Abstract
Background
Earlier studies have raised the notion that docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) supplementation could be a useful intervention in autosomal dominant Stargardt macular dystrophy (STGD3). We sought to assess whether fish oil supplementation has a beneficial effect on the clinical course of STGD3 secondary to a mutation in the ELOVL4 gene.
Materials and Methods
Eleven patients with STGD3 were enrolled in an 8-year open-label, clinical interventional study of over-the-counter fish oil supplements at a recommended daily dose of 650 mg EPA and 350 mg DHA (NCT00420602). Subjects had annual eye examinations with complete imaging, visual function testing and blood lipid analyses. Compliance with therapy was measured by periodic patient self-report and with serum and red blood cell biomarkers of lipid consumption. Paired sample t-tests were used to measure differences in mean values of visual acuity, lipid biomarkers, and contrast sensitivity obtained at baseline and the last follow-up.
Results
All subjects showed progression of their maculopathy, and we could not discern a beneficial effect of the intervention. Compliance with the recommended fish oil supplement intervention was poor as assessed by patient self-report and biomarkers of lipid consumption.
Conclusions
Our inability to detect a benefit of fish oil could be the result of small subject numbers, poor compliance, or intervention too late in the course of the disease. We still advise STGD3 patients to consume fish or fish oil regularly, and we recommend that pre-symptomatic children with ELOVL4 mutations should be especially targeted for these interventions.
Keywords: genetics, Stargardt disease, macular dystrophy, retinal degeneration, ELOVL4
Introduction
Stargardt disease (STGD) is a group of early onset inherited macular dystrophies that generally present with visual loss in childhood or in early adulthood accompanied by foveal atrophy and other perimacular changes such as fleck-like deposits at the level of the retinal pigment epithelium (RPE). 1, 2
The vast majority of Stargardt disease is inherited in an autosomal recessive fashion (STGD1) secondary to homozygous and compound heterozygous mutations in the ABCA4 gene that encodes for a protein involved in retinoid transport out of the rod outer segments. 1, 3
Rarer forms of STGD are inherited in an autosomal dominant manner. The most common dominant form (STGD3) affects hundreds of people in the United States and is due to a mutation in ELOVL4, a gene that encodes for a protein that is involved in fatty acid metabolism in the retina. 2, 4, 5 The ELOVL4 protein is an elongase enzyme that appears to be essential for the biosynthesis of non-dietary very long chain polyunsaturated fatty acids (VLC-PUFAs) in the human retina. These rare lipids with greater than 26 carbons are synthesized from dietary long chain polyunsaturated fatty acid (LC-PUFA) precursors such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and arachidonic acid (AA). 2 Mouse model studies indicate that VLC-PUFAs are required for normal structure and function of photoreceptors. 6
Knowledge of the genetic and molecular origins of STGD3 has already led to suggestions of possible early intervention strategies for these patients. Previous investigations from our center that focused on a large Utah/California family with two one-base-pair deletions in ELOVL4 found that biomarkers of long-term dietary intake of omega-3 LC-PUFAs such as EPA and DHA were inversely associated with severity of phenotype in STGD3 patients, presumably as a result of bypass of the ELOVL4 defect. 7 Specifically, we found that family members who regularly consumed fish had a much milder phenotype than those who rarely ate fish. Thus, it has been proposed that dietary supplementation with DHA and/or EPA supplements could be a useful intervention in STGD3 patients, and a Canadian case report indicates that this may indeed be the case. 8 In this prospective, interventional study, we investigated whether fish oil supplementation could slow the course of STGD3.
Materials and Methods
Study Design
The study was conducted at the John A. Moran Eye Center in Salt Lake City, Utah between August 2006 and December 2015. All subjects were members of the previously published Utah/California STGD3 family who lived in the Salt Lake City area. The study protocol was approved by the Moran Eye Center Institutional Review Board, and consent of the patient to participate in the study was requested, received, and documented prior to enrollment to the study.
