Abstract
Introduction
Diabetes and hypertension are two leading non-communicable conditions, which are suboptimally managed in India. Thus, innovative comprehensive approaches that can concomitantly improve their detection, prevention and control are warranted.
Methods and analysis
UDAY, a 5-year initiative, aims to reduce the risk of diabetes and hypertension and improve management by implementing a comprehensive intervention programme in the two selected study sites, Sonipat and Visakhapatnam (Vizag). It has a pre-post evaluation design with representative cross-sectional surveys before and after intervention. Within these two sites, urban and rural subsites each with a total population of approximately 100 000 people each were selected and a baseline and postintervention assessment was conducted deploying five surveys [among general population (including body measurements or biosamples), patients, healthcare providers including physicians and pharmacists, health facilities], which will determine the knowledge levels about diabetes and hypertension, the proportion treated and controlled; the patient knowledge and self-management skills; healthcare providers’ management practices; the level of access and barriers to obtaining care.
The interventions will include: tailored health promotion for improving public knowledge; screening of adults aged ≥ 30 years for identifying those at high risk of diabetes and/or hypertension for linkage to the healthcare system; patient education using technology enabled community health workers, geographic information system (GIS) based mapping of the communities, healthcare provider training on management guidelines, community based diabetes registry and; advocacy to improve access to healthcare. The baseline surveys have been completed, the study areas mapped using GIS and the interventions are being implemented. UDAY is expected to increase over baseline the levels of: public knowledge about diabetes and hypertension; those treated and controlled; patient self-management skills; the use of guideline based management by providers and; access to healthcare, leading to improved health outcomes and inform development of a India relevant chronic care model.
Ethics and dissemination
Ethical clearance for conduct of the study was obtained from the Institutional Ethics Committee (IEC) of the Public Health Foundation of India. The findings will be targeted primarily at public health policymakers and advocates, but will be disseminated widely through other mechanisms including conference presentations and peer-reviewed publications, as well as to the participating communities.
Keywords: hypertension, general diabetes, epidemiology
Strengths and limitations of this study.
Unique large community-based study in geographically and culturally diverse ‘real world’ settings in India for improving prevention and control of diabetes and hypertension.
Employs a comprehensive multilevel, multicomponent intervention approach to target the general population, patients, providers and to strengthen the health system for improving prevention and control of diabetes and hypertension.
Concomitantly addresses detection, prevention and management of both diabetes and hypertension.
Extensively leverages mobile technology to enable health workers in task sharing as well as for electronic data capture.
Quasi-experimental design with complex intervention may hinder attribution of specific effect to individual intervention components.
Introduction
Non-communicable diseases (NCDs) are currently the leading cause of preventable death and disability in India, accounting for two out of every three deaths.1 Among NCDs, the prevalence of type 2 diabetes mellitus and hypertension has been rising rapidly. Currently there are about 69 million people with diabetes, a figure that is projected to further increase to a staggering 124 million by 2040.2 The number of individuals with hypertension is projected to increase to 214 million by 2030 from 118 million in 2000.3 4 Furthermore, diabetes and hypertension are important risk factors for both the major forms of cardiovascular disease (CVD (coronary heart disease and stroke)), highly prevalent and attributable to nearly half of all the deaths from NCDs.4
Despite the availability of proven and effective treatments, diabetes as well as hypertension detection and control rates are abysmally low in India with blood pressure control among those with diabetes being even more suboptimal, with a huge gap between detection and adequate treatment.1 3 4 Thus, there is great potential and opportunity to reduce the rising burden of diabetes and hypertension as well as the associated vascular risk in India through concomitantly improving their detection, prevention and control.
To address this huge gap in the prevention and management of both these conditions, we are undertaking a 5-year initiative entitled ‘UDAY’ (meaning dawn in Sanskrit) in epidemiologically transitioning communities that aims to reduce the risk of diabetes and hypertension and concomitantly improve the management of either conditions by implementing a comprehensive community-based innovative intervention programme in the two geographically and culturally distinct study sites, Sonipat (Haryana, north India) and Visakhapatnam or Vizag (Andhra Pradesh, south India). This paper describes the design and methods of UDAY- A Comprehensive Diabetes and Hypertension Prevention and Management Program In India.
Methods
Study design
UDAY has a pre-post evaluation design with representative cross-sectional surveys before (in year 1 at baseline, preintervention) and after the intervention (in year 5). The main research question is whether a multicomponent, multilevel, cost-effective, comprehensive intervention programme will improve the prevention, detection and optimal management of diabetes and hypertension in the two selected study sites.
We selected a pre-post evaluation design due to the following reasons. We wanted to evaluate if multicomponent interventions delivered at multiple levels in a comprehensive manner can improve outcomes. Further, we also wanted to understand and examine the operational part of the programme implementation to gain insights into underpinning factors behind success or failure that can inform possible replication and scale up in the future. The options for an implementation study of this nature with process outcomes are either a pre-post design or quasi-experimental design or step wedge design. We deemed a step wedge to be too complicated for this evaluation, and given that quasi-experimental design would not have enough power to decipher real differences, we chose a pre-post design. This was also aimed at cutting down costs.
At baseline, in year 1, five surveys were conducted among the general population, among patients, among healthcare providers including physicians and pharmacists and in health facilities to guide intervention development and impact assessment. Similar assessment is planned after the intervention, in year 5.
