Key Points
Question
Is there a benefit of tamsulosin to promote ureteral stone expulsion for emergency department patients who present with a symptomatic stone less than 9 mm in diameter?
Findings
In this randomized clinical trial of 512 adults, the proportion achieving ureteral stone expulsion by 28 days was 50% with tamsulosin vs 47% with placebo, a nonsignificant difference.
Meaning
For emergency department patients who present with renal colic owing to ureteral stones smaller than 9 mm, tamsulosin does not appear to promote stone passage.
Abstract
Importance
Urinary stone disease is a common presentation in the emergency department, and α-adrenergic receptor blockers, such as tamsulosin, are commonly used to facilitate stone passage.
Objective
To determine if tamsulosin promotes the passage of urinary stones within 28 days among emergency department patients.
Design, Setting, and Participants
We conducted a double-blind, placebo-controlled clinical trial from 2008 to 2009 (first phase) and then from 2012 to 2016 (second phase). Participants were followed for 90 days. The first phase was conducted at a single US emergency department; the second phase was conducted at 6 US emergency departments. Adult patients were eligible to participate if they presented with a symptomatic urinary stone in the ureter less than 9 mm in diameter, as demonstrated on computed tomography.
Interventions
Participants were randomized to treatment with either tamsulosin, 0.4 mg, or matching placebo daily for 28 days.
Main Outcomes and Measures
The primary outcome was stone passage based on visualization or capture by the study participant by day 28. Secondary outcomes included crossover to open-label tamsulosin, time to stone passage, return to work, use of analgesic medication, hospitalization, surgical intervention, and repeated emergency department visit for urinary stones.
Results
The mean age of 512 participants randomized to tamsulosin or placebo was 40.6 years (range, 18-74 years), 139 (27.1%) were female, and 110 (22.8%) were nonwhite. The mean (SD) diameter of the urinary stones was 3.8 (1.4) mm. Four hundred ninety-seven patients were evaluated for the primary outcome. Stone passage rates were 50% in the tamsulosin group and 47% in the placebo group (relative risk, 1.05; 95.8% CI, 0.87-1.27; P = .60), a nonsignificant difference. None of the secondary outcomes were significantly different. All analyses were performed according to the intention-to-treat principle, although patients lost to follow-up before stone passage were excluded from the analysis of final outcome.
Conclusions and Relevance
Tamsulosin did not significantly increase the stone passage rate compared with placebo. Our findings do not support the use of tamsulosin for symptomatic urinary stones smaller than 9 mm. Guidelines for medical expulsive therapy for urinary stones may need to be revised.
Trial Registration
ClinicalTrials.gov Identifier: NCT00382265
In this randomized clinical trial, participants were randomized to treatment with either tamsulosin, 0.4 mg, or matching placebo daily for 28 days to determine if tamsulosin promotes the passage of urinary stones within 28 days among emergency department patients.
Introduction
Urinary stone disease affects nearly 1 in 11 people over a lifetime in the United States, with estimated annual medical costs of $5 billion.1 The prevalence has nearly doubled over the past 15 years, attributed primarily to the increase in diabetes, obesity, and metabolic syndrome.2,3,4,5 The rate of emergency department visits for urinary stone disease has also nearly doubled over the period 1992 to 2009, from 178 to 340 visits per 100 000 persons.6 Initial treatment of urinary stone disease often occurs in the emergency department and frequently includes administration of α-adrenergic receptor blockers (α-blockers) to promote stone passage, commonly referred to as medical expulsive therapy. The presumed mechanism of action of α-blockers is inhibition of smooth muscle contraction in the ureter, facilitating passage of the stone into the bladder. Current North American and European treatment guidelines support the use of the α-blocker tamsulosin as medical expulsive therapy, with the most recent rigorous systematic review and meta-analysis concluding that its effectiveness is mainly for larger stones, not smaller stones, because the latter have a high likelihood of passage without any intervention.7,8,9,10,11,12,13 However, these guidelines have been called into question by 3 recent large clinical trials.14,15,16 We conducted a multicenter, randomized, double-blind, placebo-controlled clinical trial among emergency department patients with a confirmed symptomatic ureteral stone to determine whether tamsulosin initiated at the time of diagnosis increases the proportion of participants who report passing their stone during a subsequent 28-day treatment period.