Eleven patients with STGD3 in Utah were enrolled in an open-label, clinical interventional study of over-the-counter fish oil supplements at a recommended dose of 650 mg of EPA and 350 mg of DHA per day (NCT00420602). Inclusion criteria included: 1) patient must have a genetically confirmed mutation in ELOVL4; 2) subject must be willing and able to undergo the proposed intervention and attend follow up; 3) patient must be at least 18 years of age. Subjects were instructed to purchase an over-the-counter fish oil supplement compliant with the study’s protocol. No other dietary intervention was asked of the patient.
Patients were instructed to follow up annually. At each annual visit, a complete slit lamp examination of the eye was performed. Other tests included contrast sensitivity, and retinal imaging (color photography, blue light autofluoresence, OCT). Compliance with therapy was measured by periodic patient self-report and with biomarkers of lipid consumption, which include DHA and EPA levels in serum and red blood cell (RBC) lipids.
Region finder software, a semi-automated software embedded in Spectralis (Heidelberg Engineering, Heidelberg, Germany), was used to analyze fundus autofluorescence images and determine the rate of change of geographic atrophy.
Chemicals
All chemical reagents, such as methanol, hydrochloric acid, isopropanol, n-hexane and diethyl ether, were of gas chromatography mass spectrometry (GC-MS) grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). All standards including the internal standards such as tridecanoic acid (13:0), and Supelco-37 (a commercial mixture of fatty acid methyl esters (FAMEs)) were purchased from Matreya (Pleasant Gap, PA, USA). The internal standard, tridecanoic acid, was dissolved in nonane at a concentration of 1.0 mg/ml.
Lipid extraction and purification of lipids with solid-phase extraction
Sera and RBC membrane lipids were extracted using the procedure previously adopted in our laboratory.9 The samples (100 μl) and internal standards (50 μg of tridecanoic acid) were added in 12 ml glass tubes with 4 ml hexane-isopropanol (3:2 v: v) and sonicated for 5 min in an ice water bath. After centrifugation at 3,000 rpm for 5 min, the extracted solution supernatant was transferred to a clean vial and then dried under a stream of nitrogen. The dried film was dissolved in 200 μl hexane, and 2 ml of 4% HCl in methanol was added. The tubes were flushed with argon and incubated at 80°C for 4 hr to form FAMEs and then allowed to cool. The FAME mixture was extracted three times with 1 ml distilled water and 2 ml hexane. The hexane layers were combined and dried under nitrogen gas.
The dry film was dissolved in 200 μl of hexane and centrifuged for 3 min at 14,000 rpm to remove particles prior to GC-MS analysis. 1 μl of sample was injected into the GC-MS instrument for LC-PUFAs analysis.
GC-MS Instrumentation and Chromatographic Conditions
The Thermo Trace GC-DSQ II system (ThermoFisher Scientific, Waltham, MA, USA) consists of an automatic sample injector (AS 3000), gas chromatograph (GC), single quadrupole mass detector, and an analytical workstation. The chromatographic separation was carried out with an Rxi-5MS coated 5% diphenyl/95% dimethyl polysiloxane capillary column (30 m × 0.25 mm i.d, 0.25 μm film thickness) (Restek, Bellefonte, PA, USA). For LC-PUFA analyses, the following mass spectrometry conditions were used (Method A): 1 μl from a 200 μl sample was injected into the GC-MS using a splitless mode; the septum purge was on; and the injector temperature was set at 200°C. The column temperature was programmed as follows: initial temperature 60°C; 5 degree/min to 170°C; 1 degree/min to 180°C; 2 degree/min to 240°C; 4 degree/min to 290°C; and a hold at 290°C for 5 min. Transfer line temperature was 290°C. Helium was used as the carrier gas at a flow rate of 1.0 ml/min. Mass spectrometry conditions were as follows: electron ionization mode with full scan and selected ion monitoring (SIM, m/z 79, 108, and 150) because m/z 79, 108, and 150 are typical ions of PUFAs, and n-3 and n-6 PUFAs can be distinguished by comparing the ratio of ions of 108 and 150 m/z; ion source temperature, 200°C; multiplier voltage, 1182 V; and detector delay, 10 min. For peak identification, the data were obtained by collecting the full-scan mass spectra within the scan range of 50–650 amu, and these peaks were identified by comparing their mass spectra with those in the standard solution and the National Institute of Standards and Technology library. For the quantification of LC-PUFAs, the data were obtained by SIM. Authentic reference compounds were used to calculate the mol percentage of each peak.
