Abstract
Despite intensive research, the causes of the obesity epidemic remain incompletely understood and conventional calorie-restricted diets continue to lack long-term efficacy. According to the Carbohydrate-Insulin Model (CIM) of obesity, recent increases in the consumption of processed, high-glycemic load carbohydrates produce hormonal changes that promote calorie deposition in adipose tissue, exacerbate hunger and lower energy expenditure. Basic and genetic research provides mechanistic evidence in support of the CIM. In animals, dietary composition has been clearly demonstrated to affect metabolism and body composition, independently of calorie intake, consistent with CIM predictions. Meta-analyses of behavioral trials report greater weight loss with reduced-glycemic load versus low-fat diets, though these studies characteristically suffer from poor long-term compliance. Feeding studies have lacked the rigor and duration to test the CIM, but the longest such studies tend to show metabolic advantages for low-glycemic load vs low-fat diets. Beyond the type and amount of carbohydrate consumed, the CIM provides a conceptual framework for understanding how many dietary and non-dietary exposures might alter hormones, metabolism and adipocyte biology in ways that could predispose to obesity. Pending definitive studies, the principles of a low-glycemic load diet offer a practical alternative to the conventional focus on dietary fat and calorie restriction.
For decades, consideration of “energy balance” has informed efforts to prevent and treat obesity in the clinic and public health arena. Indeed, a recent scientific statement from the Endocrine Society concludes that “the answer to the question, ‘Is a calorie a calorie?’ is ‘yes.’”1 In other words, diets high in added sugar or other processed carbohydrates should have no special adverse effects upon metabolism or body composition, after considering total calorie consumption. However, rates of obesity remain intractably high despite intensive focus on reducing calorie intake (“eat less”) and increasing calorie expenditure (“move more”), with major implications to wellbeing, life-expectancy and health care costs.
A central problem with the Conventional Model of obesity (Figure 1a) is its inability to provide a satisfactory explanation for the obesity epidemic, beyond the difficulty many people have maintaining self-control in the modern environment. With weight loss, hunger predictably increases and energy expenditure declines – physiological adaptations that tend to push body weight back up.2 Why is the average person in the US and Western Europe “defending,” from a biological perspective, a body weight 25 to 30 lb greater today than 50 years ago? An answer to this question may point the way to more effective prevention, with practical implications for clinical treatment.
Explanatory Models of Obesity.
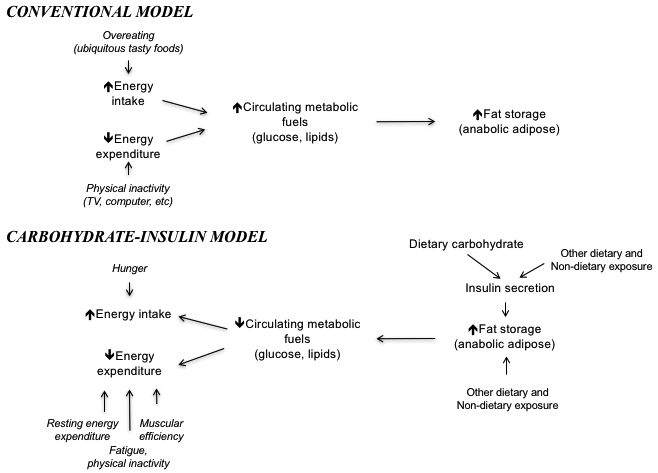
Panel A: Conventional Model; Panel B: Carbohydrate-Insulin Model
The Carbohydrate-Insulin Model
According to an alternative view, changes in dietary quality since the 1970s produce hormonal responses that shift the “partitioning” of calories (metabolic fuels) consumed in a meal toward deposition in fat tissue.3–5 Consequently, fewer calories remain available in the blood stream for use by the rest of the body, driving hunger and overeating. Importantly, this model considers fat cells as central to the etiology of obesity, not passive storage sites of calorie excess.