The specific objectives these assessments are to
Determine the prevalence, awareness, the knowledge levels about diabetes and hypertension, the proportion treated and controlled among a representative sample (n=12 000) of adults aged ≥30 years in the selected study areas. (Population survey)
Determine the patient knowledge levels and self-management skills among a convenience sample (n=400) of those diagnosed with diabetes and hypertension in the selected study areas. (Patient survey)
(a) Determine healthcare providers’ (physicians’) knowledge and practices related to diabetes and hypertension management among a convenience sample (n=50) of healthcare providers’ in the selected study areas. (b) Determine pharmacists’ knowledge related to diabetes and hypertension and dispensing practices among a convenience sample of pharmacists (n=350). (Provider survey)
Determine the level of access and potential barriers to diabetes and hypertension care provided by the public healthcare system in the selected study areas (n=50). (Facility survey)
Determine the cost-effectiveness of the intervention programme in improving diabetes and hypertension treatment and management outcomes in the study areas. (Using population survey, Geographical Information System (GIS) data and project implementation data)
Study sites
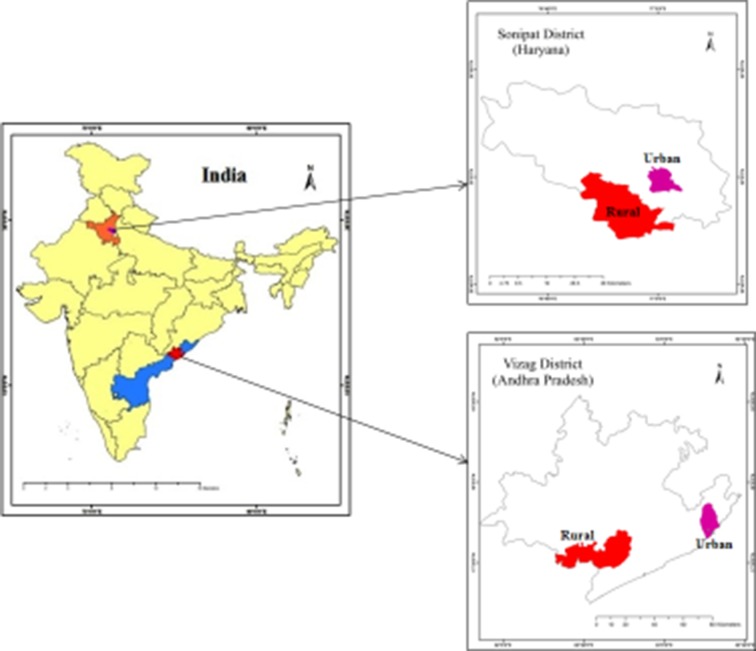
We are undertaking this comprehensive diabetes and hypertension prevention and management programme in two epidemiologically transitioning areas located in Sonipat in Haryana and Vizag in Andhra Pradesh (figure 1, online supplementary file 1).
Figure 1.
Study sites.
bmjopen-2017-015919supp001.pdf (178.7KB, pdf)
We defined the areas and their subareas using the following terminology:
Sites—A bounded geographic area within which we have defined distinct rural and urban subsites for the study. Each site has been defined such that it contains approximately 200 000 people. Sonipat and Vizag are each a site.
Subsites—Within each site, we have defined one rural and one urban subsite for study. Each subsite is geographically bounded and contains a population of approximately 100 000. Within Sonipat and Vizag, we have defined two subsites each (rural and urban).
Total project—The total project is the summation of the two sites, including the four subsites (two subsites within each site). Therefore, the total population under the study is approximately 400 000.
Intervention programme
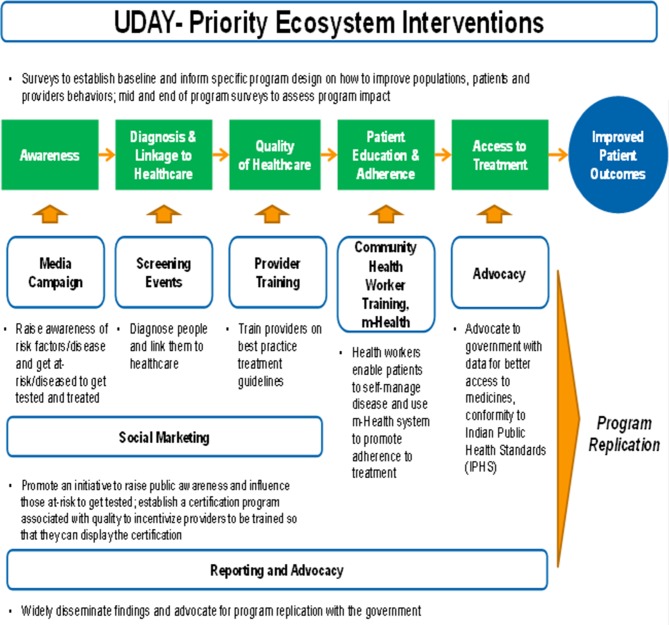
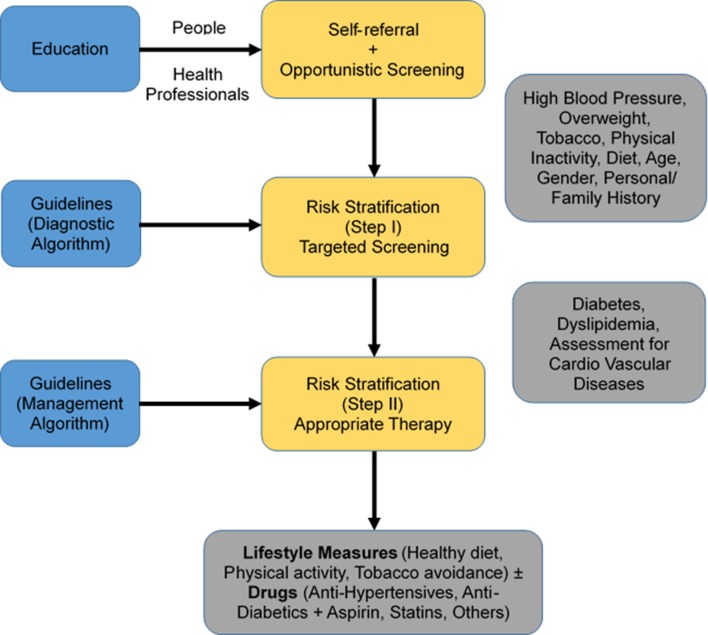
We propose to implement a priority set of synergistic innovative ecosystem interventions (figure 2 and table 1), with the overarching goal to prevent, detect, reduce the risk of diabetes and hypertension and to improve the treatment and management of individuals with either of the conditions in the study sites. We hypothesise (figure 3) that education of the public on diabetes, hypertension and related risks will lead to increased self-referral and prevention, while education of the healthcare providers will promote opportunistic screening for diabetes, hypertension and related risks, and purposeful implementation of evidence-based diagnostic (to screen, stratify by risk) and management guidelines (to initiate appropriate therapy).
Figure 2.
Priority interventions in UDAY.
Table 1.
Innovations in UDAY
| Intervention domain | Approach | Target |
| Prevention, early detection and referral | Culturally tailored health promotion, screening | 400 000 population |
| Capacity building, task shifting of healthcare providers | CME, short trainings, distance learning, QIP | Healthcare providers |
| Early diagnosis and prevention of complications | Registry | 10 000 patients |
| m-health system | Electronic data system+DSS | Adult population |
| Electronic data capture | Tablet-based surveys | 12 000 |
| Spatial and built environment assessment | Geographical Information System mapping | All study areas |
| Improved access to medicines by social marketing initiatives | Improving quality of services | 300 pharmacists |
| Culturally tailored patient education and networks for enabling self-care | Using health workers | Patients |
Figure 3.
Intervention framework.