Statistical Analysis
BCVA Snellen’s visual acuity values were converted into logMAR for statistical analysis. Descriptive statistics included mean and standard deviation for BCVA, RBC DHA, RBC EPA, and contrast sensitivity. Paired sample t-tests were used to measure differences in mean values of BCVA, RBC DHA, RBC EPA, and contrast sensitivity obtained at baseline and the last follow-up. Statistical analysis was performed using free software (R Foundation for Statistical Computing, version 3.3.3, R Core Team, Vienna, Austria). A P-value less than 0.05 was considered statistically significant. Four subjects did not complete the entire nine years of the study. For these subjects, we used their last study for their end of study comparisons.
Results
Initially, there were five females and six males in the study. The ages ranged from 29 to 59 years at the start of the trial. The ethnicities of the patients were all Caucasian, and all had the previously reported two one-base-pair deletion ELOVL4 mutation.4, 7 Three of the eleven subjects did not follow up after the initial visit and were excluded from further analysis. The other eight subjects returned annually over the initial four-year period for follow-up, but there were a number of missed and late visits. Three subjects began their initial visit during the second year of the study, and one during the third year of the study. Four subjects returned for a close-out visit at eight years.
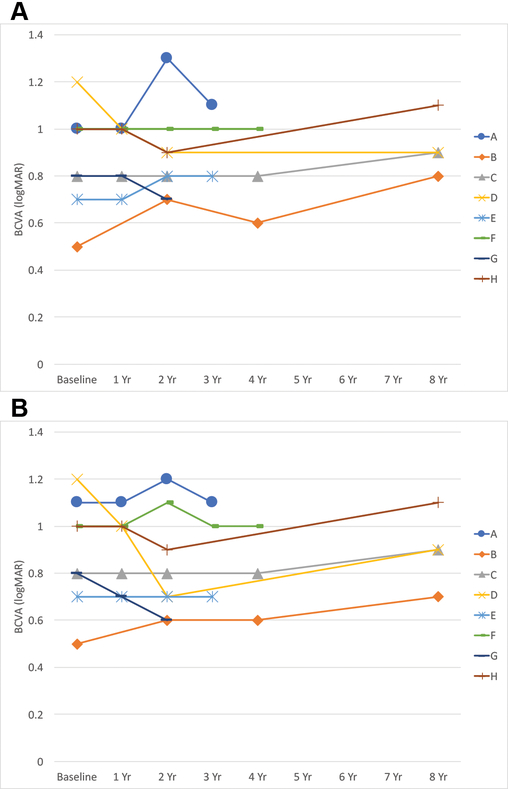
The initial average best-corrected visual acuity (BCVA) was 0.88 ± 0.21 and 0.89 ± 0.23 in the right and left eyes, respectively. The final average BCVA was 0.91 ± 0.15 and 0.88 ± 0.19 in the right and left eyes, respectively (Figure 1). Overall there was little change in BCVA over the duration of the study with P-values of P = 0.57 and P = 0.84, when comparing the mean baseline BCVA to end-of-study BCVA in the right and left eyes, respectively.
Figure 1:
BCVA over time in years (Yr) from baseline in the right (A) and the left (B) eyes.
