and also sensitive to detecting changes in a group of patients within a comparable stage of rehabilitation, age, physical severity and diagnosis as in this cohort.
Participation, measured with the Reintegration of Normal Living Index (RNL-Index).28 29 We used this measurement to measure participation because we have had very good clinical experience with the RNL-Index in severely affected individuals (comparable with participants in this cohort) and have used this outcome measure for more than 15 years to measure the success of participation after discharge from inpatient rehabilitation. The RNL-Index appears to be a very useful tool to measure participation and was recently used in a cohort study investigating the long-term consequences after neurosurgery.30 In the literature, however, we could not find information on test–retest reliability and information on the criterion or concurrent validity of the RNL-Index in the specific population of critically ill people with ICUAW.
Physical activities, measured with the Physical Activity Scale for Individuals With Physical Disabilities (PASIPD).31 32 The PASIPD has not yet been conclusively investigated for chronically critically ill patients with ICUAW.33–35 Despite this limitation we used the PASIPD because our pilot investigation found excellent inter-rater reliability (ϱ>0.8) when comparing the results of two independent raters who examined 20 patients with ICUAW. In addition we have had very good clinical experience in using this scale especially in patients with ICUAW who have severe speaking and swallowing dysfunction,24 36 and we found the PASIPD particularly useful as a postal questionnaire to get appropriate information on important long-term physical activity information from our patients who are chronically critically ill. Although we could not find investigations on the reliability of the PASIPD in the specific population of critically ill people with ICUAW, the PASIPD has been described to be reliable and valid when used in patients with disabilities (eg, subacute and/or and chronic stroke), with a good test–retest reliability of r=0.77.37 38 In the literature, however, we could not find information on the criterion or concurrent validity of the RNL-Index in the specific population of critically ill people with ICUAW. One study provided information on factor analysis of the PASIPD in people with disabilities, and the Cronbach’s alpha coefficients ranged from 0.37 to 0.65, indicating low-to-moderate internal consistency within factors.39
Cognitive function with the Montreal Cognitive Assessment (MoCA)40 at 6 months and the Clock Drawing Test (CDT)41 at 6 and 12 months. The MoCa and the CDT have been shown to have excellent reliability and concurrent validity in neurological patient cohorts which are comparable with the participants of the present study.42 43 However, the reliability and concurrent validity of the MoCA and the CDT have not yet been conclusively investigated for chronically critically ill patients with ICUAW.33–35 Despite this limitation we used both scales as pragmatic cognitive assessments to examine the reduced attention span and reduced cognitive capacity of our participants. In our pilot investigation we found excellent inter-rater reliability for both the MoCA (ϱ>0.8) and the CDT (ϰ >0.8). In addition we have had very good clinical experience in using these scales especially in patients with ICUAW with severe speaking and swallowing dysfunction.24 36
In addition, we decided to use these four outcomes in our protocol because they appear to be neither closely contextually nor statistically related to one another.
Our four main outcomes were assessed via standardised postal questionnaires at 6 and 12 months after discharge from rehabilitation, with up to three telephone reminders. These four measures were designated as our dependent variables.
To explain these dependent variables at 12 months in a multivariate logistic regression analysis, we critically discussed and reached consensus in our team to set the following cut-offs:
We defined good health-related quality of life as a score of ≥70 points on the EQ-5D Visual Analogue Scale (VAS) (0–100). This specific cut-off was used according to the results of a recent study including 3109 individuals where the mean score of ≥70 points visibly distinguished between healthy and non-healthy people of a similar age (eg, after myocardial infarct or stroke).44 Additionally we found this specific cut-off very useful in distinguishing patient groups with good or poor recovery in a recent multicentre cohort study including 754 people from 16 postacute hospitals and inpatient rehabilitation units of comparable age and severity.27
We defined good participation (reintegration in normal living) as a score of ≥75 points on the RNL-Index (0–100).
We defined good physical activity as a score of ≥20 points on the PASIPD (0–100).
We defined good cognitive function (ie, within the normal range) as the best possible score of 1 point on the CDT (score of 1–6); the best score in this test is 1 point and the worst score is 6 points.
We assessed the following outcomes on admission to our post-ICUaR as independent (ie, predictor) variables (to explain the above-mentioned dependent variables):
Activities of daily living measured with the Barthel Index (10 items).45
Clinical severity measured with Apache II score (a lower score is better than a higher score).
Muscle strength of the upper (shoulder, elbow and wrist) and lower limb (hip, knee and ankle) using the MRC for the upper and lower limbs.3 46
Summed grip strength of both hands (measured bilaterally using a dynamometer).47 48
Functional Status Score for the Intensive Care Unit (FSS-ICU; scores range from 0 to 35, with higher scores indicating better physical functioning).49 50
Physical Function ICU Test (scored) (PFIT-s; scores range from 0 to 10, with higher scores indicating better physical functioning).51 52
Pain using a Numeric Pain Rating Scale.53
Walking speed (m/s) and walking endurance (metres walked in 6 min).6 14
Ability to walk measured with the Functional Ambulation Categories (FAC; score of 0–5, with higher scores representing better function).54
Time of regaining walking ability (defined as the time between study entry on admission to our post-ICUaR and the date of reaching an FAC equal or more than 3; the time between these dates is the time span used here).