Methods
The Study of Tamsulosin for Urolithiasis in the Emergency Department (STONE) was initiated at a single site with 109 participants who were randomized from 2008 to 2009. This first phase allowed an assessment of the feasibility of recruitment and provided the opportunity to determine the rate of stone passage in the placebo group to revise our original estimate of the sample size for the trial. The primary outcome was not analyzed at the end of phase 1. The second phase of the study was conducted from 2013 to 2016 at 6 emergency department recruiting sites, including the original site in phase 1. The data from participants enrolled in both phases were analyzed together. Both protocols were approved by the institutional review boards of the participating institution or institutions. The study was registered at ClinicalTrials.gov (NCT00382265). The safety and progress of each phase was monitored by an independent Data and Safety Monitoring Board (DSMB) established by the sponsor. SAS software (version 9.4; SAS Institute Inc) was used in the data analysis.
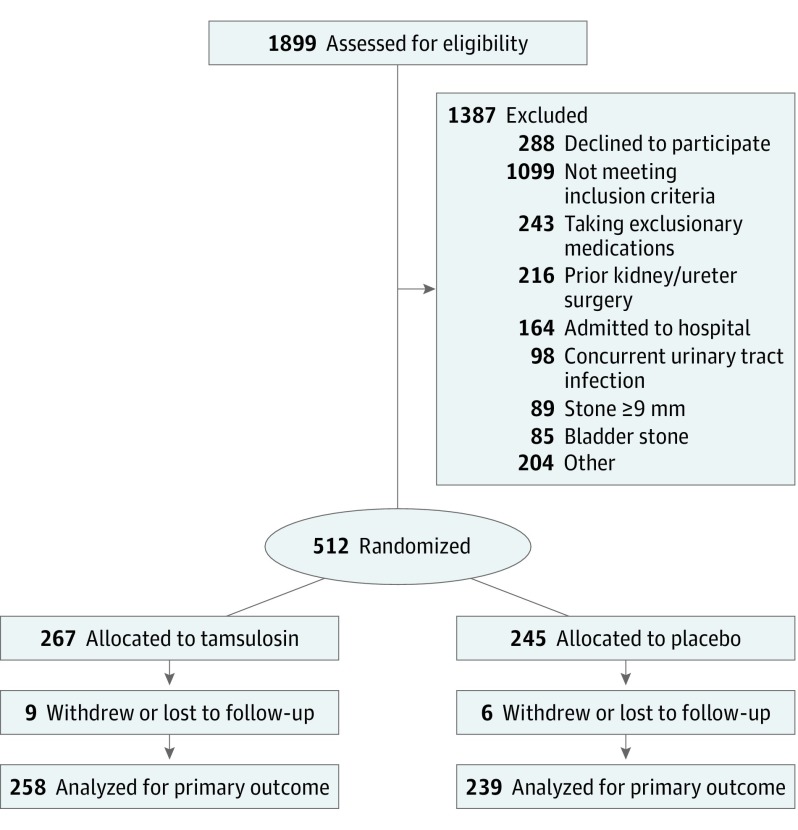
Adults at least 18 years of age were eligible for the study if they presented to the emergency department with a symptomatic urinary stone determined by computed tomography (CT) to be less than 9 mm in diameter and located in the ureter. At each participating emergency department patients with a CT-confirmed ureteral stone were screened for eligibility during study enrollment hours (approximately 60-116 hours per week at each site). The initial CT imaging was ordered as part of normal clinical care for emergency department patients with symptoms suggestive of renal colic. For patients determined to have multiple stones, one ureteral stone had to be identified as the symptomatic stone by the site principal investigator to be eligible. Full eligibility criteria for the study have been previously published in detail. Major reasons for exclusion are summarized in the CONSORT diagram (Figure), and the less common reasons are available in the trial protocol (Supplement).17 Patients provided written informed consent and were compensated for their participation.