Following the baseline assessments, a comprehensive intervention programme is being currently implemented. The intervention components include
tailored health promotion using social marketing approaches for increasing public awareness and promoting population risk factor modification and reduction;
community-based screening of all adults aged ≥30 years for identifying those at high risk of diabetes and/or hypertension or with disease to link to the healthcare system, leveraging task-sharing approaches using technology-enabled health workers;
patient education using technology-enabled health workers,
healthcare provider training on evidence-based management guidelines;
implementation of a quality improvement programme and diabetes registry;
advocacy with governments and other stakeholders to improve access to healthcare.
It is expected that the comprehensive interventions will increase over baseline the levels of public awareness and knowledge about diabetes and hypertension; those aware, diagnosed, treated and controlled to recommended targets; and the use of guideline-based management by providers. Detailed outcome assessment metrics is provided in table 2. This is anticipated to improve health outcomes and access to healthcare for people living with diabetes and hypertension in India as well as provide a model of healthcare which is low-cost, community-based and context-relevant in a milieu of rapid rise in these chronic conditions.
Table 2.
Assessment of intervention outcomes
| Indicator | Target population | Metric | Evaluation methodology |
| 1. Patient outcomes | Diabetes and hypertension patients | % implementing lifestyle change (meet the recommended levels of physical activity and intend to and/or implement dietary changes) | Baseline and endline surveys, diabetes registry |
| % engaging in self-monitoring/testing | Baseline and endline surveys, diabetes registry | ||
| % increase in correct self-management practices | Baseline and endline surveys, diabetes registry | ||
| % increase in knowledge on diabetes and hypertension | Baseline and endline surveys, diabetes registry | ||
| % of patients on treatment, whose diabetes, hypertension is successfully controlled, that is, HbA1C≤7 %/blood pressure ≤130/80 mm Hg | Baseline and endline surveys, diabetes registry | ||
| 2. Awareness and knowledge about diabetes and hypertension | General population | % increase in knowledge of diabetes, hypertension and their risk factors | Baseline and endline surveys |
| % increase in detection rate and in seeking healthcare | Baseline and endline surveys, screening programme | ||
| % implementing lifestyle change (meet the recommended levels of physical activity and intend to and/or implement dietary changes) | Baseline and endline surveys, screening programme | ||
| % exposed to health promotion campaign | Baseline and endline surveys, screening programme | ||
| 3. Provider knowledge and practices | Physicians, other health workers | Numbers who participate in training programmes | Training participation data |
| % increase in knowledge related to diabetes and hypertension management | Baseline and endline surveys of providers, diabetes registry | ||
| % increase in practices related to diabetes and hypertension management and providing lifestyle advice | Baseline and endline surveys of providers, diabetes registry | ||
| Pharmacists | % of pharmacists who identify people at risk of and with diabetes, hypertension | Baseline and endline surveys of providers | |
| % increase in pharmacists dispensing and filling prescriptions correctly | Baseline and endline surveys of providers, diabetes registry | ||
| 4. Programme cost-effectiveness | Patients with diabetes | Cost per diabetic patient treated to recommended target | Baseline and endline surveys of patients, programme cost data, diabetes registry |
| % reduction in out-of-pocket expenditure | Baseline and endline surveys of patients, diabetes registry | ||
| General population | Cost per diabetes case identified | Surveys, screening programme, programme cost data | |
| 5. Access to treatment | Healthcare system | Improvements in access to and availability of medications | Baseline and endline surveys of patients, facility survey, diabetes registry |
| % increase in the proportion patients who report that medicines are easily available | Baseline and endline surveys of patients, facility survey, diabetes registry | ||
| % reduction in stock outs of medicines | Baseline and endline surveys of patients, facility survey, diabetes registry | ||
| Adherence to Indian Public Health Standards guidelines on drugs, services | Facility survey, diabetes registry |
Intervention development
We used evidence based interventions and leveraged the results from the baseline surveys (population, patient, facility and providers) to develop and refine the interventions, which were subsequently piloted. For example, from population survey we found that there were differences in the awareness of risk factors for developing diabetes/hypertension across rural/urban areas and the two study sites in north and south India. Therefore, taking this into cognizance, we designed the tailored health promotion programme and messages to be delivered by trained health workers to increase awareness about the risk factors. Facility and providers’ surveys helped us to design the training programme for training healthcare providers as well as to conduct advocacy to improve access to the health system. Similarly, findings from the patients’ survey helped us to focus the training of health workers on building self-management skills of people with diabetes/hypertension and for developing patient networks.
Sample for the population survey
We based our sample size calculation on the prevalence of diabetes, which has a lower prevalence than hypertension, in previously reported studies in India. Anticipating a response rate of 85% with a design effect factor of 1.5 (to account for cluster sampling) and a confidence level of 1.96, the sample size estimates were generated for males and females in three age strata (30–49, 50–69, 69 and above) in each setting (urban, rural). About 2968 subjects were estimated to be required per urban subsite (Sonipat and Vizag) and 2942 subjects per rural subsite (Sonipat and Vizag). The total estimated sample was 11 820, which was increased to 12 000 to obtain equal samples in both urban and rural areas. Sample weights were generated for estimating the prevalence of diabetes, hypertension and related cardiometabolic (CMD) risk factors at the district level.
Survey sampling and participant selection
Population survey
Two representative population-based cross-sectional surveys with independent samples and identical methodologies at baseline and at the end of intervention will be conducted, which will provide estimates of the burden and changes in the prevalence of diabetes, hypertension and related CMD risk factors. In addition, those recruited for the first survey at baseline (which has been completed) will be followed up as a cohort to estimate the incidence of new CMD risk factors, disease, healthcare utilisation, diabetes and hypertension-related morbidity and mortality.
The first population survey (baseline survey) was done among a representative sample of adults aged ≥30 years residing in the selected study areas of Sonipat and Vizag. Inclusion criteria were (a) adults aged ≥30 years residing in the sampled urban and rural areas of Sonipat and Vizag, respectively; and (b) willing to participate and provide informed consent.
We excluded individuals who were unwilling to provide informed consent and those with serious chronic illnesses [such as that of the liver (cirrhosis), kidneys (renal failure) or malignancies], and pregnant women.
For the baseline survey, a multistage random sampling technique was deployed to obtain a representative sample of adults aged ≥30 years, using data from the most recent census of 2011. In addition, a manual enumeration and mapping of all households and structures was conducted in all the study areas (all census enumeration blocks (CEBs) in urban areas and villages in rural) to identify households and structures constructed since the last census (table 3). CEBs are considered as the primary sampling unit in urban areas and villages in the rural areas, respectively. On average, about 100–125 households with a population of 650–700 persons would generally constitute a CEB. This enabled a complete sampling frame for the selection of households for the survey and thus provided an equal chance of selection to each household. Besides, it also helped identify potential recipients of the intervention programme (ie, adults aged ≥30 years) in the study sites.