All assessments and standardised measures were administered by trained and experienced assessors (clinical experience of more than 10 years) during the first week in our post-ICUaR. All examiners where trained for 2 weeks prior to the study onset. After this we used specific training videos of patients with different severity to train the assessor again, and as a result we achieved a high concordance (reliability coefficients of greater than 0.8) between the examiners.
We defined baseline as the first admission to our post-ICUaR. Based on this definition the duration of illness was defined as the time between the very first day in ICU (first admission to the acute hospital due to the onset of primary illness) until the study onset (baseline, admission to our post-ICUaR).
Patient involvement
No patients were directly involved in the development of our research question, the selection of the outcome measures, the design and the implementation of our study, or the interpretation of our results.
Statistical analyses
We used descriptive analyses (eg, median and IQR, and mean and SD) of continuous variables and frequencies and proportions of categorical variables as appropriate.55 The global alpha level was set at 0.05.
Our four main dependent variables were (1) health-related quality of life (EQ-5D VAS), (2) participation (RNL-Index), (3) physical activities (PASIPD) and (4) cognitive function (CDT) at 12-month follow-up. We used univariate and multivariate logistic regression analyses with a selection of possible predictor variables to predict the outcome of our four dependent variables.56
According to Stoltzfus57 we tested and confirmed the following key assumptions for logistic regression:
Dependent variable is binary.
Observations are independent of each other and do not come from repeated measurements or matching data.
A low or no multicollinearity between the independent variables.
The independent variables are linked linearly to the log odds.
An adequate sample size.
To assess the final regression model fit, we examined the residuals of the final model (ie, we examined the difference between the estimated and observed values of the dependent variable).
Univariate analysis
In our univariate logistic regression analysis, we included the following as possible predictor variables: age at study onset, body mass index, sex, duration of illness, number of medical tubes (catheters and vascular access), duration of mechanical ventilation, number secondary diagnosis, Apache II, ability to reach forward, FSS-ICU score, PFIT-s score, grip strength, MRC sum score of the upper limb, MRC sum score of the lower limb and VAS. We did univariate logistic regression analysis of these possible predictor variables and listed the results.
Multivariate analysis and model building
After the univariate logistic regression analysis and listing of the results, we selected all clinically meaningful and statistically significant variables (ie, alpha level of 0.2 for selection) as candidate predictor variables. Afterwards we used a stepwise logistic regression analysis with all candidate predictor variables. Variables with the highest global scores were selected first into a multivariable model.58–60 In the process of stepwise regression, a predictor variable had to be significant at the 0.2 level to be entered into the multivariate model, and a variable in the model had to be significant at the 0.1 level to remain in the multivariate model. The aim of our analysis was to explain the dependent variable by a multivariate logistic model with a small number of variables. Therefore we limited this model-building process to a maximum of four variables in the multivariate model. After this process we compared the multivariate models (with the remaining variables) on the global score χ2 statistic (ie, best subset selection) and on the Hosmer-Lemeshow goodness-of-fit test to decide for our final multivariate model.56 We expressed the effects of our final multivariate model as ORs with 95% CIs. We used SAS/STAT V.9.3 for all statistical procedures, and the statistical assumptions were tested with the implemented function of the PROC LOGISTIC of SAS/STAT V.9.3 according to specific recommendations for this approach.60
Results
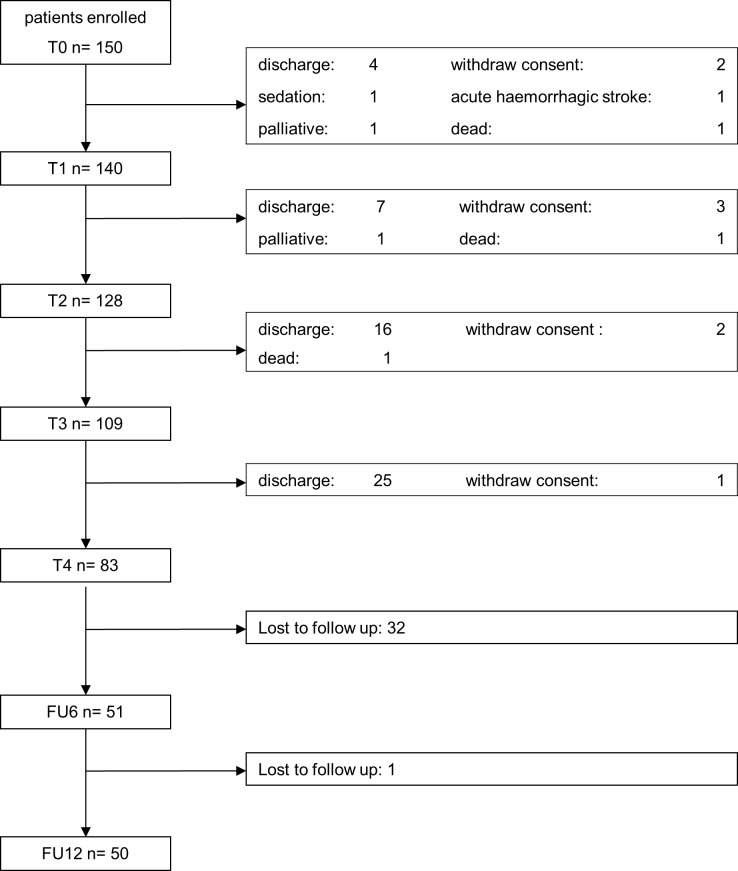
We screened 1387 patients between January 2013 and March 2015 and included 150 participants with ICUAW (30% female) in our cohort study (see figure 1 and table 1). From these initially 150 recruited participants, we were able to follow 51 (34%) at 6 months and 50 (33%) at 12 months after their postacute inpatient treatment. The demographic and clinical characteristics at baseline are shown in table 1.