Figure. CONSORT Participant Flow Diagram.
Eligible patients were randomized to either tamsulosin at a dose of 0.4 mg daily or a matching placebo in a 1:1 allocation. Both treatments consisted of identically encapsulated pills with identical packaging (1 bottle with 30 capsules per patient). Neither the participant nor the study staff knew to which group the participant was randomized. The randomization sequence was generated using the simple urn method, stratified by site.18 Study participants were contacted by telephone to collect data at 2, 7, 15, 20, 29, and 90 days after randomization. Study participants enrolled in the second phase were also asked to undergo a follow-up CT scan after the 28-day treatment period to determine whether their stone had passed based on this imaging modality. Patients who had already received a second CT outside the study protocol with a confirmed passage of their stone were not asked to return for a follow-up CT.
The primary outcome was passage of a ureteral stone within 28 days after randomization, as determined by the participant’s visualization or physical capture of the stone. Secondary outcomes included an assessment of urinary stone passage, as determined by a follow-up CT scan after 28 days (second phase only); the number of participants who discontinued their assigned study medication and crossed over to open-label tamsulosin; the proportion of participants who returned to work; the rate of surgical procedures (including lithotripsy); the rate of hospitalization; the percentage who returned to the emergency department for ureteral stone–related symptoms; and the duration of pain defined by the time until participants reported stopping use of analgesic medication or the time to stone passage.
Participants who underwent surgery for their symptomatic ureteral stone were considered “treatment failures.” Follow-up CT scans were read by radiologists at participating institutions who were unaware of treatment assignment. An outcome review committee composed of 3 urologists (P.M., S.V.J., and Scott Hubosky, who is listed in the acknowledgments) from recruiting sites who were blinded to the treatment group assessed the passage status in the follow-up CT scan reports. This committee was required to agree unanimously whether the symptomatic stone had passed and created an algorithm for categorizing the results as follows: In cases when stones were either not visualized or there were no stones on the symptomatic side, the participant was considered to have passed the stone. When the symptomatic stone was at the same location or lower in the ureter than the initial symptomatic stone, participants were considered not to have passed their stone. In the 11 cases for which this algorithm did not yield a clear result, the CT scans were individually reviewed and a consensus was reached.
Based on the placebo passage rate from the first phase of the study, the original target sample size of 250 per treatment group was maintained. Since only the passage rate in the placebo group of phase 1 data was obtained, the phase 1 participants were included in the overall sample. This provided a power of at least 90% (with a 2-sided type 1 error of 5%) to detect a 15% absolute increase in the primary outcome, assuming a 45% stone passage rate in the placebo group. Analyses were performed according to the intention-to-treat principle; however, participants lost to follow-up before stone passage status could be ascertained (n = 15) were not included in the analysis of the primary outcome. A group sequential method was used to characterize the rate at which the type 1 error was spent; the chosen spending function was the Lan-DeMets generalization of the O’Brien-Fleming boundary.19 Two interim analyses were performed at the request of the DSMB. As a result, the final critical value for the primary outcome was adjusted, and a 2-tailed P value of less than .04 was considered to indicate statistical significance. For the primary outcome, we thus report a 95.8% CI for the relative risk. The primary outcome analysis was also adjusted for imbalances in baseline characteristics of the participants and recruitment sites using logistic regression.
We compared categorical variables using the χ2 test or Fisher exact test, as appropriate. Continuous variables were compared using the Wilcoxon test. The time to stone passage and time to pain relief were analyzed using Kaplan-Meier survival curves and log-rank tests. For all secondary outcomes, a nominal P value of less than .05 was considered to indicate statistical significance, without adjustment for multiple comparisons. Relative risks and 95% CIs are reported for dichotomous variables.