Table 3.
Manual enumeration of study areas
| Study site | Structures | Households | Population≥30 years | Total population (census 2011) |
| Sonipat urban subsite | 24 408 | 20 406 | 41 981 | 102 292 |
| Sonipat rural subsite | 28 813 | 17 283 | 40 850 | 100 935 |
| Vizag urban subsite | 16 888 | 39 504 | 70 735 | 153 721 |
| Vizag rural subsite | 33 799 | 30 817 | 59 540 | 121 209 |
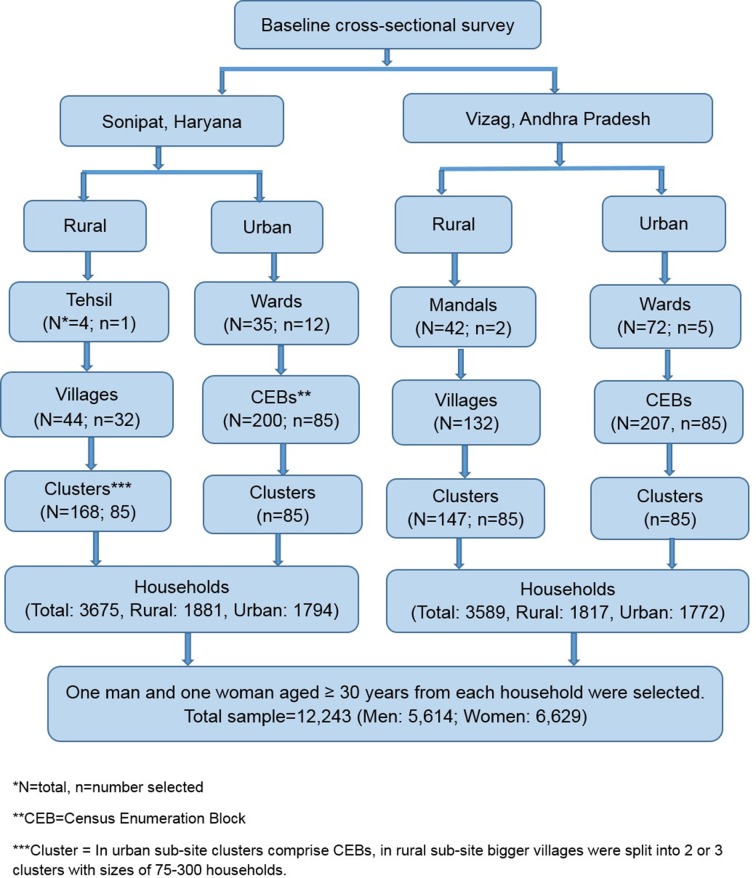
In each subsite, 85 clusters were randomly selected (urban Sonipat: 85/200 CEBs; urban Vizag: 85/207 CEBs; and rural Sonipat: 85/168 clusters; rural Vizag: 85/147 clusters) according to probability proportional to size (figure 4). In rural subsites, bigger villages were divided into clusters with 75–300 households. From each cluster in the urban and rural subsites, 18–25 households were randomly selected and one eligible male and female were selected randomly within each household using Kish table. In Sonipat district, 3675 households participated in baseline survey (rural: 1881; urban: 1794) with total being 6208 participants (rural: 3104; urban: 3104). In Vizag district, 3589 households (rural: 1817; urban: 1772) participated in the baseline survey with total being 6035 participants (rural: 3069; urban: 2966). Thus, we had 12 000 individuals from both urban and rural areas of north and south India.
Figure 4.
Sample selection for the baseline survey.
Data collection for the population survey
For the baseline survey, trained health workers visited the selected participants at their homes. Written informed consent was sought and obtained from the selected individuals prior to data collection and a unique identification number was assigned. For each participant, data collection comprised an interviewer-administered questionnaire, a brief physical examination (height, weight, waist circumference, hip circumference, blood pressure, body fat percentage), blood and urine collection as detailed below.
Information on demographics, socioeconomic characteristics, behavioural risk factors (tobacco use, alcohol use, diet and physical inactivity), family history of various NCDs, female reproductive history, general awareness about diabetes and hypertension, risk factors, prevention, symptoms and diagnosis, complications, treatment and management, medical history of NCDs (diabetes, hypertension, coronary artery disease, myocardial infraction, stroke, heart failure, chronic kidney disease, peripheral vascular disease, chronic obstructive pulmonary disease, asthma), quality of life, mental health, social support, cost of healthcare and healthcare utilisation was collected using a tablet-based application (table 4).
Table 4.
Summary of indicators, measures, methods and instruments for baseline survey
| Indicators | Measures | Methods | Instruments |
| Demographics and socioeconomic characteristics | Age, sex, marital status, religion, education, income, occupation, contact details and household assets | Questionnaires | CARRS Surveillance Study10
Establishment of Sentinel Surveillance System for CVD in Indian Industrial Populations (Sentinel Surveillance Study)11 National Family Health Survey, 2005–200612 |
| Behavioural risk factors | Tobacco use Alcohol use |
Questionnaire | CARRS, Sentinel Surveillance Study |
| Physical activity | Questionnaire | Global Physical Activity Questionnaire13 | |
| Dietary habits | Questionnaire | CARRS, INTERHEART study14 | |
| Family history | Prevalence of CMDs among family members related to participants, mortality | Questionnaire | CARRS |
| Female reproductive history | Menarche/gestational history, menopause (surgical/physiological/ whether on hormone replacement therapy)/contraception |
Questionnaire | CARRS |
| Awareness and knowledge | General awareness about diabetes and hypertension, risk factors, prevention, symptoms and diagnosis, complications, treatment and management | Questionnaire | CARRS |
| Physiological and biochemical risk factors | Hypertension | Blood pressure measurements | Standardised method (American Heart Association) and validated instrument (certified by British Hypertensive Society and Association for the Advancement of Medical Instrumentation) Standardised across both the sites |
| Diabetes | Laboratory estimation of fasting plasma glucose, glycated haemoglobin (HbA1c) | Standardised across both the sites | |
| Dyslipidaemia | Laboratory estimation of serum total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides | Standardised across both the sites | |
| Obesity | Anthropometry (height, weight, waist and hip circumferences, body fat) | Standard procedures based on National Health and Nutrition Examination Survey III with instruments used in epidemiological studies on South Asian population | |
| Medical history | Chronic kidney disease Stroke, myocardial infraction, congestive heart failure, chronic stable angina |
Serum creatinine, urea, urine microalbumin and urine creatinine Questionnaires including medical history |
Standardised across both the sites Rose Angina, CARRS |
| Treatment history, health services, quality of care and healthcare costs | Awareness and risk factor control Access to healthcare services, utilisation of services, health insurance coverage, costs of treating CMDs and their risk factors |
Questionnaire | CARRS |
| Prevalence | COPD and asthma | Questionnaire | NHANES III and present standards of the American Thoracic Society |
| Health-related quality of life | Mobility, self-care, usual activities, pain/discomfort, anxiety/depression (related to CMDs and risk factors) | Questionnaire | European Quality of Life 5 Dimensions questionnaire 15 |
| Mental health | Depression | Questionnaire | Modified from Patient Health Questionnaire 916 |
| Social well-being | Social support | Questionnaire | Developed for UDAY |
CARRS, Centre for cArdiometabolic Risk Reduction in South Asia; CMDs, cardiometabolic diseases; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Body measurements such as height, weight, waist and hip circumferences, blood pressure and body fat measurement by bio impedance using TANITA were also obtained using standardised equipment.