Figure 1.
Flow chart. FU, follow-up.
Table 1.
Baseline characteristics (measured at first admission to our postacute hospital or our inpatient rehabilitation)
| Variable (n=150) | Median (IQR) | Mean (SD) |
| Age (years) | 71 (12) | 69.16 (9.02) |
| BMI (points) | 27.4 (6.7) | 29.11 (8.25) |
| Duration of illness (days)* | 41 (30) | 49.13 (29.13) |
| Duration of mechanical ventilation (days) | 53 (42) | 65.22 (45.14) |
| Apache II (points) | 16 (5) | 16.45 (4.08) |
| Barthel Index (points) | 5 (25) | 14.68 (19.20) |
| MRC sum score at baseline, upper limb | 9.5 (3.25) | 0.5 (0.8) |
| MRC sum score at baseline, lower limb | 9 (3.25) | 0.5 (0.8) |
| MoCA score at baseline (points) | 16 (10) | 14.3 (7.0) |
| CDT score at baseline (points) | 4 (4) | 3.9 (1.8) |
*Duration of illness was defined as the time between the very first day on ICU (first admission to the acute hospital due to the onset of primary illness) until the study onset (admission to our postacute hospital or our inpatient rehabilitation).
BMI, body mass index; CDT, Clock Drawing Test; ICU, intensive care unit; MoCA, Montreal Cognitive Assessment; MRC, Medical Research Council.
The detailed results for the four main outcome variables—(1) health-related quality of life (EQ-5D), (2) participation (RNL-Index), (3) physical activities (PASIPD) and (4) cognitive function (MoCA/CDT)—at 6-month and 12-month follow-up are shown in table 2.
Table 2.
Results of follow-up at 6 and 12 months
| Variable (n=51) | Median (IQR) | Mean (SD) |
| Health-related quality of life | ||
| EQ-5D, score (0–60) at 6 months | 40 (20) | 41.4 (11.7) |
| EQ-5D, score (0–60) at 12 months | 40 (20) | 39.8 (11.2) |
| EQ-5D VAS (0–100) at 6 months | 60 (30) | 59.9 (22.2) |
| EQ-5D VAS (0–100) at 12 months | 60 (29) | 61.9 (20.8) |
| Participation, RNL-Index (0–100) at 6 months |
65.5 (42) | 64.3 (27.9) |
| Participation, RNL-Index (0–100) at 12 months | 68.2 (53) | 65.9 (27.2) |
| Physical activities, PASIPD (0–100) at 6 months | 7.9 (17.4) | 16.2 (22.4) |
| Physical activities, PASIPD (0–100) at 12 months | 7.9 (17.3) | 12.3 (13.6) |
| Cognitive function | ||
| MoCA (0–30) at 6 months | 24 (5) | 22.8 (5.2) |
| MoCA (0–30) at 12 months | NA | NA |
| CDT (0–6) at 6 months | 1 (1) | 1.7 (1.1) |
| CDT (0–6) at 12 months | 1 (0) | 1.4 (0.8) |
| Pain VAS (0–10) at 6 months | 4 (4) | 4.2 (3.0) |
| Pain VAS (0–10) at 12 months | 3 (6) | 3.5 (3.1) |
CDT, Clock Drawing Test; EQ-5D, health-related quality of life; MoCA, Montreal Cognitive Assessment; NA, not available; PASIPD, Physical Activity Scale for Individuals With Physical Disabilities; RNL-Index, Reintegration of Normal Living Index; VAS, Visual Analogue Scale.
