Abstract
The chance discovery of hydroxymethylglutaryl (HMG)-CoA reductase inhibitors has revolutionized the care of patients with cardiovascular disease. The unexpected finding that these cholesterol-lowering drugs (or ‘statins’) also posses pleiotropic immunomodulatory properties, has opened a new area of research which investigates the anti-inflammatory and anti-proliferative properties of statins. In this brief commentary, we discuss the potential application of these drugs in asthma, where metabolic pathways pertinent to lung inflammation, in addition to the mevalonate cascade, may be targeted. We review mechanisms of action, discuss the potential therapeutic use of statins in asthma, share some preliminary data from our laboratory, discuss results from recent clinical trials in asthma, and propose a new target asthma subpopulation that could potentially benefit. We conclude our essay by highlighting the mevalonate-dependent and –independent pathways that may be modulated by statins, including the emerging area of cholesterol, sphingolipid, and lipid raft biology in lung disease. In this is an opportunity to develop new treatments for asthma, where innovative therapies are urgently needed to prevent acute exacerbations and alter disease progression.
Keywords: Statins, asthma, mevalonate, metabolic, novel therapy, HMG-CoA reductase, anti-inflammatory, immunomodulatory, eotaxin, obese asthmatic, lipid
A GROUNDBREAKING DISCOVERY
The discovery of compactin (now known as mevastatin) by Dr. Akira Endo in 1973, led to the development of the ‘statin’ class of drugs. In the last two decades these medications have revolutionized the care of patients with cardiovascular disease. Endo’s work on cholesterol metabolism in fungi revealed that Penicillium citrinum can produce organic molecules, i.e. the statins, which naturally inhibit hydroxymethylglutaryl (HMG)-CoA reductase (HMGR) via competitive inhibition. Subsequent studies on cholesterol homeostasis and the low density lipoprotein (LDL) receptor by Drs. Joseph L. Goldstein and Michael S. Brown garnered them the Nobel Prize in 1985 and lay the foundation for the use of statins in clinical trials. The relatively recent realization that statins also have anti-inflammatory and immunomodulatory properties has resulted in a new and intriguing avenue of research relevant to lung diseases such as asthma and chronic obstructive pulmonary disease (COPD).
MECHANISMS AND BIOLOGICAL IMPLICATIONS
The statins directly inhibit HMGR, the rate-limiting step in the cholesterol biosynthesis pathway in the mevalonate (MA) cascade (Fig. 1). Depletion of MA by statins affects critical downstream intermediates, such as the isoprenoids farnesyl- and geranylgeranylpyrophosphate (FPP and GGPP). These lipid metabolites post-translationally modify the small guanosine triphosphatases (GTPases) Rho, Ras, Rac, and Cdc42, which can then associate with the cell membrane leading to intracellular signal transduction [1, 2]. These GTPases are important in a variety of key biological activities that include recruitment of inflammatory cells, cellular proliferation and transmigration, vesicular trafficking, cytoskeletal dynamics, apoptosis and phagocytosis, antigen uptake and processing, and cell cycle regulation [3]. Thus, the MA cascade is a major metabolic pathway that regulates manifold cellular processes important to many diseases beyond cardiovascular disease [4]. The statins modulate this pathway in different cell types which has created an opportunity for novel and innovative investigations in several fields outside of cardiovascular medicine.
Fig. 1.
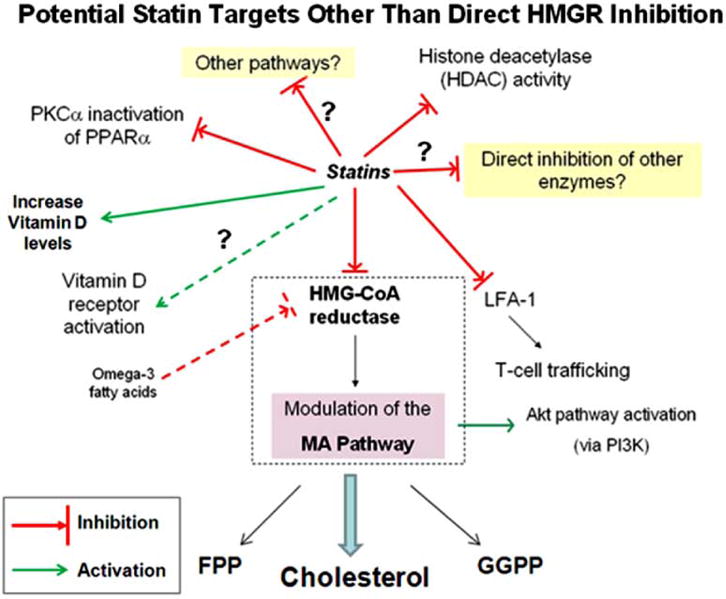
The statins may inhibit or activate various other pathways beyond the MA cascade. Direct HMGR inhibition depletes cellular MA and downstream metabolites (cholesterol, FPP, and GGPP) – i.e. the classical target of statins, while HMGR-independent pathways represent novel statin targets. (Abbreviations: phosphatidylinositol 3-kinase (P13K), peroxisome proliferators-activated receptor (PPAR), protein kinase C (PKC)α, mevalonate (MA), hydroxymethylglutaryl (HMG)-CoA reductase (HMGR), histone deacetylase (HDAC), leukocyte function antigen-1 (LFA-1), farnesylpyrophosphate (FPP), geranygeranylpyrophosphate (GGPP)).