Although many factors affect fat cells, the hormone insulin exerts dominant anabolic control. Insulin decreases the circulating concentration of all major metabolic fuels by stimulating glucose uptake into tissues, suppressing release of fatty acids from adipose tissue, inhibiting production of ketones in the liver, and promoting fat and glycogen deposition. Consistent with these effects, states of increased insulin action (such as insulin-producing tumors, initiation of insulin treatment of type 2 diabetes or overtreatment of type 1 diabetes) are predictably associated with weight gain. Interestingly, a component of insulin-induced weight gain in diabetes relates to changes in metabolism, not just reduction in calorie loss from glycosuria.6 Conversely, inadequate insulin treatment of type 1 diabetes and drugs that inhibit insulin secretion7 cause weight loss.
Among the many influences on insulin secretion, dietary carbohydrate has the most potent effects, which vary by amount and type. With regard to carbohydrate type, the glycemic index (GI)3 describes how fast specific foods raise blood glucose (and therefore insulin) in the 2 hours after consumption. Most refined grains, potato products and added sugars digest quickly and have a relatively high GI, whereas non-starchy vegetables, legumes, whole fruits and intact whole grains tend to have a moderate or low GI. A related measure, the glycemic load (GL, the multiplicative product of carbohydrate amount and GI) is the best single predictor of postprandial blood glucose levels, explaining up to 90% of the variance.8 Protein, depending upon amino acid composition, stimulates insulin secretion, but this macronutrient also elicits the secretion of glucagon, a catabolic hormone that antagonizes insulin. Dietary fat has little direct effect on insulin, providing a theoretical basis for the efficacy of high-fat diets.
Thus, the Carbohydrate-Insulin Model of obesity (CIM) proposes that a high-carbohydrate diet – including large amounts of refined starchy foods and sugar, as commonly consumed in the low-fat diet era9,10 – produces postprandial hyperinsulinemia, promotes deposition of calories in fat cells instead of oxidation in lean tissues, and thereby predisposes to weight gain through increased hunger, slowing metabolic rate, or both.3–5 Like the Conventional Model, CIM obeys the First Law of Thermodynamics specifying conservation of energy. However, CIM considers overeating a consequence of increasing adiposity, not the primary cause. That is, the causal pathway relating energy balance to fat storage flows opposite to the conventional direction (as depicted in Figure 1b). From this perspective, calorie restriction can be viewed as symptomatic treatment, destined to fail for most people in the modern food environment. Low-calorie/low-fat diets may actually exacerbate the underlying metabolic problem by further restricting energy available in the blood – triggering the starvation response comprised of rising hunger, falling metabolic rate and elevated stress hormone levels.3
Animal research
Insulin injection into the central nervous system produces anorexia and weight loss. However, peripheral insulin administration, a more relevant model of insulin’s whole body actions, typically11 (but not always12) promotes fat deposition, increases hunger and causes weight gain. Even when calorie-restricted to prevent excessive weight gain, insulin-treated animals still developed excessive body fat,13 consistent with a prediction of the CIM regarding fuel partitioning.
Diets that intrinsically raise insulin secretion have metabolic effects similar to insulin injection. Rodents fed high- vs low-GI diets controlled for macronutrients (carbohydrate, fat and protein) manifest progressive abnormalities in this sequence: hyperinsulinemia; increased adipocyte diameter and other anabolic changes; greater adiposity; lower energy expenditure; and finally, increased hunger.14–17 Analogous to the insulin administration studies, calorie restriction to prevent excessive weight gain in animals on a high-GI diet did not prevent excessive adiposity or the associated cardiometabolic risk factors17 – findings for which the Conventional Model has no explanation. Moreover, energy expenditure increased and weight decreased among mice consuming a very-low-carbohydrate vs standard diet, despite no difference in food intake, suggesting the existence of “a unique metabolic state congruous with weight loss.”18
Genetic models
High insulin levels in blood may arise from primary hypersecretion (postulated to cause weight gain) or as a compensatory response to insulin resistance (a mechanism that may protect against weight gain, especially if present in adipose tissue19). Therefore, simple observational studies of fasting insulin and body weight do not provide a meaningful test of the CIM. Genetic studies offer an approach to disentangle cause and effect. In a recent report,20 bi-directional Mendelian Randomization was used to examine the relationship between insulin secretion and BMI, potentially free from confounding by socio-demographic and behavioral factors inherent to most conventional associational analyses. This study found that genetically-determined insulin secretion strongly predicted BMI , whereas genetically-determined BMI did not predict insulin secretion. In addition, variants in the insulin promotor gene associated with insulin hypersecretion in humans predict weight gain during adolescence.21 Furthermore, transgenic mice with reduced insulin secretion had increased energy expenditure and were protected from diet-induced obesity, leading the investigators to conclude, in accordance with the CIM, that “circulating hyperinsulinemia drives diet-induced obesity and its complications.”22
Behavioral trials and observational studies
Contrary to prediction of the Conventional Model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat (and thus low-GL) comparisons.23,24 However, these studies characteristically rely upon dietary counseling, a method with limitations for testing mechanistic hypotheses due to varying levels of non-compliance over the long term. Of note, two major trials that employed special measures to improve compliance, Diogenes25 and the DIRECT trial26 found greater weight loss on low- vs high-GL diets A third major study, DIETFITS,27 reported non-significantly more weight loss on a Healthy Low-Carbohydrate Diet vs Healthy Low-Fat Diet, but both groups were counselled to avoid refined grains, sugar and other processed foods. Consequently, the GL of the Healthy Low-Fat Diet was exceptionally low for a higher-carbohydrate diet – similar to that of the lowest-GL diet in Diogenes.