Good health-related quality of life, with a score of ≥70 points on the EQ-5D-VAS (0–100), was reached by 44% of all followed patients 12 months after discharge from rehabilitation. Good participation, with a score of ≥75 points on the RNL-Index (0–100), was reached by 38% of all followed patients. A good physical activity, defined as a score of ≥20 points on the PASIPD (0–100), was reached by 22% of all followed patients. Cognitive function within the normal range (CDT=1) was reached by 88% of all followed patients.
After inspecting our data we found that the statistical key assumptions for logistic regression analysis were met. There were no violations of the assumptions for logistic regression, and there was no potential outlier for the multivariate model as assessed with residual analysis.
The best predictors for good health-related quality of life 1 year after discharge were the time until regaining walking ability (OR=0.96, OR per day, 95% CI 0.93 to 0.99) and the mean MoCA score at start of rehabilitation (OR=1.25, 95% CI 1.02 to 1.52) (Hosmer-Lemeshow goodness-of-fit test, p=0.7372, r2=0.4561). Individuals who achieved good health-related quality of life at 12-month follow-up had a shorter time until regaining their walking ability (52.6±23.96 vs 101.4±60.0 days) and a higher MoCA score (18.00±5.08 vs 13.66±7.07 points) compared with those not achieving good health-related quality of life, respectively. These findings suggest that a shorter time to regaining walking ability and a higher MoCA score on admission to our post-ICUaR increase the likelihood of good health-related quality of life 1 year after discharge. The MoCA score and the time to regaining walking explained approximately 45% of the health-related quality of life 1 year after discharge.
The best predictor for good participation 1 year after discharge was the MoCA sum score on admission to our post-ICUaR (OR=0.85, 95% CI 0.72 to 1.00) (Hosmer-Lemeshow goodness-of-fit test, p=0.83, r2=0.19). Individuals who achieved good participation had a mean MoCA sum score of 17.86±5.17 points at the beginning of rehabilitation compared with a mean MoCA sum score of 13.63±7.08 points for those not achieving good participation 12 months later. These findings suggest that the higher the MoCA sum score on admission to our post-ICUaR, the more likely an individual will have good participation 1 year after discharge. The MoCA score explained approximately 19% of participation 1 year after discharge. The best predictor for good physical activity 1 year after discharge was the Apache sum score (OR=1.68, 95% CI 0.89 to 3.13) (Hosmer-Lemeshow goodness-of-fit test, p=0.83; r2=0.23). Individuals who achieved good physical activity had a mean Apache II sum score of 14.27±4.03 points on admission to our post-ICUaR compared with a mean Apache II sum score of 16.63±4.05 points for those not achieving good physical activity 1 year after discharge. These findings suggest that the lower the Apache sum score on admission to our post-ICUaR, the more likely individuals will have good physical activity 1 year after discharge. The Apache II sum score explained approximately 23% of physical activity 1 year after discharge.
The best predictor for normal cognitive function 1 year after discharge was regaining walking function (OR=8.0, 95% CI 0.49 to 13.69) (Hosmer-Lemeshow goodness-of-fit test, p=0.54; r2=0.11). Of the individuals who achieved walking function after admission to our post-ICUaR, 76% regained cognitive function within the expected normal range at 1 year compared with 24% of the individuals who did not achieve walking function. These findings suggest that regaining walking function is related to good cognitive function 1 year after discharge. Regaining walking function after admission to our post-ICUaR explained approximately 11% of cognitive function 1 year after discharge.
At 12-month follow-up, 27% of all followed participants lived alone and 73% lived with a partner.
After discharge up to 12 months later, 86% of all participants received no occupational therapy and 71% received no physiotherapy, while 14% received at least one appointment for occupational therapy or physiotherapy. We found that no cognitive therapy was received for any of the included participants over the 12 months of follow-up.
Discussion
The present study describes the outcomes of individuals with ICUAW at 12 months after discharge from an inpatient rehabilitation. The main result is that health-related quality of life, participation and physical activity were still very limited 12 months after discharge from rehabilitation. Less than half of our followed patients reached good health-related quality of life, participation and physical activity (44%, 38% and 24%, respectively).
We also found that health-related quality of life, participation, physical activity and cognitive function did not change much between 6 and 12 months after discharge from rehabilitation. This is in line with the results of Chan et al,61 who described that the walking velocity of 19 patients with ICUAW did not differ between 6 and 12 months after ICU discharge.
Health-related quality of life and participation
There are several studies describing the health-related quality of life of individuals after ICU stay.6 14 62 Studies describing the quality of life of individuals with ICUAW after discharge from rehabilitation are, however, somewhat rare. In a prospective analysis of 293 survivors of critical illness, Griffith et al described in a multicentre questionnaire-based study the health-related quality of life 6 and 12 months after ICU discharge.63 The median score was 64 out of 100 on the EQ-5D VAS at 6 months, and 66 out of 100 at 12 months, with no significant changes during follow-up. The health-related quality of life of our participants was more limited (ie, approximately 40).