Bio-samples (fasting blood and morning urine) were collected for the laboratory estimation of fasting plasma glucose, glycated haemoglobin (HbA1c), lipids, serum creatinine, urea, urine microalbumin and urine creatinine. We have also stored the biosamples for future analysis of emerging biomarkers and genetics.
Data collection for the patient survey
Trained research staff visited health facilities located in the aforementioned selected study areas and approached patients attending outpatient section of the health facilities and identified those with the diagnosis of diabetes and/or hypertension based on their prescription note for participating in the study. Written informed consent was sought and obtained from the individuals prior to data collection, and data collection comprised an interviewer-administered questionnaire (table 5).
Table 5.
Summary of indicators, measures, methods and instruments for the patient survey
| Indicators | Measures | Methods | Instruments |
| Demographics and socioeconomic characteristics | Age, sex, education, income, occupation, contact details | Questionnaires | CARRS, Sentinel Surveillance Study |
| Behavioural risk factors | Tobacco use Alcohol use |
Questionnaire | CARRS, Sentinel Surveillance Study |
| Awareness and knowledge of diabetes and hypertension | Awareness of risk factors, symptoms and diagnosis, cut-off levels for diagnosis, complications, treatment and management | Questionnaire | Developed for UDAY |
| Diabetes and hypertension related medical history | Diagnosis, healthcare utilisation, control, self-management practices, complications, comorbidities and treatment adherence | Questionnaire | Developed for UDAY |
| Health-related quality of life | Mobility, self-care, usual activities, pain/discomfort, anxiety/depression (related to CMDs and risk factors) | Questionnaire | European Quality of Life 5 Dimensions questionnaire |
| Mental health | Depression | Questionnaire | Modified from Patient Health Questionnaire 9 |
| Social well-being | Social support | Questionnaire | Developed for UDAY |
| Healthcare utilisation | Hospital visits in the past 12 months and healthcare expenditure | Questionnaire | CARRS |
CARRS, Centre for cArdiometabolic Risk Reduction in South Asia; CMDs, cardiometabolic diseases.
The patient survey was done among a convenience sample of 400 patients having diabetes and/or hypertension attending health facilities and residing in the selected study areas of Sonipat and Vizag.
Inclusion criteria: We included patients with diabetes and or hypertension residing/attending health facilities in the sampled urban and rural areas of Sonipat and Vizag, respectively, and were willing to participate and provide informed consent.
Exclusion criteria: We excluded those unwilling to provide informed consent.
Data collection for the provider surveys
Trained research staff visited health facilities located in the aforementioned selected study areas and approached healthcare providers (physicians and pharmacists). Written informed consent was sought and obtained from the individuals prior to data collection, and data collection comprised an interviewer-administered questionnaire (table 6).
Table 6.
Summary of indicators, measures, methods and instruments for the provider survey (physicians)
| Indicators | Measures | Methods | Instruments |
| Socio-demographic details | Age, gender, qualification, years of practice, patient load, training in diabetes and hypertension management | Questionnaire | Developed for UDAY |
| Knowledge and practice pertaining to diabetes and hypertension diagnosis and evaluation of complications | Signs and symptoms, diagnosis and cut-off levels for diagnosis, evaluation for complications | Questionnaire | Developed for UDAY |
| Treatment practices for diabetes and hypertension | Lifestyle modifications, prevention and management of complications, names of medicines prescribed commonly | Questionnaire | Developed for UDAY |
The physician survey was done among a convenience sample of 50 physicians working in the health facilities located in the selected study areas of Sonipat and Vizag.
Inclusion criteria: We included physicians working in health facilities located in the sampled urban and rural areas of Sonipat and Vizag, respectively, and were willing to participate and provide informed consent.
Exclusion criteria: Those unwilling to provide informed consent were excluded.
The pharmacist survey was conducted among 350 pharmacists by trained research staff in the aforementioned selected study areas. Written informed consent was sought and obtained from the individuals prior to data collection, and data collection comprised an interviewer-administered questionnaire.
Inclusion criteria: We included pharmacists who were located in the sampled urban and rural areas of Sonipat and Vizag, respectively, dispensing medicines for diabetes and hypertension, were willing to participate and provide informed consent.
Exclusion criteria: Those not dispensing medicines for diabetes and hypertension and unwilling to provide informed consent were excluded.
Data collection for the health facility survey
Permission was sought and obtained from respective district medical/health officers prior to data collection, and data collection comprised an interviewer-administered questionnaire (table 7). Trained research staff visited public healthcare facilities located in the aforementioned selected study areas and approached the person/medical officer in charge.
Table 7.
Summary of indicators, measures, methods and instruments for the health facility survey
| Indicators | Measures | Methods | Instruments |
| Coverage statistics | Types of services offered, number of hours and days services provided per week, population covered, average daily outpatient department attendance, number of beds available, status of National Program for Control and Prevention of Diabetes, Cardiovascular Disease and Stroke implementation | Questionnaire | Adapted from SARA tool of WHO |
| Recommended manpower list | Numbers working at district hospitals, community health centres, primary health centres and subcentres against recommended numbers and reasons for lack of recommended personal Additional manpower required for NCDs |
Checklist, questionnaire | Adapted from IPHS and SARA |
| Recommended medication list | Availability of medicines at district hospitals, community health centres, primary health centres and subcentres against recommended medicines and reasons for lack for recommended medicines Additional medicines required for NCDs |
Checklist, questionnaire | Adapted from IPHS and SARA |
| Recommended equipment list | Numbers of equipment available at district hospitals, community health centres, primary health centres and subcentres against recommended equipment and their functional status Additional equipment required for NCDs |
Checklist, questionnaire | Adapted from IPHS and SARA |
| Recommended investigative services list | Investigative services available at district hospitals, community health centres, primary health centres and subcentres against recommended services and reasons for their unavailability Additional investigative services required for NCDs |
Checklist, questionnaire | Adapted from IPHS and SARA |
| Recommended activities list | Frequency of recommended activities conducted and methods of conducting at district hospitals, community health centres, primary health centres and subcentres for current diagnosis, treatment and health promotion Reasons for not conducting the activities |
Checklist, questionnaire | Adapted from IPHS and SARA |
| Availability of national guidelines and training of healthcare providers | Availability of national guidelines for diagnosis and management of diabetes, hypertension and CVD and training of healthcare providers in the facility to diagnose and manage diabetes, hypertension and CVD | Questionnaire | Adapted from IPHS and SARA |
CVD, cardiovascular disease; IPHS, Indian Public Health Standards; NCD, non-communicable diseases; SARA, Service Availability and Readiness Assessment.