In large, long-term cohort studies, some high-fat foods with exceptionally high energy density (e.g., nuts, full-fat dairy) have either null or inverse associations with weight gain. In contrast, many commonly-consumed high-GL foods (e.g., potato products, refined grains, sweet desserts, sugary beverages and 100% fruit juice) are directly associated with weight gain.28,29
Feeding studies
According to the CIM, a high-GL meal would limit the availability of metabolic fuels in the late postprandial period (approximately 3 to 5 hour after eating), decrease fat oxidation, lower energy expenditure, stimulate stress hormone secretion and increase voluntary food intake. These effects have been reported in several studies.3,30,31
Over the long-term, increased fat storage may occur with repeated postprandial cycles following high-GL meals. Aiming to test this possibility, a recent meta-analysis reported no meaningful differences between low-fat and low-carbohydrate diets and claimed to have “falsified” the CIM.32,33 However, this analysis of very short studies (most ≤ 2 weeks) suffers from major methodological flaws that preclude a definitive finding. Most importantly, the authors did not account for the physiological processes involved in adaptation to a low-carbohydrate diet over time, confounding transient with chronic effects.
On a conventional high-carbohydrate diet, the brain is critically dependent on glucose, requiring more than 100 g/d. With severe carbohydrate restriction, the body must initially break down protein from lean tissue for conversion into glucose. However, this catabolic response is only temporary because, over time, the concentration of ketones (produced in the liver from fatty acids) increases markedly, replacing glucose as the primary fuel for the brain. For this reason, the hallmark of a very-low-carbohydrate diet (and prolonged fasting) is development of ketosis – giving rise to the term “ketogenic diet.”
Studies of human starvation provide insights into the time course of fat adaptation. As reviewed by Owen et al,34 the total ketone concentration – including ß-hydroxybutyric acid, acetoacetic acid and acetone – rises progressively for 10 days, reaching steady state only after about 3 weeks of fasting. Yang et al35 showed that urinary excretion of ketones also rose throughout 10 days on a very-low-carbohydrate diet, but at a slower rate than during fasting. And Vazquez et al36 showed that nitrogen balance was more negative on a hypocaloric ketogenic diet compared to a non-ketogenic diet for about 3 weeks, then reached a net neutral balance (i.e., no net loss of lean body mass). Thus, the process of fat adaption requires at least 2 to 3 weeks, and perhaps longer. Studies of shorter duration have no bearing on the chronic effects of macronutrients.
Among the 25 unique studies in the meta-analysis of energy expenditure, only 4 had durations of 2.5 weeks or longer. Each of these reported at least a numerical advantage for the low-carbohydrate diet, as described in the Supplement, averaging about 50 kcal/day per 10% decrease in dietary carbohydrate as a proportion of total energy intake.
Criticisms
As with the metabolic studies, other commonly-cited criticisms of the CIM warrant reexamination.
Overeating does cause obesity.