Even less is known about participation of patients with ICUAW. We are not aware of any study measuring participation over a long duration of individuals with ICUAW. Jolley et al64 pointed out that a significant knowledge gap exists concerning long-term outcomes of individuals with ICUAW after critical illness.
Physical activity
To our knowledge cohort studies describing the recovery of physical activity in individuals with ICUAW are quite rare. However a few studies have investigated physical activity in individuals after ICU stay. Wright et al65 recruited 308 participants over 34 months: 150 were assigned to an intervention and 158 to the control group. At 6 months there was no difference in the Physical Component Summary measure of SF-36. Fan et al17 analysed the physical function of the SF-36 questionnaire and 6 min walking test within the first 24 months after ICU discharge in 18 acute lung injury survivors who developed ICUAW. Patients had a 80% decrease in the physical functioning subscale and achieved only 40% of normal walking distance in the 6 min walking test within the first 12 months after discharge from ICU. Wieske et al13 reported a significant lower physical functioning score of individuals with ICUAW. Chan et al61 compared 19 patients with ICUAW with 290 with normal muscle function at 6 and 12 months after ICU discharge. Patients with ICUAW were much slower in the 4 m walking test, while individuals without muscle impairment reached a normal gait velocity.
Cognitive function
In a landmark study cognition was assessed on 821 patients after a stay in an acute ICU hospital and then followed over a 1-year period.66 At the beginning of the acute stay, only 6% had cognitive impairment, but 3 months later 40% of all patients had a reduced global cognition score. Deficits persisted for about 30% of all patients at 12 months.66 There are, however, no cohort studies describing the recovery of cognitive function especially for people with ICUAW. Our results showed that 88% of our participants reached the best possible result in the CDT. Our findings are difficult to compare with other studies and also to interpret as there is no reasonable threshold for this specific sample. Furthermore our participants who reached the 12-month follow-up possibly differ from those who did not reach this time point. This might be interpreted as the so-called ‘healthy worker’ effect, which explains another result: 27% of all patients followed lived alone and 73% lived with a partner.
We used a wide range of functional variables to predict and found that health-related quality of life, participation, physical activity and cognitive function can be predicted by clinical variables during post-ICUaR. However, the predictions can be seen as poor for participation, physical activity and cognitive function, and this suggests that this is an area for further study in individuals with ICUAW. The main predictor variables in our multivariate model were clinical scales or functional scores, such as cognitive function (MoCA Score), clinical severity (Apache II sum score) and walking ability (regaining walking and time until walking was regained). Those scales or scores can be used very early and oftentimes easily in patients in the ICU and may predict important patient goals of individuals with ICUAW.
The strong aspects of the GymNAST study are its prospective design and that various clinical assessments for individuals with ICUAW were used to predict patients’ long-term outcomes. The present study might therefore provide new information about the long-term results of physical rehabilitation of individuals with ICUAW.
A potential limitation of this study is that we could not include sedated or very agitated individuals because they were unable to perform the assessments. This reduces the possibility of generalising the results to the entire critically ill population. Another point is that the diagnosis of CIP and CIM requires clinical evaluation and electrophysiological investigations.67 Therefore another limitation is that a clinical but not always both a clinical and an electrophysiological evaluation was provided. The limitations of this study are that electromyography was not used for differential diagnostics of muscle weakness (eg, between CIM and CIP and for other reasons of acquired muscle weakness) and that creatine kinase was not measured.
Additionally one could argue that the participants in our study were much more chronically affected and therefore not directly comparable with other published clinical trials in the field of ICU research. A consequence of this could be that our participants do not have the identical potential for recovery that patients in the first week of the acute stage might have. Moreover our participants might be seen as less severe as shown by the Apache score, but had a longer duration of primary illness and a longer ventilation period compared with studies focusing on the first weeks of ICU.13 17 Our participants were chronically critically ill and might have started later with mobilisation and rehabilitation than other critically ill individuals in the ICU, due to a necessarily extended weaning period. Further studies, however, should investigate certain differences between patient groups weaned off early and those weaned off later in respect to long-term outcomes (ie, health-related quality of life, participation, and physical and cognitive function).
Although the features of the instruments chosen (eg, for health-related quality of life, activities and participation) are established in some studies, these instruments, up to the present, have not been used extensively in different contexts and populations. Therefore it is important to examine the validity and reliability of these instruments for chronically critically ill individuals.