The facility survey was done in 50 public healthcare facilities (subcentres, primary and community health centres, taluk/district/tertiary hospitals) situated in the selected study areas of Sonipat and Vizag.
Biosample collection and storage
Blood samples (in fasting state) were collected in plain, fluoride and EDTA vacutainers (Becton & Dickinson, USA) by trained phlebotomists in households or in camps arranged close to the households. Samples for estimation for blood glucose were kept in ice and transferred to laboratories within 2 hours of sample collection and immediately centrifuged. Samples were centrifuged at 2500 rpm for 15 min and serum/plasma were transferred into the cryo-vials which were stored in −20°C immediately. Buffy coat from EDTA vacutainers were separated and stored into cryo-vials for DNA extraction. Red blood cells were washed thrice with normal saline and were stored for analysis of fatty acids in future. First early morning voided urine was collected from the participants and has been stored in deep freezer for future analysis of metabolites. All these samples were transported to the biochemistry laboratory at the Public Health Foundation of India (PHFI) in dry ice on the day of collection from Sonipat and Vizag, where they were stored at −80°C.
All clinical chemistry parameters were analysed on autoanalyzer Cobas 311 using reagents from Roche Diagnostics, Switzerland. Glucose was estimated using Hexokinase method, cholesterol by enzymatic cholesterol oxidase method, triglycerides by GPO-PAP method and high-density lipoprotein and low-density lipoprotein by direct method, aspartate aminotransferase and alanine transaminase were estimated according to International Federation of Clinical Chemistry (IFCC) method without pyridoxal phosphate, bilirubin total by Diazonium ion method, bilirubin direct by Diazo with sulphanilic acid method, urea by kinetic method, creatinine by Jaffe’s method and urinary microalbumin using immuno turbidimetric method. HbA1c was assayed by high-performance liquid chromatography method using reagents from Bio-Rad Laboratories, Hercules, California, USA.
Two levels of internal controls were run with every batch of samples. The intra-assay and inter-assay coefficient of variation for all the parameters were <3% and <5%, respectively.
The biochemistry laboratory is part of External Quality Assurance program from RIQAS for clinical chemistry parameters and HbA1c assay.
Evaluation of cost-effectiveness
This will be evaluated by assessing the costs and benefits of the multicomponent, multilevel comprehensive interventions in improving diabetes-related health outcomes. Data on healthcare utilisation and costs, as well as that of out-of-pocket expenditure will be collected in the baseline and endline surveys. In addition, data on direct costs including the cost of personnel, provider training, medications, lab tests and supplies, screening, outpatient visits and costs related to the social marketing campaign will be obtained during the implementation process. The total costs entailed to identify a person with diabetes as well as to appropriately treat that person to recommended targets based on guidelines will be measured. In addition, we will model the costs accrued from the use of drugs and other related interventions, based on results of other such comprehensive programmes and do a comparison to assess effectiveness. Based on the aforesaid indicators, we will develop a comprehensive cost-effectiveness model to assess the overall programme effectiveness.
GIS-based mapping of study households and neighbourhood-built environment
All households as well as the study areas, including various points of interest as indicated below, were geocoded using GIS mapping. This will be used to comprehensively assess the built environment (BE) and its impact on diabetes hypertension and risk factors. GIS-based techniques will be employed to study the association between BE features and diabetes, hypertension and related risk factors.
Specially trained field staff visited all participant households and used handheld Garmin Global Positioning System (GPS) receivers to capture GPS locations of the households. The captured GPS coordinates were verified using Google Earth to see if the locations matched the actual locations.
Table 8 shows the BE features from the study areas that were located and mapped.
Table 8.
Characteristics of the built environment in study sites
| Environment features | Points of interest | N | Sonipat | Vizag |
| Healthcare facilities | Government health facilities | 64 | 36 | 28 |
| Private hospitals and clinics | 228 | 147 | 81 | |
| Registered medical practitioners and unqualified practitioners | 220 | 195 | 25 | |
| Other health professionals | 118 | 115 | 3 | |
| Pharmacies | 337 | 224 | 113 | |
| Medical laboratories | 46 | 30 | 16 | |
| Food outlets | Hotels | 165 | 4 | 161 |
| Restaurants | 33 | 10 | 23 | |
| Small eateries | 97 | 40 | 57 | |
| Provision/department stores | 313 | 196 | 117 | |
| Fruit/vegetable/juice outlets | 254 | 36 | 218 | |
| Meat/fish shops | 128 | 24 | 104 | |
| Public distribution system (ration) shops | 52 | 344 | 52 | |
| Milk outlets | 128 | 6 | 122 | |
| Bakeries/sweet shops | 123 | 73 | 50 | |
| Tobacco outlets | Pan shop | 713 | 17 | 696 |
| Alcohol outlets | Authorised government outlets | 45 | 31 | 14 |
| Unauthorised alcohol outlets | 100 | 100 | ||
| Recreational facilities | Parks | 91 | 62 | 29 |
| Walking tracks | 6 | 6 | ||
| Play grounds | 50 | 43 | 7 | |
| Fitness / yoga centres | 10 | 6 | 4 | |
| Other | Anganwadis, schools, temples, and so on | 633 | 124 | 509 |
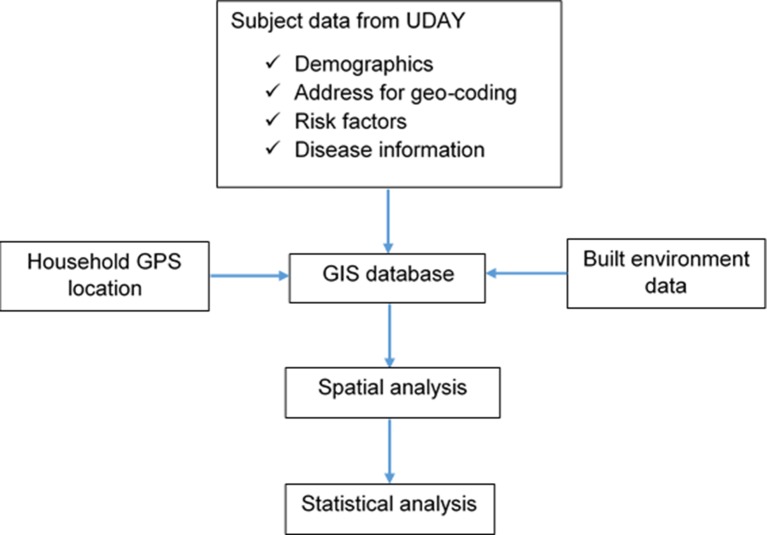
Household GPS locations and neighbourhood BE features were integrated with participant data collected as part of the baseline data collection. An overview of the GIS mapping is provided in figure 5. Area boundaries were obtained from government records and digitised. All spatial data were integrated into a spatial database, and ArcGIS software was used to carry out the following spatial analysis methods.