Intentionally increasing calorie consumption will result in weight gain, as dictated by the First Law of Thermodynamics. However, over the long term, the body responds dynamically to overfeeding with increased energy expenditure and decreased hunger – physiological mechanisms (opposite to underfeeding) that resist ongoing weight gain. In the classic overfeeding studies,2,37,38 volunteers reported feeling uncomfortable and had difficulty with compliance. When the protocol ends, body weight spontaneously returns to or near baseline. Research in animals and humans confirms that biological factors limit excessive weight gain, just as they do with weight loss. The CIM argues that a high-GL diet alters these homeostatic mechanisms, shifting defended body weight upward.
Obesity is typically associated with normal or elevated circulating glucose and fatty acid levels.1
Unfortunately, cross-sectional studies after development of obesity may also confound understanding of etiology. The CIM proposes that metabolic fuel concentration is reduced with a high-GL diet in the late postprandial period (approximately 2.5 to 5 hr after eating) due to excessive adipose anabolic activity during the dynamic stage of obesity development.3,31 Eventually, fat cells reach a limit, beyond which they cannot effectively expand storage capacity.39 At this stage, weight gain plateaus (at the cost of increasing insulin resistance and chronic inflammation) and circulating metabolic fuel concentrations consequently rise.
The natural history of hypothalamic obesity – resulting from damage to brain areas controlling food intake and energy expenditure – provides an illustrative example. Following ventromedial hypothalamus lesion in rodents, fat cells are initially insulin sensitive, directing calories to fat storage in the presence of hyperinsulinemia.40 Insulin sensitivity decreases later, with progressive weight gain. This sequence of events shows how static analyses late into disease development can be misleading.
Nevertheless, circulating metabolic fuels provide only an indirect and imperfect measure of cellular metabolism, as demonstrated by the catabolic state characteristic of uncontrolled diabetes despite elevated blood glucose. With newer methods for determination of tissue-specific metabolic activity, a key prediction of the CIM might be directly testable.
Some populations consume a high-carbohydrate diet with low obesity prevalence.
In the US, absolute intakes of protein and fat have not changed since the 1970s, whereas carbohydrate (predominantly high-GL refined grains, potato products and add sugars) intake has increased markedly – resulting in major increases in total calorie consumption and the proportion of calories from carbohydrate.9 As of 2003–2006, the top 3 food sources of energy for US adults were breads and rolls; cakes, cookies, quick bread, pastry and pie; and sugary beverages.10
However, international epidemiological data do not always show such a clear parallel between GL and obesity prevalence. Historically, Asian farming societies remained lean on white rice-based diets, though these populations typically had high levels of physical activity and experienced seasonal limitations in food availability. As physical activity level decreases with urbanization (e.g., China), rates of obesity and diabetes have rapidly risen. In Australia, GL declined moderately since 1995, according to self-reported survey data, despite ongoing increases in obesity prevalence.41 Perhaps there is a threshold above which GL remains sufficiently high to promote ongoing weight gain; or other factors predominant at this stage of the epidemic in some populations, as considered below.
Other considerations
Some heterogeneity within nutrition research is attributable to methodological limitations or other design issues. However, as with many complex traits, biological variability within a population – related to genes, perinatal factors, health status or other exposures – may affect how a specific individual responds to a specific diet. The CIM predicts that people with an intrinsically high insulin response to carbohydrate (assessed as insulin concentration 30 minutes into a standard oral glucose tolerance test) will gain the most weight on a high-GL diet, whereas those with low response may do relatively well on a low-fat diet. This possibility receives support from animal research17, a cohort study42 and several,43,44 but not all,27 clinical trials.
Of course, no one dietary factor can fully explain variations in body weight among individuals and populations; furthermore, many hormones (notably including leptin and ghrelin) and the gut microbiome may affect body composition related to, or independently of, GL. The CIM focuses on high-GL carbohydrates, because these elicit a greater insulin response calorie for calorie than any other category of food. However, as indicated in Figure 1b other aspects of diet (e.g., protein amount and type, fatty acid profile, micronutrients) and non-dietary factors (e.g., sleep, stress, physical activity, environmental endocrine-disrupting chemicals) can affect insulin secretion or adipocyte biology directly. Thus, the CIM offers a comprehensive paradigm, beyond a focus on one macronutrient, to address major drivers of fat accumulation and metabolic dysfunction.