We used a consensus-based definition of ‘good outcome’ for our dependent variables. Our definitions were not tested for validity and reliability in the ICU population. This could be a limitation of our study. Since the initiation of this study published core outcome sets that recommend the EQ-5D and the SF-36 to measure health-related quality of life in the chronically ill population have been performed.68
Another limitation of this study is that the reliability and concurrent validity of the EQ-5D, PASIPD, MoCA and CDT have not been established for chronically critically ill patients with ICUAW.33–35 However, in one of our pilot studies, we had good clinical experience with the application of these measures in this specific population with very good reliability. We decided therefore a priori to use the PASIPD, MoCA and CDT for this study. In the future, however, large studies will be required to investigate the reliability and validity of these scales in individuals with ICUAW.
Currently not much is known about the relation between the EQ-5D, PASIPD, RNL-Index and MoCA in people with ICUAW. Future studies should therefore investigate the concurrent validity of these measures in the specific population of individuals with ICUAW.
Further studies should use a randomised controlled design including individuals with ICUAW with a defined reason for muscle weakness (ie, defined diagnosis of CIP and/or CIM) to investigate specific rehabilitation therapies to enhance recovery.
Supplementary Material
Footnotes
Contributors: JM and ST planned the study, contributed to the procurement of funding and developed the protocol. ST and JM evaluated and interpreted the data and did the statistical analysis. Both authors contributed to writing and checked the final draft of the manuscript.
Funding: This work was supported by Klinik Bavaria Kreischa, GymNAST.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: We conducted this study in accordance with the ’Helsinki Declaration' and received ethical approval from the local ethic commission (Sächsische Landesärztekammer EK-BR-32/13–1/1 06 755), and registered the study before publication (German Register of Clinical Trials, DRKS00006528).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data set supporting the conclusions of this article is available on request. Requests for data access can be submitted to the corresponding author, who will evaluate the request with the funding organisation (Klinik Bavaria Kreischa) as well as the GymNAST consortium.
References
- 1. Elliott D, Davidson JE, Harvey MA, et al. . Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med 2014;42:2518–26. 10.1097/CCM.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2. De Jonghe B, Sharshar T, Lefaucheur JP, et al. . Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859–67. [DOI] [PubMed] [Google Scholar]
- 3. Nordon-Craft A, Moss M, Quan D, et al. . Intensive care unit-acquired weakness: implications for physical therapist management. Phys Ther 2012;92:1494–506. 10.2522/ptj.20110117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015;19:274 10.1186/s13054-015-0993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014;370:1626–35. 10.1056/NEJMra1209390 [DOI] [PubMed] [Google Scholar]
- 6. Herridge MS, Tansey CM, Matté A, et al. . Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 7. Fan E. Critical illness neuromyopathy and the role of physical therapy and rehabilitation in critically ill patients. Respir Care 2012;57 933–46. 10.4187/respcare.01634 [DOI] [PubMed] [Google Scholar]
- 8. Herridge MS. The challenge of designing a post-critical illness rehabilitation intervention. Crit Care 2011;15:1002 10.1186/cc10362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohtake PJ, Strasser DC, Needham DM. Rehabilitation for people with critical illness: taking the next steps. Phys Ther 2012;92:1484–8. 10.2522/ptj.2012.92.12.1484 [DOI] [PubMed] [Google Scholar]
- 10. Tolep K, Getch CL, Criner GJ. Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest 1996;109:167–72. 10.1378/chest.109.1.167 [DOI] [PubMed] [Google Scholar]
- 11. Hermans G, De Jonghe B, Bruyninckx F, et al. . Clinical review: Critical illness polyneuropathy and myopathy. Crit Care 2008;12:238 10.1186/cc7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tipping CJ, Young PJ, Romero L, et al. . A systematic review of measurements of physical function in critically ill adults. Crit Care Resusc 2012;14:302–11. [PubMed] [Google Scholar]
- 13. Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, et al. . Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care 2015;19:196 10.1186/s13054-015-0937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herridge MS, Cheung AM, Tansey CM, et al. . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–93. 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- 15. Hermans G, Van Mechelen H, Clerckx B, et al. . Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2014;190:410–20. 10.1164/rccm.201312-2257OC [DOI] [PubMed] [Google Scholar]