THERAPEUTIC POTENTIAL
We are interested in the therapeutic potential of statins in lung disease, in particular inflammatory airway diseases such as asthma and COPD. We and others have demonstrated that simvastatin in the allergic mouse model attenuates eosinophilic airway inflammation [5, 6] via inhibition of HMGR in the MA pathway [7]. Interestingly, improvements in airway hyperreactivity (AHR) and lung compliance appeared to be MA- or HMGR-independent [7]. This suggests other statin targets exist beyond the HMGR enzyme (Fig. 1).
Although systemic treatment with simvastatin has a known potent anti-inflammatory effect in our asthma model (Fig. 2), additional work in our lab will explore lung-targeted modalities of delivering statins. Beyond the asthma model, simvastatin also attenuates the production of cytokines important in neutrophilic recruitment and airway remodeling [8]. Ongoing studies will test the anti-inflammatory and anti-remodeling effects of simvastatin and lovastatin in a rat model of cigarette smoke-induced inflammation. Our preliminary data lead us to believe that statins affect a profound inhibition of Th1/Th2/Th17 cytokine and chemokine expression in both mouse and human airway epithelial cells (Fig. 3), where the epithelium is known to play a central role in asthma mucosal immunity [9].
Fig. 2.
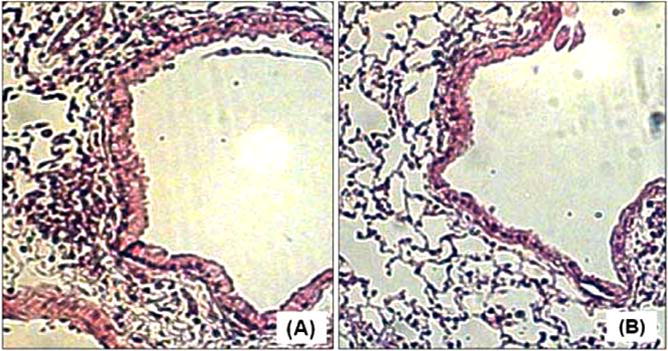
Lung histology of ovalbumin (OVA)-sensitized/exposed BALB/c mice (H&E statin at 100× magnification). Panel (A) shows the influx of peribronochiolar inflammatory cells in the OVA control group. Panel (B) shows a marked reduction of peribronchiolar inflammation in OVA mice treated with simvastatin (40 mg/kg) intraperitoneally.
Fig. 3.
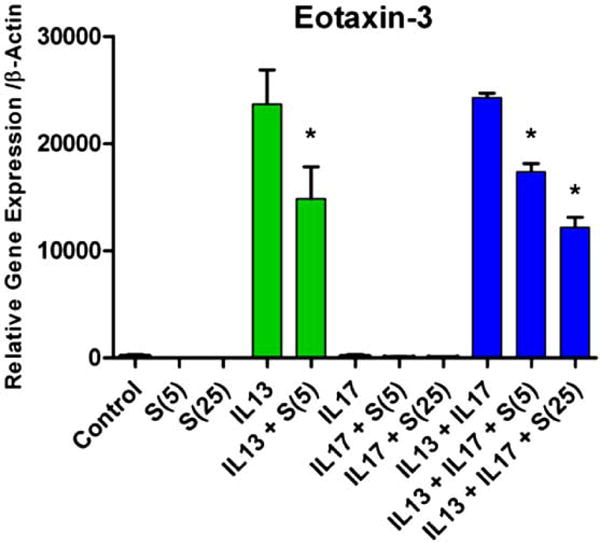
Eotaxin-3 expression in primary human airway epithelial cells. Simvastatin (5 uM and 25 uM, abbreviated as S(5) and S(25), respectively) attenuated IL-13-induced eotaxin-3-expression (IL-13 dose = 20 ng/mL) (*p<0.05). Co-stimulation with IL-17 did not alter the effect of IL-13 on eotaxin-3 gene expression.