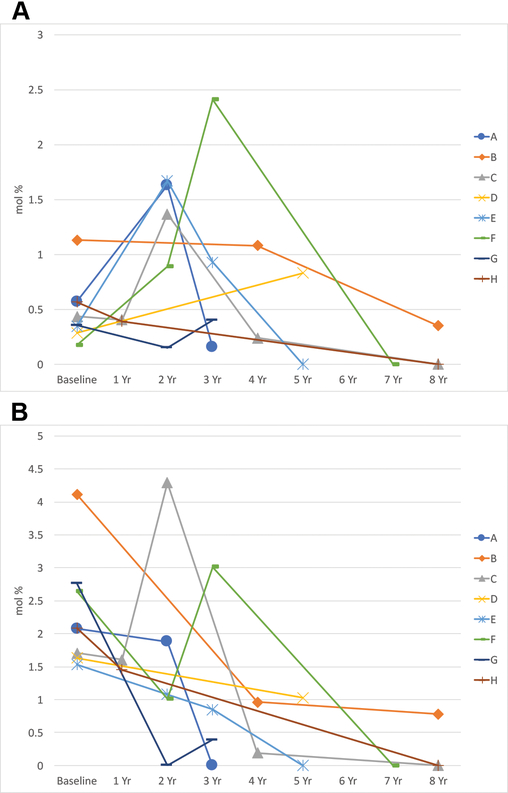
The pattern of serum markers of fish oil ingestion varied dramatically from visit to visit, and we were unable to measure a consistent trend. The average initial RBC EPA was 0.48 ± 0.29 mol %, and the final RBC EPA was 0.27 ± 0.29 mol % (P = 0.16, Figure 2). The average initial RBC DHA was 2.31 ± 0.85 mol %, and the average final DHA was 0.28 ± 0.42 mol % (P=0.0001, Figure 2). This suggests poor compliance with the recommended fish oil supplementation, consistent with subject self-report.
Figure 2:
RBC EPA levels (A), and RBC DHA levels (B) over time in years (Yr).
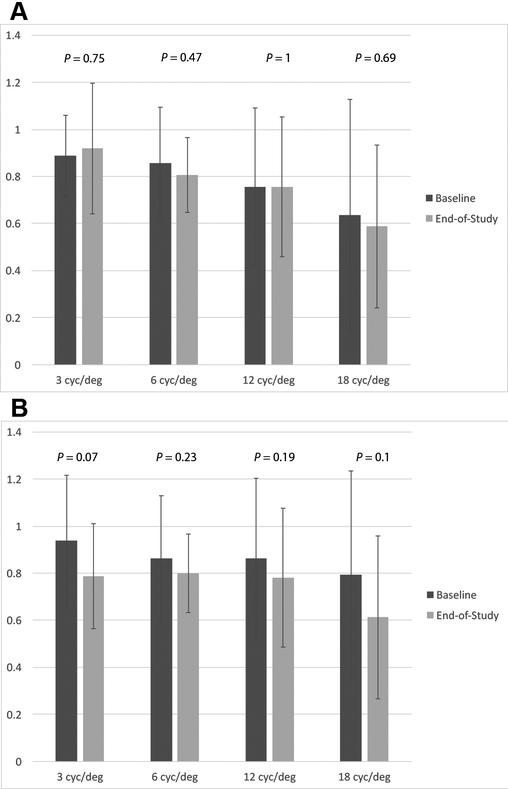
Contrast sensitivity measurements ceased at the 4-year appointment. However, there was no statistically significant difference between the average values at the beginning and end of the study in either eye (Figure 3).
Figure 3:
Mean contrast sensitivity measurements for different frequencies in cycles per degree (cyc/deg) at baseline and end-of-study in the right (A) and left eye (B). P-values are provided above each frequency.