Figure 5.
GIS mapping overview.
Distance calculations: Distance between participant households and features of interest such as healthcare facilities, food and alcohol outlets, and parks, and their association between CMD and risk factors.
Spatial aggregation: Aggregation of features such as number of food outlets and parks in the neighbourhood and relationship with CMD and risk factors.
Clustering: Identify clustering of CMD and risk factors (hotspots) and determine if disease clusters are of sufficient geographic size and concentration to have not occurred by chance.
Spatial smoothing and interpolation: Used to derive a spatial surface from sampled data points (filling in where data are unobserved) or to smooth across polygons (aggregate data) to create more robust estimates.
Spatial regression: Use of spatial regression methods such as geographically weighted regression to further understand the relationship between BE and CMD risk factors as standard statistical regression models, which assume independence of the observations, are not appropriate for analysing spatially dependent data.
Data management
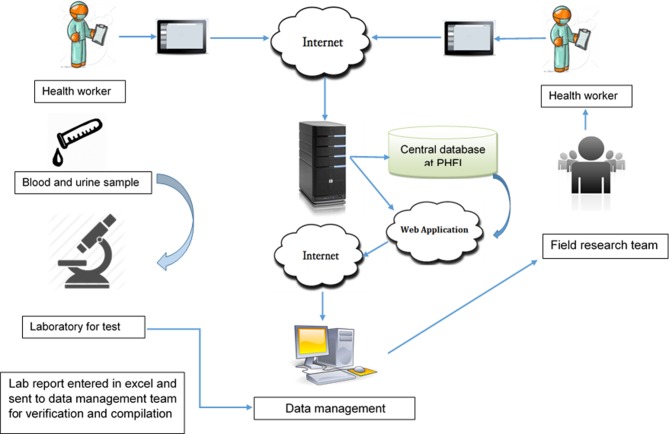
Data were collected in electronic format using customised android-based software on a tablet platform and uploaded to server on a real-time basis (figure 6). For ensuring quality control, all validation, range and logical checks were in-built in the software. Error reports were generated biweekly and sent to the study sites for rectification. Errors were checked against completion of the questionnaire, identifiers and adherence to the sampling strategy of interviews (only one man and one woman from single household).
Figure 6.
Data collection and management pathway.
Further, site research teams identified any other issues and reported to centralised team for the corrections. Data correction took place concomitantly with the conduct of the baseline survey. Similarly, biosample reports were matched with participant questionnaires and the final data were locked after all matching and rectification of errors.
Analysis plan
Data were entered into a database designed specifically for the project, housed at PHFI and accessible only to investigators and designated study staff. Data will be analysed using Stata/SE V.12.1 for Windows software. Descriptive statistics will be done, and the data expressed as frequencies and percentages for categorical variables and means and SD for normally distributed continuous variables or IQRs otherwise. Differences between gender groups, age groups, socioeconomic groups, study sites, time periods and individual hypotheses will be tested using appropriate analytical statistical tests (χ2 tests for categorical variables, t-tests continuous variables, multiple linear regression for continuous variables and multiple logistic regression for categorical variables). Stratified analysis will be done to assess for potential confounding and effect modification by other variables. A P value of <0.05 will be considered to indicate statistical significance. Multilevel modelling will be undertaken by clusters and households as potential levels.
Discussion
UDAY is one of the largest community-based intervention studies established in India to implement and evaluate a multicomponent, multilevel, cost-effective, comprehensive intervention programme to concomitantly improve the prevention, detection and optimal management of diabetes and hypertension, which together constitute the leading NCDs in India. Their high and rising burden, coupled with huge healthcare costs, underlines the need for cost-effective community-based approaches supplemented by measures to strengthen the health system to address both these NCDs effectively as envisaged in UDAY.
Most of the evidence on community interventions for chronic NCDs are from developed countries.5 6 In the last two decades, some evidence has emerged from low-income, middle-income countries as well but not quite commensurate to the disproportionate burden borne by them (80% NCD mortality).7 8 This is due to several reasons including resources to conduct such large projects as well as the technical capacity.7 8 However, available information indicates that results are likely better in low-income, middle-income countries (eg, Isfahan Healthy Heart Program in Iran, diabetes prevention programmes in China and India).7–9 We have taken into account findings of such prior research and attempted to address the reported gaps by adding relevant elements to the design of our study. For instance, most such intervention programmes have entailed community-based interventions (largely targeting lifestyle modification) but have not had active healthcare system and advocacy interventions as proposed in our study. In addition, many of the diabetes prevention programmes have targeted high-risk groups and not the general free-living population as envisaged in this programme. Further, we have used several innovations (see table 1) including task shifting/sharing of care to non-physician health workers by the extensively leveraging low-cost m-health technology to enable and empower them to screen and deliver interventions as well as physicians to treat patients as per evidence-based algorithms. We have also used GIS mapping to characterise the sites, BE, healthcare facilities and providers to examine the influence of BE on diabetes/hypertension and their risks factors as well as care pathways that patients undertake in order to deliver interventions in a more focused way. In addition, we have built-in extensive stakeholder and community engagement in the study implementation which should aid in improving acceptability and buy-in for the intervention programme.
Further, the community-based cohorts established will not only facilitate tracking of the trends in risk factors and diseases in rapidly transitioning populations in India, but also serve as well-characterised population platforms for embedding and evaluating new research questions of public health relevance in combating the rise of NCDs.
Notably, a unique strength of UDAY besides occurring in a low-income, middle-income country setting where there are limited community-based projects compared with various other community-based projects conducted in both developed countries and low-income, middle-income countries, which have either addressed prevention or management of select chronic conditions, is that UDAY aims to address both prevention and management of concurrently and comprehensively.