EMERGING CLINICAL TRIAL DATA
Recent observational studies have linked statin use with improvements in lung health (e.g. exacerbations, COPD mortality, and decline in forced expiratory volume in the first second (FEV1)), the largest studies being done in COPD and asthma [10–13]. A recent large retrospective study found that statin exposure in patients with asthma using inhaled corticosteroids (ICS) was independently associated with a significant reduction in asthma-related hospitalizations and emergency room events over 12 months [14]. However, no human randomized clinical trials have definitively reproduced the benefits seen in animal models.
Several ongoing clinical trials are investigating exacerbation rates, lung function, inflammatory markers, and quality of life in asthmatics treated with statins compared to placebo (www.clinicaltrials.gov). So far, four small clinical trials in asthma have been reported, three using simvastatin [15–17] and one using atorvastatin [18]. Overall, the results are mixed where in one study an anti-inflammatory effect (as measured by sputum markers, e.g. macrophage count and leukotriene B4) was observed absent clinical benefit [18]. And in another study, despite a lack of a steroid-sparing effect, there were some improvements in asthmatic symptoms, lung function (as measured by FEV1), and the number of sputum eosinophils (in those who reached the 0 μg/day inhaled corticosteroid dose) [16]. However, in a recent double-blinded study, simvastatin (10 mg daily for 8 weeks) was given as add-on therapy to low-dose inhaled budesonide (200 μg) in patients with mild asthma. Simvastatin enhanced the anti-inflammatory effect of budesonide where sputum eosinophil counts were significantly reduced by the combined therapy (budesonide and simvastatin) compared to the control group (budesonide and placebo) (p=0.02) [17]. Although the study was not powered to detect changes in lung function, there was a trend toward higher FEV1 in the budesonide and simvastatin group compared to the control population. A major limitation of these trials is that these were small, relatively short-term studies (4-8 weeks [15, 17, 18]), and 3 months [16]) where the subpopulation of asthmatics was not defined beyond allergic asthma.
It is important to remain cautious with these results given no definitive improvement in clinical outcomes as of yet. However, the observation that statins attenuate airway inflammation in asthma as measured by sputum markers is noteworthy. Whether this translates into reduced exacerbations and improved lung function remains an open and worthwhile research question. It also raises the following question: What would happen if the statin was given for a longer period of time and/or at a higher dose? This has implications for an aspect of severe asthma that remains without a viable treatment – irreversible airway remodeling.
Thus, the hypothesis that the statins may have benefit in a subpopulation of asthmatics has not been adequately tested in clinical trials of longer-term duration, where effects on asthma pathogenesis (e.g. chronic inflammation and remodeling) and clinical outcomes (e.g. acute exacerbations) require additional evaluation.
PROPOSED TARGET ASTHMA POPULATION
Why is asthma a potential disease target for statins? Asthma being a heterogeneous disease presents particular difficulties for clinical trials designed to test novel therapies. However, in this limitation is a hidden opportunity. Although one can propose many different arguments for why statins could benefit patients with asthma, we choose to focus on a subpopulation that may be underappreciated.
Epidemiologic studies have linked obesity with asthma [19–22]. Obesity is also strongly associated with metabolic syndrome, where a link to asthma is also emerging [23]. Using multidimensional cluster analyses, an obese subgroup of female asthmatics has been described [24]. The Severe Asthma Research Program has also described a cluster of older obese women with late onset non-atopic asthma [25]. Obesity being linked to systemic inflammation [26, 27], dyslipidemia, and metabolic syndrome [28] (where statins may be indicated) [29, 30], presents a unique opportunity for those with the obese-asthma phenotype. This subpopulation of asthmatics could potentially benefit from statins – a safe and widely used treatment that would also treat other comorbidities (e.g. cardiovascular disease, dyslipidemia). Adding to this complexity, emerging epidemiologic data link serum cholesterol levels with asthma risk in U.S. populations [31].