Clinical implications
With failure of conventional low-fat, calorie-restricted diets to stem the obesity epidemic, the CIM provides a practical alternative for public health and clinical medicine. Primary emphasis should be placed on the quality rather than quantity of calories consumed, to shift calorie partitioning away from storage in adipose tissue and improve metabolic fuel availability to the rest of the body. This shift would, according to the CIM, lower the apparent “body weight set point” – the weight at which antagonistic physiological adaptations (including rising hunger and slowing metabolic rate) kick in. In this way, a negative energy balance and weight loss might be achieved with less difficulty and greater sustainability. The Panel provides practical recommendations to achieve a diet based on the CIM, without severe carbohydrate restriction. Most of these line items are broadly consistent with key messages from the recent 2015 USDA Dietary Guidelines, including abandoning prior advice to limit intake of fat.45
Conclusions
A spate of recent reviews claim to refute the CIM,1,32,33,46,47 but these attacks are premised on a misunderstanding of physiological mechanisms, misinterpretation of metabolic studies and disregard for much supportive data. In animals, dietary composition has been shown to affect metabolism and body composition, controlling for calorie intake, in a manner consistent with CIM predictions. Admittedly, the evidence for these effects in humans remains inconclusive.
Limited evidence notwithstanding, the Conventional Model has an implicit conflict with modern research on the biological control of body weight. The rising mean BMI among genetically stable populations suggests that changing environmental factors have altered the physiological systems defending body weight. After all, inexorable weight gain isn’t the inevitable consequence of calorie abundance, as demonstrated by many historical examples (e.g., the US, Western Europe and Japan from the end of World War II until at least the 1970s).
Diets of varying composition, apart from calorie content, have varying effects on hormones, metabolic pathways, gene expression and the gut microbiome in ways that could potentially influence fat storage. By asserting that all calories are alike to the body, the Conventional Model rules out the environmental exposure with the most plausible link to body weight control. What other factors could be responsible for such massive changes in obesity prevalence? The Conventional Model offers no compelling alternatives.
Ultimately, high-quality research will be needed to resolve the debate, which has been ongoing for at least a century.5 In 1941, the renowned obesity expert Julius Bauer described a key component of the CIM (the reverse direction of causality depicted in Figure 1b), writing in this journal: “The current energy theory of obesity, which considers only an imbalance between intake of food and expenditure of energy, is unsatisfactory…. An increased appetite with a subsequent imbalance between intake and output of energy is the consequence of the abnormal anläge [fat tissue] rather than the cause of obesity.”48 In view of the massive and rising toll of obesity-related disease, this research should be given priority.
PANEL
Dietary Recommendations Based on the Carbohydrate-Insulin Model
Reduce refined grains, potato products and added sugars – high-GL carbohydrates with low overall nutritional quality
Emphasize low-GL carbohydrates, including non-starchy vegetables, legumes and non-tropical whole fruits*
When consuming grain products, choose whole kernel or traditionally processed alternatives (e.g., whole barley, quinoa, traditionally fermented sourdough made from stone ground flour†)
Increase nuts, seeds, avocado, olive oil and other healthful high-fat foods
Maintain an adequate, but not high, intake of protein, including from plant sources§
Reduce potential exposure to endocrine-disrupting chemicals (e.g., with use of a water filter and glass rather than plastic containers for food storage, and avoidance of potentially “obesogenic” food additives)
For individuals with severe insulin resistance, metabolic syndrome or type 2 diabetes
Restriction of total carbohydrate intake, and replacement with dietary fat, may provide greatest benefit49
Supplementary Material
KEY POINTS
Question:
Does a diet low in total or processed carbohydrates facilitate weight loss, as proposed in the Carbohydrate-Insulin Model of obesity?
Findings:
In laboratory and animal studies, consumption of processed carbohydrates elicits adverse effects on energy expenditure and body composition, after controlling for calorie intake. Feeding studies and behavioral trials suggest superiority of lower-carbohydrate, higher-fat diets for weight control, but the existing scientific evidence has major limitations.
Meaning:
Reducing consumption of processed carbohydrates may provide metabolic benefits beyond consideration of calorie intake, a possibility that warrants testing in high-quality clinical research.