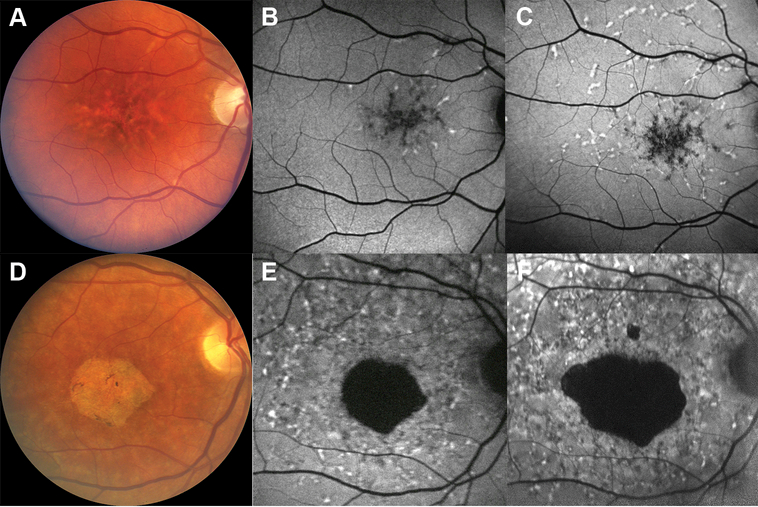
At the inception of the study, fundus examination and autofluorescence revealed phenotypic variation ranging from a pattern-like dystrophy (subject B) to foveal atrophy with flecks (subject F) (Figure 4). Near the end of the study, there was progression of the disease with an increase in the number of whitish macular flecks (8 years from the start of the study, subject B) and size of foveal atrophy (7 years from the start of the study, subject F) as is highlighted on fundus autofluorescence as hyperautofluorescent and hypoautofluorescent regions, respectively (Figure 4). The rate of change of geographic atrophy in subject F over a period of 7 years was 0.591 mm2 per year for the main region of atrophy.
Figure 4:
Color fundus photographs of patients at baseline exhibiting the variation in phenotype from a pattern-like dystrophy (A, subject B) to foveal atrophy (D, subject F), and the corresponding fundus autofluorescence images (B, E). Fundus autofluorescence images showing disease progression over (C, 8 years from baseline; F, 7 years from baseline) compared to baseline (B, E).
Discussion
Stargardt macular dystrophy (STGD) is an appealing target for clinical interventions because its major disease-causing genetic defects are well established, and the biochemical mechanisms of retinal dysfunction are reasonably well understood. 1 Ultimately, the vision typically stabilizes at legal blindness (20/200 best corrected vision in the better eye), although peripheral vision is preserved. Because Stargardt macular dystrophy is a vision threatening disease, discovery of effective interventions will directly improve patient care.
STGD3 is autosomal dominant, which means that one copy of ELOVL4 may still be functional for synthesis of VLC-PUFAs. This suggests that individuals with high intakes of VLC-PUFA precursors could be able to bypass the defect and potentially slow the progression of the macular dystrophy, and an epidemiology study and a case report support this approach. 7, 8 We therefore undertook an open-label interventional study to evaluate whether dietary supplementation with fish oil could alter the course of the disease.
In this investigation, we observed no significant change in BCVA or contrast sensitivity, and serum markers of fish and fish oil ingestion were highly variable. At the beginning of our study, dilated fundus examination and autofluorescence imaging did reveal that all of our patients were in the advanced disease stages. Many of these fundus findings, including macular atrophy and parafoveal whitish flecks are consistent with the results from a recent study using multimodality diagnostic imaging to characterize STGD3 patients. 10 Because of the marginal compliance and irregular follow up, we cannot draw conclusions about fish oil’s impact on the progression of STGD3, but we find that this study provides valuable insights on the long-term progression of a rare ocular disease and insights on the design of future interventions against similar ocular disorders.
Several factors complicated the results of this study. First, STGD3 is a rare disease, and it was difficult to recruit a large population of subjects. Ultimately only eight of eleven patients continued participation in the study beyond the initial visit. This small sample size complicates statistical analysis. Second, compliance was particularly poor in this study. Only four of the eight patients reported regular intake of fish oil supplements, and among the patients who took supplements, the brand, dose, and frequency of intake were also variable. These discrepancies can lead to variations in dosing that can probably account for the wide fluctuations in lipid biomarkers during the study. Over an eight-year period, it was difficult to enforce a consistent change in behavior (taking an oral medication), and because the goal of the study was to slow further of progression of disease, rather than improving vision, many patients who had already suffered advanced vision loss may not have been motivated to follow through with the intervention. In our study, the patients had to purchase their own supply of fish oil; so the financial cost of the medication may have also reduced compliance. Thirdly, follow up study visits were inconsistent. In a trial that lasted eight years, patients only followed up consistently for the first four years and the last year. This left a significant section of time that was not documented. Fourthly, we did not randomize the patients to intervention and non-intervention groups because the family members indicated that they would not join the study if there was a placebo arm. Fifth, some patients in this study already had evidence of advanced macular pathology at the start of the study, leaving little room for slowing of progression. In other words, the disease in these patients may already have been too advanced for meaningful nutritional intervention. Lastly, STGD3 is a slowly progressive disorder, and the trial period may have been insufficient to measure the supplement’s effect.