Thus, we believe that future clinical trials to assess the potential therapeutic use of statins in asthma should focus on the obese asthmatic as one target subpopulation or phenotype that could potentially benefit. Albeit the underling mechanisms in this specific population have not yet been fully described, we feel that parallel work in animal models and humans may accelerate the application of this innovative therapy.
ROLE OF THE MEVALONATE PATHWAY: FERTILE GROUND AND FUTURE DIRECTIONS
The MA pathway is important not only for its regulation of small GTPases, but also for the biosynthesis of cholesterol which is the precursor to many crucial metabolites and is a critical component of cell membranes. Numerous cholesterol products have emerging roles in asthma (and possibly other lung diseases), including vitamin D [32], steroid hormones, lipid rafts [33], and lipoproteins [34, 35]. Cholesterol itself may have a role in allergic lung inflammation [36] and lung host defense [37] which may be important in the pathogenesis of asthma.
Beyond this and given the pleiotropic effects of statins [38], an opportunity also exists to investigate HMGR- or MA-independent pathways thereby unraveling other important mechanisms. Emerging data suggest that statins can also inhibit LFA-1 [39], HDAC activity [40], and PKCα inactivation of PPARα [41], while potentially activating vitamin D receptors [42] or increasing serum vitamin D levels [43] (Fig. 1, see for Abbreviations). Omega-3 fatty acids may also inhibit HMGR [44] independently of statins, indicating crosstalk among different metabolic pathways with the attendant dietary considerations. This highlights the broad-ranging, pleiotropic statin effects and alternative metabolic pathways which may be relevant to asthma or other lung diseases.
Finally, the potential for statins to modulate cell signaling and subsequent immune responses by altering cholesterol content in lipid rafts [45] remains a relatively unexplored area in lung biology. Lipid raft trafficking and stabilization of receptor-to-ligand binding, a fundamental event in cellular inflammation and proliferation, is an area of intense exploration with direct relevance to asthma [46, 47]. In addition to cholesterol, the sphingolipids are also a key component of lipid rafts [48], where they may play a role in cigarette smoke-induced lung injury [49, 50] and asthma [51]. Thus, the statins, the pathways they affect, and cholesterol/ sphingolipid biology [52, 53] emerge as a fertile ground for investigation in lung diseases, particularly in asthma where targeted, novel, and innovative therapies are urgently needed.
Acknowledgments
We would like to thank Professor Reen Wu and Dr. Phil Thai for their technical input and insightful discussions regarding some of our in vitro experiments.
GRANT FUNDING
NIH (T32) HL07013, NCRR UL1 RR024146 (CTSC K30), HL-076415 (K08), ATS Fellows Career Development Award, CTSC K12 Award (KL2 RR 024144), and NIH-R01-HL066189.
References
- 1.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6(5):358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takai Y, Sasaki T, Matozaki T. Small GTP-Binding Proteins. Physiological Reviews. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 4.Almuti K, Rimawi R, Spevack D, Ostfeld RJ. Effects of statins beyond lipid lowering: potential for clinical benefits. Int J Cardiol. 2006;109(1):7–15. doi: 10.1016/j.ijcard.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Kim DY, Ryu S, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur J Pharmacol. 2007;557(1):76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Mc Kay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A Novel Anti-Inflammatory Role of Simvastatin in a Murine Model of Allergic Asthma. The Journal of Immunology. 2004;172:2903–2908. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 7.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin Inhibits Airway Hyperreactivity: Implications for the Mevalonate Pathway and Beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy DM, Forrest IA, Corris PA, Johnson GE, Small T, Jones D, Fisher AJ, Egan JJ, Cawston TE, Ward C, Lordan JL. Simvastatin attenuates release of neutrophilic and remodeling factors from primary bronchial epithelial cells derived from stable lung transplant recipients. Am J Physiol Lung Cell Mol Physiol. 2008;294:L592–L599. doi: 10.1152/ajplung.00386.2007. [DOI] [PubMed] [Google Scholar]