Acknowledgments
Financial Disclosures: Both authors received grants (to Boston Children’s Hospital) from the National Institutes of Health, Nutrition Science Initiative, the Laura and John Arnold Foundation and other philanthropic organizations unaffiliated with the food industry. Both authors have conducted research studies examining the Carbohydrate-Insulin Model. Dr. Ludwig received royalties for books on obesity and nutrition that recommend a low-glycemic load diet.
Funding/Support: Dr. Ludwig is supported in part by award K24DK082730 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Role of Sponsors: The funders had no role in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Author Contributions:
Concept and design: Both.
Acquisition, analysis, or interpretation of data: Both authors.
Drafting of the manuscript: Ludwig.
Critical revision of the manuscript for important intellectual content: Both authors.
Administrative, technical, or material support: Ludwig.
Additional Contributions: The authors acknowledge Dr. Dariush Mozaffarian for advice on an earlier version of this manuscript. He received no financial compensation.
Tropical fruits (e.g., banana, papaya) have higher GL than temperate fruits (e.g., berries, apple).
Because digestion rate is inversely related to particle size, coarsely milled flour has a lower GI than finely-milled modern industrial flours. Long fermentation reduces rapidly digestible carbohydrate content and produces organic acids, thereby lowering GI.
By eliciting glucagon secretion, protein tends to balance carbohydrate from a metabolic perspective. However, large amounts of protein can also raise insulin secretion. Preliminary evidence suggests plant proteins stimulate less insulin, and may have a lesser anabolic effect, than animal proteins50
References
- 1.Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocrine Reviews. 2017;38:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167–2168. [DOI] [PubMed] [Google Scholar]
- 5.Taubes G The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. BMJ. 2013;346:f1050. [DOI] [PubMed] [Google Scholar]
- 6.Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in IDDM. Diabetes. 1993;42(12):1700–1707. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JB, Arkhammar PO, Bodvarsdottir TB, Wahl P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Curr Med Chem. 2004;11(12):1595–1615. [DOI] [PubMed] [Google Scholar]
- 8.Wolever TM, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996;126(11):2807–2812. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Dietz WH. Trends in energy intake among adults in the United States: findings from NHANES. Am J Clin Nutr. 2013;97(4):848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. 2012;4(12):2097–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cusin I, Rohner-Jeanrenaud F, Terrettaz J, Jeanrenaud B. Hyperinsulinemia and its impact on obesity and insulin resistance. Int J Obes Relat Metab Disord. 1992;16 Suppl 4:S1–11. [PubMed] [Google Scholar]
- 12.VanderWeele DA, Haraczkiewicz E, Van Itallie TB. Elevated insulin and satiety in obese and normal-weight rats. Appetite. 1982;3(2):99–109. [DOI] [PubMed] [Google Scholar]
- 13.Torbay N, Bracco EF, Geliebter A, Stewart IM, Hashim SA. Insulin increases body fat despite control of food intake and physical activity. Am J Physiol. 1985;248(1 Pt 2):R120–124. [DOI] [PubMed] [Google Scholar]
- 14.Kabir M, Rizkalla SW, Champ M, et al. Dietary amylose-amylopectin starch content affects glucose and lipid metabolism in adipocytes of normal and diabetic rats. J Nutr. 1998;128(1):35–43. [DOI] [PubMed] [Google Scholar]
- 15.Kabir M, Rizkalla SW, Quignard-Boulange A, et al. A high glycemic index starch diet affects lipid storage-related enzymes in normal and to a lesser extent in diabetic rats. J Nutr. 1998;128(11):1878–1883. [DOI] [PubMed] [Google Scholar]
- 16.Lerer-Metzger M, Rizkalla SW, Luo J, et al. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. Br J Nutr. 1996;75(5):723–732. [DOI] [PubMed] [Google Scholar]
- 17.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364(9436):778–785. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy AR, Pissios P, Otu H, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292(6):E1724–1739. [DOI] [PubMed] [Google Scholar]
- 19.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. [DOI] [PubMed] [Google Scholar]