This study highlights the challenges of conducting a clinical trial on a very rare disease such as STGD3, and we do not intend to repeat the trial under its current protocol. The rarity of the disease, the protracted treatment course, and difficulties in enforcing compliance complicated the execution of this study. STGD3 is inherited in an autosomal dominant pattern, and it is feasible to genotype and identify affected children prior to clinical manifestation of maculopathy. It may therefore be better to study the next generation of patients with STGD3 at a young age, before there is significant macular damage from the disease. Instituting intervention at a young age over a long period would allow the investigators to compare progression between intervention and control groups at an earlier stage of the disease. Furthermore, parents may be more compliant with the intervention if they believe that the intervention could reduce long-term vision loss in their child. Still the challenges of such a study would be daunting. Subjects would need to be followed for decades, and in our experience, there would be strong resistance to participating in a placebo-controlled trial when the study intervention is safe and readily available without a prescription.
In the absence of definitive prospective evidence of fish oil’s benefit for STGD3, what should a clinician recommend to his or her patient with an ELOVL4 mutation? We contend that there is currently sufficient biochemical and epidemiological rationale to support increasing VLC-PUFA precursor intake in all STGD3 patients either by diet or by consumption of fish oil supplements. Such an intervention could be particularly beneficial in young children with STGD3, emphasizing the importance of performing genetic testing for ELOVL4 mutations in children of STGD3 patients soon after birth.
Acknowledgements:
We would like to thank our clinical study coordinators Kelliann Farnsworth and Bonnie Carlstrom who greatly assisted the research.
Funding:
Supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah by a core grant from the National Institutes of Health EY-14800.
Footnotes
Publisher's Disclaimer: Statement of Originality: This work has not been published elsewhere and has not been submitted simultaneously for publication elsewhere.
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Koenekoop RK. The gene for Stargardt disease, ABCA4, is a major retinal gene: a mini-review. Ophthalmic Genet. 2003;24:75–80. [DOI] [PubMed] [Google Scholar]
- 2.Logan S, Anderson RE. Dominant Stargardt Macular Dystrophy (STGD3) and ELOVL4. Adv Exp Med Biol. 2014;801:447–453. [DOI] [PubMed] [Google Scholar]
- 3.Burke TR, Tsang SH. Allelic and phenotypic heterogeneity in ABCA4 mutations. Ophthalmic Genet. 2011;32:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein PS, Tammur J, Singh N et al. Diverse macular dystrophy phenotype caused by a novel complex mutation in the ELOVL4 gene. Invest Ophthalmol Vis Sci. 2001;42:3331–3336. [PubMed] [Google Scholar]
- 5.Zhang K, Kniazeva M, Han M et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27:89–93. [DOI] [PubMed] [Google Scholar]
- 6.Marchette LD, Sherry DM, Brush RS et al. Very long chain polyunsaturated fatty acids and rod cell structure and function. Adv Exp Med Biol. 2014;801:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard AF, Askew EW, Singh N et al. Association of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutation. Arch Ophthalmol. 2006;124:257–263. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald IM, Hébert M, Yau RJ et al. Effect of docosahexaenoic acid supplementation on retinal function in a patient with autosomal dominant Stargardt-like retinal dystrophy. Br J Ophthalmol. 2004;88:305–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu A, Terry R, Lin Y et al. Comprehensive and sensitive quantification of long-chain and very long-chain polyunsaturated fatty acids in small samples of human and mouse retina. J Chromatogr A. 2013;1307:191–200. [DOI] [PubMed] [Google Scholar]
- 10.Palejwala NV, Gale MJ, Clark RF et al. Insights into autosomal dominant Stargardt-like macular dystrophy through multimodality diagnostic imaging. Retina. 2016;36:119–130. [DOI] [PubMed] [Google Scholar]