- 9.Holgate ST. The Airway Epithelium is Central to the Pathogenesis of Asthma. Allergology International. 2008;57:1–10. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- 10.Alexeeff SE, Litonjua AA, Sparrow D, Vokonas PS, Schwartz J. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med. 2007;176:742–747. doi: 10.1164/rccm.200705-656OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blamoun AI, Batty GN, DeBari VA, Rashid AO, Sheikh M, Khan MA. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: evidence from a retrospective cohort study. Int J Clin Pract. 2008;62(9):1373–1378. doi: 10.1111/j.1742-1241.2008.01731.x. [DOI] [PubMed] [Google Scholar]
- 12.Soyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007;29(2):279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 13.Janda S, Park K, FitzGerald JM, Etminan M, Swiston J. Statins in COPD: A Systematic Review. Chest. 2009;136:734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- 14.Stanek E, Aubert R, Xia F, Frueh F, Sanders C, Epstein R, Weiss S. Statin Exposure Reduces the Risk of Asthma-Related Hospitalizations and Emergency Room Visits in Asthmatic Patients on Inhaled Corticosteroids. J Allergy Clin Immunol. 2009;123(2):S65. (Abstract) [Google Scholar]
- 15.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119:328–335. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax. 2010;65:891–896. doi: 10.1136/thx.2010.138990. [DOI] [PubMed] [Google Scholar]
- 17.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 23-dioxygenase. J Allergy Clin Immunol. 2010;126:754–762. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, Weir CJ, Meiklejohn J, Sattar N, McInnes I, Wood S, Thomson NC. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–1075. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 19.Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, Calatroni A, Zeldin DC. Association of Childhood Obesity With Atopic and Nonatopic Asthma: Results From the National Health and Nutrition Examination Survey 1999-2006. Journal of Asthma. 2010;47:822–829. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Weiss ST. Obesity: insight into the origins of asthma. Nature Immunology. 2005;6(6):537–539. doi: 10.1038/ni0605-537. [DOI] [PubMed] [Google Scholar]
- 22.Von BJ, Lipsett M, Horn-Ross PL, Delfino RJ, Gilliland F, McConnell R, Bernstein L, Clarke CA, Reynolds P. Obesity, waist size, and prevalence of current asthma in the California Teachers Study cohort. Thorax. 2009;64:889–893. doi: 10.1136/thx.2009.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal AMU, Ahmad T, Ghosh B. Emerging Interface Between Metabolic Syndrome And Asthma. American Journal of Respiratory Cell and Molecular Biology. 2010:1–12. doi: 10.1165/rcmb.2010-0141TR. (Epub Online) [DOI] [PubMed] [Google Scholar]
- 24.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster Analysis and Clinical Asthma Phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SAA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta AK, Johnson WD. Prediabetes and prehypertension in disease free obese adults correlate with an exacerbated systemic proinflammatory milieu. Journal of Inflammation. 2010;7:36–40. doi: 10.1186/1476-9255-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-Reactive Protein to Abdominal Adiposity. Am J Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Wildman RP, McGinn AP, Lin J, Wang D, Muntner P, Cohen HW, Reynolds K, Fonseca V, Sowers MFR. Cardiovascular Disease Risk of Abdominal Obesity vs. Metabolic Abnormalities. Obesity. 2010:1–8. doi: 10.1038/oby.2010.168. (Epub Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bays HE, Roth EM, McKenney JM, Kelly MT, Thakker KM, Setze CM, Obermeyer K, Sleep DJ. The Effects of Fenofibric Acid Alone and With Statins on the Prevalence of Metabolic Syndrome and its Diagnostic Components in Patients With Mixed Dyslipidemia. Diabetes Care. 2010;33(9):2113–2116. doi: 10.2337/dc10-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jialal I, Amess W, Kaur M. Management of Hypertriglyceridemia in the Diabetic Patient. Curr Diab Rep. 2010;10:316–320. doi: 10.1007/s11892-010-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fessler MD, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, Arbes SJ, Calatroni A, Zeldin DC. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J Allergy Clin Immunol. 2009;124:967–974. doi: 10.1016/j.jaci.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DYM. Vitamin D Levels, Lung Function, and Steroid Response in Adult Asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin JS, Shelburne CP, Jin C, LeFurgey EA, Abraham SN. Harboring of particulate allergens within secretory compartments by mast cells following IgE/FcepsilonRI-lipid raft-mediated phagocytosis. J Immunol. 2006;177(9):5791–5800. doi: 10.4049/jimmunol.177.9.5791. [DOI] [PubMed] [Google Scholar]