- 16. Hermans G, Van Mechelen H, Bruyninckx F, et al. . Predictive value for weakness and 1-year mortality of screening electrophysiology tests in the ICU. Intensive Care Med 2015;41:2138–48. 10.1007/s00134-015-3979-7 [DOI] [PubMed] [Google Scholar]
- 17. Fan E, Dowdy DW, Colantuoni E, et al. . Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014;42:849–59. 10.1097/CCM.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Needham DM, Wozniak AW, Hough CL, et al. . Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med 2014;189:1214–24. 10.1164/rccm.201401-0158OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Semmler A, Okulla T, Kaiser M, et al. . Long-term neuromuscular sequelae of critical illness. J Neurol 2013;260:151–7. 10.1007/s00415-012-6605-4 [DOI] [PubMed] [Google Scholar]
- 20. Mehrholz J, Thomas S, Burridge JH, et al. . Fitness and mobility training in patients with Intensive Care Unit-acquired muscle weakness (FITonICU): study protocol for a randomised controlled trial. Trials 2016;17:559 10.1186/s13063-016-1687-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehrholz J, Mückel S, Oehmichen F, et al. . The General Weakness Syndrome Therapy (GymNAST) study: protocol for a cohort study on recovery on walking function. BMJ Open 2014;4:e006168 10.1136/bmjopen-2014-006168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oehmichen F, Ragaller M. Beatmungsentwöhnung bei Chronisch- Kritisch-Kranken. Intensiv- und Notfallbehandlung 2012;37:118–26. 10.5414/IBX00376 [DOI] [Google Scholar]
- 23. Nelson JE, Cox CE, Hope AA, et al. . Chronic critical illness. Am J Respir Crit Care Med 2010;182:446–54. 10.1164/rccm.201002-0210CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehrholz J, Mückel S, Oehmichen F, et al. . First results about recovery of walking function in patients with intensive care unit-acquired muscle weakness from the General Weakness Syndrome Therapy (GymNAST) cohort study. BMJ Open 2015;5:e008828 10.1136/bmjopen-2015-008828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sessler CN, Gosnell MS, Grap MJ, et al. . The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–44. 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 26. EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 27. Pohl M, Berger K, Ketter G, et al. . [Long-term course of patients in neurological rehabilitation Phase B. Results of the 6-year follow-up in a multicenter study]. Nervenarzt 2011;82:753–63. 10.1007/s00115-010-3119-0 [DOI] [PubMed] [Google Scholar]
- 28. Wood-Dauphinee SL, Opzoomer MA, Williams JI, et al. . Assessment of global function: The Reintegration to Normal Living Index. Arch Phys Med Rehabil 1988;69:583–90. [PubMed] [Google Scholar]
- 29. Wood-Dauphinee S, Williams JI. Reintegration to Normal Living as a proxy to quality of life. J Chronic Dis 1987;40:491–9. 10.1016/0021-9681(87)90005-1 [DOI] [PubMed] [Google Scholar]
- 30. Sonesson B, Kronvall E, Saveland H, et al. . Long-term reintegration and quality of life in patients with subarachnoid hemorrhage and a good neurological outcome: findings after more than 20 years. J Neurosurg 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Salisbury LG, Merriweather JL, Walsh TS. Rehabilitation after critical illness: could a ward-based generic rehabilitation assistant promote recovery? Nurs Crit Care 2010;15:57–65. 10.1111/j.1478-5153.2010.00382.x [DOI] [PubMed] [Google Scholar]
- 32. van den Berg-Emons RJ, L’Ortye AA, Buffart LM, et al. . Validation of the Physical Activity Scale for individuals with physical disabilities. Arch Phys Med Rehabil 2011;92:923–8. 10.1016/j.apmr.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 33. Sommers J, Engelbert RH, Dettling-Ihnenfeldt D, et al. . Physiotherapy in the intensive care unit: an evidence-based, expert driven, practical statement and rehabilitation recommendations. Clin Rehabil 2015;29:1051–63. 10.1177/0269215514567156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Schaaf M, Beelen A, de Vos R. Functional outcome in patients with critical illness polyneuropathy. Disabil Rehabil 2004;26:1189–97. 10.1080/09638280410001724861 [DOI] [PubMed] [Google Scholar]
- 35. van der Schaaf M, Beelen A, Dongelmans DA, et al. . Functional status after intensive care: a challenge for rehabilitation professionals to improve outcome. J Rehabil Med 2009;41:360–6. 10.2340/16501977-0333 [DOI] [PubMed] [Google Scholar]
- 36. Thomas S, Sauter W, Starrost U, et al. . Regaining water swallowing function in the rehabilitation of critically ill patients with intensive-care-unit acquired muscle weakness. Disabil Rehabil 2018;40:1494–500. 10.1080/09638288.2017.1300341 [DOI] [PubMed] [Google Scholar]
- 37. Rand D, Eng JJ, Tang PF, et al. . Daily physical activity and its contribution to the health-related quality of life of ambulatory individuals with chronic stroke. Health Qual Life Outcomes 2010;8:80 10.1186/1477-7525-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Ploeg HP, Streppel KR, van der Beek AJ, et al. . The Physical Activity Scale for Individuals with Physical Disabilities: test-retest reliability and comparison with an accelerometer. J Phys Act Health 2007;4:96–100. 10.1123/jpah.4.1.96 [DOI] [PubMed] [Google Scholar]