However, the study has some limitations. First, we used a pre-post study design for evaluating the effect of our interventions. Though a randomised controlled trial is a better design to evaluate the effectiveness of interventions, providing a higher level of evidence than a pre-post design, to study the effect of multicomponent interventions delivered at multiple levels in a comprehensive manner in a large population over a vast geographic area, we considered the pre-post design as more appropriate for our study. Further, we also wanted to understand and examine the operational part of the programme implementation to gain insights into underpinning factors behind success or failure that can inform possible replication and scale up in the future.
Second, our study does not include controls for the comparison. Given the size of the population covered by the interventions, we would have had to recruit control communities of similar size and numbers, which was not feasible from an implementation and resources availability point of view. However, our baseline and endline surveys that evaluate the impact are done on independent random samples of the population, which should provide robust data regarding potential changes over baseline in the levels of public awareness and knowledge about diabetes and hypertension; those aware, diagnosed, treated and controlled to recommended targets; and the use of guideline-based management by providers leading to improved health outcomes and access to healthcare for people living with diabetes and hypertension in India. In addition, we will be comparing our results with ongoing national survey data on NCDs and their risk factors (National Family Health Survey, Annual Health Survey, District Level Household Survey) as well as a New National NCD survey which is being implemented currently. This will help assess secular trends and evaluate our findings in conjunction with such trends if any. Also we did not account for the regression to the mean as there would be at least some people both in the endline and baseline. We will do sensitivity analysis to explore this bias.
Third, one of the major interventions of our programme is to implement a community-based screening, follow-up and educational programme through health workers. We specifically hired and trained health workers to implement this interventional component, which might add to the cost of implementing a community-based diabetes and hypertension prevention and management programme. However, the additional cost is likely to be minimal as indicated by previous modelling estimates of training and using health workers.
Fourth, we are using multicomponent interventions at multiple levels (health promotion campaigns, health workers-led home-based screening, follow-up and education, training of healthcare providers, registry for facility-based improvement in quality of care, patient networks and advocacy to strengthen the health system) which makes it difficult to evaluate the individual contribution of each intervention. However, the purpose is to deliver it in a comprehensive manner to improve outcomes, which to our knowledge has hitherto not been implemented in similar settings, and not to tease out impact of individual interventions in a milieu where many individuals have elevations of multiple NCD risk factors and suffer often from comorbid conditions that require to be addressed comprehensively.
It is anticipated that the results obtained from the study will inform policymakers on the most appropriate community and health system-based approaches that are effective in stemming the rising burden of diabetes and hypertension in India and countries with similar challenges.
Supplementary Material
Acknowledgments
The UDAY project is led by the Public Health Foundation of India, Gurgaon, India in collaboration with Population Services International, India and Project HOPE. We acknowledge the contributions of the field and research staff of the project. The funder has no role in the design, implementation, and evaluation of the project as well as in the writing of this paper. All contents of this paper are solely the responsibility of the authors.
Footnotes
Contributors: SM, KSR, DP and NT conceived the study. SM wrote the first draft of the manuscript. PJ, SG, NSV, RR, CM, RG, BR and SK wrote specific sections of the manuscript as well as contributed to the design and implementation of the study. BR and SK also coordinate the activities in the study sites and provide technical support. SM, SG, PJ and NSV contributed to the collection and assembly of data. All authors provided input into the study design, as well as provided critical intellectual input for revision of the manuscript and approved the final version of the manuscript.
Funding: It is supported by an unrestricted educational grant from Eli Lilly and Company under the Lilly NCD Partnership Program.
Disclaimer: The funding agency had no role in the design, conduct or analysis of the study, and no role in the decision to submit the protocol for publication.
Competing interests: None declared.
Ethics approval: Institutional Ethics Committee (IEC) of the Public Health Foundation of India.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. World Health Organization (WHO) Country Office for India. National multisectoral action plan for prevention and control of noncommunicable diseases. India, New Delhi: World Health Organization (WHO) Country Office for India, 2013. [Google Scholar]
- 2. International Diabetes Federation. IDF Diabetes Atlas. 7th edn Brussels, Belgium: International Diabetes Federation, 2015. [Google Scholar]
- 3. Srinath Reddy K, Shah B, Varghese C, et al. . Responding to the threat of chronic diseases in India. Lancet 2005;366:1744–9. 10.1016/S0140-6736(05)67343-6 [DOI] [PubMed] [Google Scholar]
- 4. Mohan S, Koller T. Hypertension and related comorbidities in India: Implications for the health system. India, New Delhi: World Health Organization (WHO) Country Office for India, 2013. [Google Scholar]
- 5. Berra K, Franklin B, Jennings C. Community-Based Healthy Living Interventions. Prog Cardiovasc Dis 2017;59:430–9. 10.1016/j.pcad.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 6. Puska P. Why did north karelia-finland work? Is it transferrable? Glob Heart 2016;11:387–91. 10.1016/j.gheart.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 7. Sarrafzadegan N, Kelishadi R, Esmaillzadeh A, et al. . Do lifestyle interventions work in developing countries? Findings from the isfahan healthy heart program in the islamic republic of Iran. Bull World Health Organ 2009;87:39–50. 10.2471/BLT.07.049841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harati H, Hadaegh F, Momenan AA, et al. . Reduction in incidence of type 2 diabetes by lifestyle intervention in a middle eastern community. Am J Prev Med 2010;38:628–36. 10.1016/j.amepre.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 9. Rawal LB, Tapp RJ, Williams ED, et al. . Prevention of type 2 diabetes and its complications in developing countries: a review. Int J Behav Med 2012;19:121–33. 10.1007/s12529-011-9162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nair M, Ali MK, Ajay VS, et al. . CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health 2012;12:701 10.1186/1471-2458-12-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reddy KS, Prabhakaran D, Chaturvedi V, et al. . Methods for establishing a surveillance system for cardiovascular diseases in Indian industrial populations. Bull World Health Organ 2006;84:461–9. 10.2471/BLT.05.027037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005–2006. Volume II India, Mumbai: IIPS, 2007. [Google Scholar]
- 13. World Health Organization. Global Physical Activity Questionnaire (GPAQ) analysis guide: World Health Organization; http://www.who.int/chp/steps/resources/GPAQ_Analysis_Guide.pdf (assessed 15 Dec 2015). [Google Scholar]
- 14. Yusuf S, Hawken S, Ounpuu S, et al. . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 15. EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 16. Poongothai S, Pradeepa R, Ganesan A, et al. . Reliability and validity of a modified PHQ-9 item inventory (PHQ-12) as a screening instrument for assessing depression in Asian Indians (CURES-65). J Assoc Physicians India 2009;57:147–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-015919supp001.pdf (178.7KB, pdf)