- 34.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic Abnormalities in Children with Asthma. Am J Respir Crit Care Med. 2010 Sep 17; doi: 10.1164/rccm.201004-0603OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak Ml, Amar MJ, Remaley AT, Levine SJ. Apolipoprotein E Negatively Regulates House Dust Mite-induced Asthma via a LDL Receptor-mediated Pathway. Am J Respir Crit Care Med. 2010;182(10):1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J Biomed Sci. 2004;11(5):599–606. doi: 10.1007/BF02256124. [DOI] [PubMed] [Google Scholar]
- 37.Draper DW, Madenspacher JH, Dixon D, King DH, Remaley AT, Fessler MB. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am J Respir Crit Care Med. 2010;182(3):404–412. doi: 10.1164/rccm.200910-1580OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemoto M, Liao JK. Pleiotropic Effects of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 39.Weitz-Schmidt G. Lymphocyte Function–Associated Antigen-1 Blockade by Statins: Molecular Basis and Biological Relevance. Endothelium. 2003;10:43–47. doi: 10.1080/10623320303360. [DOI] [PubMed] [Google Scholar]
- 40.Lin YC, Lin JH, Chou CW, Chang YF, Yeh SH, Chen CC. Statins Increase p21 through Inhibition of Histone Deacetylase Activity and Release of Promoter-Associated HDAC1/2. Cancer Res. 2008;68(7):2375–2383. doi: 10.1158/0008-5472.CAN-07-5807. [DOI] [PubMed] [Google Scholar]
- 41.Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, Staels B. Acute Antiinflammatory Properties of Statins Involve Peroxisome Proliferator–Activated Receptor-α via Inhibition of the Protein Kinase C Signaling Pathway. Circ Res. 2006;98:361–369. doi: 10.1161/01.RES.0000202706.70992.95. [DOI] [PubMed] [Google Scholar]
- 42.Grimes DS. Are statins analogues of vitamin D? Lancet. 2006;36(8):83–86. doi: 10.1016/S0140-6736(06)68971-X. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Castrillón JL, Vega G, Abad L, Sanz A, Chaves J, Hernandez G, Dueñas A. Effects of Atorvastatin on Vitamin D Levels in Patients With Acute Ischemic Heart Disease. Am J Cardiol. 2007;99:903–905. doi: 10.1016/j.amjcard.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 44.Duncan RE, El-Sohemy A, Archer MC. Regulation of HMG-CoA reductase in MCF-7 cells by genistein, EPA, and DHA, alone and in combination with mevastatin. Cancer Letters. 2005;224:221–228. doi: 10.1016/j.canlet.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Hillyard DZ, Jardine AG, McDonald KJ, Cameron AJ. Fluvastatin inhibits raft dependent Fcgamma receptor signalling in human monocytes. Atherosclerosis. 2004;172(2):219–228. doi: 10.1016/j.atherosclerosis.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Owens JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88 dependent toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51(11):3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via TLR4 triggering of airway structural cells. Nat Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Meer G. The Different Hues of Lipid Rafts. Science. 2002;296:855–857. doi: 10.1126/science.1071491. [DOI] [PubMed] [Google Scholar]
- 49.Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, Last J, Pinkerton K, Tuder R, Goldkorn T. Neutral Sphingomyelinase 2: A Novel Target in Cigarette Smoke-Induced Apoptosis and Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2010 May 6;:1–25. doi: 10.1165/rcmb.2009-0422OC. (Epub online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldkorn T, Filosto S. Lung Injury and Cancer: Mechanistic Insights into Ceramide and EGFR Signaling under Cigarette Smoke. Am J Respir Cell Mol Biol. 2010;43:259–268. doi: 10.1165/rcmb.2010-0220RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roviezzo F, D’Agostino B, Brancaleone V, De Gruttola L, Bucci M, De Dominicis G, Orlotti D, D’Aiuto E, De Palma R, Rossi F, Sorrentino R, Cirino G. Systemic administration of sphingosine-1-phosphate increases bronchial hyperresponsiveness in the mouse. Am J Respir Cell Mol Biol. 2010;42(5):572–577. doi: 10.1165/rcmb.2009-0108OC. [DOI] [PubMed] [Google Scholar]
- 52.Nixon GFS. phingolipids in inflammation: pathological implications and potential therapeutic targets. British Journal of Pharmacology. 2009;158(4):982–993. doi: 10.1111/j.1476-5381.2009.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boini KM, Zhang C, Xia M, Poklis JL, Li PL. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. J Pharmacol Exp Ther. 2010;334(3):839–846. doi: 10.1124/jpet.110.168815. [DOI] [PMC free article] [PubMed] [Google Scholar]