- 39. Washburn RA, Zhu W, McAuley E, et al. . The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil 2002;83:193–200. 10.1053/apmr.2002.27467 [DOI] [PubMed] [Google Scholar]
- 40. Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 41. Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 2000;15:548–61. [DOI] [PubMed] [Google Scholar]
- 42. Mazancova AF, Nikolai T, Stepankova H, et al. . The Reliability of Clock Drawing Test Scoring Systems Modeled on the Normative Data in Healthy Aging and Nonamnestic Mild Cognitive Impairment. Assessment 2017;24:945–57. 10.1177/1073191116632586 [DOI] [PubMed] [Google Scholar]
- 43. Wong A, Nyenhuis D, Black SE, et al. . Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke 2015;46:1059–64. 10.1161/STROKEAHA.114.007253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bach JP, Riedel O, Pieper L, et al. . Health-related quality of life in patients with a history of myocardial infarction and stroke. Cerebrovasc Dis 2011;31:68–76. 10.1159/000319027 [DOI] [PubMed] [Google Scholar]
- 45. Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 46. Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991;14:1103–9. 10.1002/mus.880141111 [DOI] [PubMed] [Google Scholar]
- 47. Mathiowetz V, Weber K, Volland G, et al. . Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984;9:222–6. 10.1016/S0363-5023(84)80146-X [DOI] [PubMed] [Google Scholar]
- 48. Mathiowetz V, Kashman N, Volland G, et al. . Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985;66:69–74. [PubMed] [Google Scholar]
- 49. Zanni JM, Korupolu R, Fan E, et al. . Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care 2010;25:254–62. 10.1016/j.jcrc.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 50. Thrush A, Rozek M, Dekerlegand JL. The clinical utility of the functional status score for the intensive care unit (FSS-ICU) at a long-term acute care hospital: a prospective cohort study. Phys Ther 2012;92:1536–45. 10.2522/ptj.20110412 [DOI] [PubMed] [Google Scholar]
- 51. Nordon-Craft A, Schenkman M, Edbrooke L, et al. . The physical function intensive care test: implementation in survivors of critical illness. Phys Ther 2014;94:1499–507. 10.2522/ptj.20130451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Denehy L, de Morton NA, Skinner EH, et al. . A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored). Phys Ther 2013;93:1636–45. 10.2522/ptj.20120310 [DOI] [PubMed] [Google Scholar]
- 53. Yu A, Teitelbaum J, Scott J, et al. . Evaluating pain, sedation, and delirium in the neurologically critically ill-feasibility and reliability of standardized tools: a multi-institutional study. Crit Care Med 2013;41:2002–7. 10.1097/CCM.0b013e31828e96c0 [DOI] [PubMed] [Google Scholar]
- 54. Holden MK, Gill KM, Magliozzi MR. Gait assessment for neurologically impaired patients. Standards for outcome assessment. Phys Ther 1986;66:1530–9. [DOI] [PubMed] [Google Scholar]
- 55. Armitage P, Colton T. Encyclopedia of Biostatistics. Chichester: Wiley, 1998. [Google Scholar]
- 56. Hosmer D, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd ed New York: John Wiley & Sons, Inc, 2008. [Google Scholar]
- 57. Stoltzfus JC. Logistic regression: a brief primer. Acad Emerg Med 2011;18:1099–104. 10.1111/j.1553-2712.2011.01185.x [DOI] [PubMed] [Google Scholar]
- 58. Spiegelhalter DJ. Probabilistic prediction in patient management and clinical trials. Stat Med 1986;5:421–33. 10.1002/sim.4780050506 [DOI] [PubMed] [Google Scholar]
- 59. Steyerberg EW, Eijkemans MJ, Harrell FE, et al. . Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med 2000;19:1059–79. [DOI] [PubMed] [Google Scholar]
- 60. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2 ed New York: John Wiley & Sons, Inc, 2000. [Google Scholar]
- 61. Chan KS, Aronson Friedman L, Dinglas VD, et al. . Evaluating Physical Outcomes in Acute Respiratory Distress Syndrome Survivors: Validity, Responsiveness, and Minimal Important Difference of 4-Meter Gait Speed Test. Crit Care Med 2016;44:859–68. 10.1097/CCM.0000000000001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Herridge MS. Recovery and long-term outcome in acute respiratory distress syndrome. Crit Care Clin 2011;27:685–704. 10.1016/j.ccc.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 63. Griffiths J, Hatch RA, Bishop J, et al. . An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care 2013;17:R100 10.1186/cc12745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jolley SE, Bunnell A, Hough CL. Intensive Care Unit Acquired Weakness. Chest 2016;150:1129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wright SE, Thomas K, Watson G, et al. . Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax 2018;73 10.1136/thoraxjnl-2016-209858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pandharipande PP, Girard TD, Jackson JC, et al. . Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306–16. 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931–41. 10.1016/S1474-4422(11)70178-8 [DOI] [PubMed] [Google Scholar]
- 68. Needham DM, Sepulveda KA, Dinglas VD, et al. . Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med 2017;196:1122–30. 10.1164/rccm.201702-0372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.