- 20.Astley CM, Todd JN, Salem RM, et al. Genetic Evidence That Carbohydrate-Stimulated Insulin Secretion Leads to Obesity Clinical Chemistry, in press 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Stunff C, Fallin D, Schork NJ, Bougneres P. The insulin gene VNTR is associated with fasting insulin levels and development of juvenile obesity. Nat Genet. 2000;26(4):444–446. [DOI] [PubMed] [Google Scholar]
- 22.Mehran AE, Templeman NM, Brigidi GS, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16(6):723–737. [DOI] [PubMed] [Google Scholar]
- 23.Mansoor N, Vinknes KJ, Veierod MB, Retterstol K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. The British journal of nutrition. 2016;115(3):466–479. [DOI] [PubMed] [Google Scholar]
- 24.Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. [DOI] [PubMed] [Google Scholar]
- 27.Gardner CD, Trepanowski JF, Del Gobbo LC, et al. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA. 2018;319(7):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397–1406. [DOI] [PubMed] [Google Scholar]
- 30.Solomon TP, Haus JM, Cook MA, Flask CA, Kirwan JP. A low-glycemic diet lifestyle intervention improves fat utilization during exercise in older obese humans. Obesity (Silver Spring). 2013;21(11):2272–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh CO, Ebbeling CB, Swain JF, Markowitz RL, Feldman HA, Ludwig DS. Effects of diet composition on postprandial energy availability during weight loss maintenance. PLoS One. 2013;8(3):e58172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall KD. A review of the carbohydrate-insulin model of obesity. Eur J Clin Nutr. 2017;71(3):323–326. [DOI] [PubMed] [Google Scholar]
- 33.Hall KD, Guo J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology. 2017;152(7):1718–1727 e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen OE, Caprio S, Reichard GA Jr., Mozzoli MA, Boden G, Owen RS. Ketosis of starvation: a revisit and new perspectives. Clin Endocrinol Metab. 1983;12(2):359–379. [DOI] [PubMed] [Google Scholar]
- 35.Yang MU, Van Itallie TB. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J Clin Invest. 1976;58(3):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992;41(4):406–414. [DOI] [PubMed] [Google Scholar]
- 37.Norgan NG, Durnin JV. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am J Clin Nutr. 1980;33(5):978–988. [DOI] [PubMed] [Google Scholar]
- 38.Sims EA, Goldman RF, Gluck CM, Horton ES, Kelleher PC, Rowe DW. Experimental obesity in man. Trans Assoc Am Physicians. 1968;81:153–170. [PubMed] [Google Scholar]
- 39.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338–349. [DOI] [PubMed] [Google Scholar]
- 40.Penicaud L, Kinebanyan MF, Ferre P, et al. Development of VMH obesity: in vivo insulin secretion and tissue insulin sensitivity. Am J Physiol. 1989;257(2 Pt 1): E255–260. [DOI] [PubMed] [Google Scholar]
- 41.Kusnadi DTL, Barclay AW, Brand-Miller JC, Louie JCY. Changes in dietary glycemic index and glycemic load in Australian adults from 1995 to 2012. Am J Clin Nutr. 2017;106(1):189–198. [DOI] [PubMed] [Google Scholar]
- 42.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87(2):303–309. [DOI] [PubMed] [Google Scholar]
- 43.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. Jama. 2007;297(19):2092–2102. [DOI] [PubMed] [Google Scholar]
- 44.Pittas AG, Das SK, Hajduk CL, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28(12):2939–2941. [DOI] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Ludwig DS. The 2015 US Dietary Guidelines: Lifting the Ban on Total Dietary Fat. Jama. 2015;313(24):2421–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howell S, Kones R. “Calories in, calories out” and macronutrient intake: The Hope, Hype, and Science of Calories. Am J Physiol Endocrinol Metab. 2017:ajpendo 00156 02017. [DOI] [PubMed] [Google Scholar]
- 47.Bosy-Westphal A, Hagele F, Nas A. Impact of dietary glycemic challenge on fuel partitioning. Eur J Clin Nutr. 2017;71(3):327–330. [DOI] [PubMed] [Google Scholar]
- 48.Bauer J Obesity: its pathogenesis, etiology and treatment. Arch Intern Med. 1941;67(5):968–994. [Google Scholar]
- 49.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31(1):1–13. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez A, Hubbard RW. Plasma amino acids and the insulin/glucagon ratio as an explanation for the dietary protein modulation of atherosclerosis. Med Hypotheses. 1991;36(1):27–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