We also performed preplanned exploratory analyses of subgroups based on age (<40 years vs ≥40 years), sex, self-report of a previous urinary stone, urinary stone size (diameter <5 mm vs 5-8 mm), and urinary stone location in the urinary tract (lower vs upper ureter). In addition, a post hoc exploratory analysis of the subgroups of urinary stone size and location combined (<5 mm proximal, <5 mm distal, ≥5 mm proximal, ≥5 mm distal) was performed. The location of a stone in the lower ureter was defined as one found in either the distal ureter (below the level of the sacroiliac vessels) or the ureterovesical junction; those found in the upper ureter were located either in the renal pelvis, ureteropelvic junction, proximal ureter, or mid-ureter.
Results
We randomized 512 participants overall (Figure): 109 in the first phase and 403 in the second phase. Their mean (SD) age was 40.6 (13.3) years; 139 (27.1%) were female, and 110 (22.8%) were nonwhite (Table 1). Symptomatic stones were most frequently observed either in the ureterovesical junction, 229 (44.7%), or the distal ureter, 126 (24.6%). One hundred ninety-six of the participants (38.3%) had multiple urinary stones detected by CT at the time of enrollment; however, in each case only 1 stone was identified to be the cause of symptoms. The mean (SD) diameter of the symptomatic urinary stone was 3.8 (1.4) mm. The baseline clinical and demographic characteristics of the 2 treatment groups were similar (Table 1) except for age (41.8 [13.6] years in the tamsulosin group and 39.3 years [12.9] years in the placebo group; P = .04).
Table 1. Characteristics of the Enrolled Participants at Study Entry by Treatment Group.
| Characteristic | No. (%) | |
|---|---|---|
| Tamsulosin (n = 267) | Placebo (n = 245) | |
| Age at screening, mean (SD), y | 41.8 (13.6) | 39.3 (12.9) |
| Female sex | 70 (26.2) | 69 (28.2) |
| Nonwhite racea | 56 of 254 (22.0) | 54 of 228 (23.7) |
| Hispanica | 19 of 266 (7.1) | 16 of 243 (6.6) |
| Personal history of kidney stones | 76 (28.5) | 76 (31.0) |
| Family history of kidney stones | 64 (24.0) | 66 (27.0) |
| Presence of flank pain at screening | 224 (83.9) | 206 (84.1) |
| Symptomatic stone location on CT | ||
| Ureteropelvic junction/renal pelvis | 4 (1.5) | 10 (4.1) |
| Proximal ureter | 46 (17.2) | 43 (17.6) |
| Mid-ureter | 32 (12.0) | 19 (7.8) |
| Distal ureter | 71 (26.6) | 55 (22.4) |
| Ureterovesical junction | 114 (42.7) | 115 (46.9) |
| Bladder | 0 (0.0) | 3 (1.2) |
| Symptomatic stone size on CT, mean (SD), mm | 3.8 (1.4) | 3.7 (1.4) |
| Symptomatic stone size distribution on CT, mm | ||
| 1-2 | 47 (17.6) | 47 (19.2) |
| 3-4 | 148 (55.4) | 137 (55.9) |
| 5-6 | 60 (22.5) | 50 (20.4) |
| 7-8 | 12 (4.5) | 11 (4.5) |
| Hydronephrosis on CT | 202 (75.7) | 181 (73.9) |
| Evidence of multiple stones on CT | 92 (34.5) | 104 (42.4) |
Abbreviation: CT, computed tomography.
Self-reported data; not all participants provided data.
At the end of the 28-day treatment period, the urinary stone passage rate of 49.6% among participants assigned to tamsulosin did not differ significantly from the placebo passage rate of 47.3% (relative risk, 1.05; 95.8% CI, 0.87-1.27; P = .60) (Table 2). After adjusting for age and recruiting site, the difference between the urinary stone passage rates remained nonsignificant (relative risk, 1.08; 95% CI, 0.92-1.26; P = .36). The rates of treatment-related adverse effects were similar between treatment groups for the 399 participants in phase 2 who had any contact after randomization, with the exception of increased ejaculatory dysfunction among men in the tamsulosin group (Table 3). No serious adverse events were reported. In the subset of participants evaluated by CT scan at 28 days who were enrolled in phase 2 (n = 238), the rate of ureteral stone passage was 83.6% in the tamsulosin group and 77.6% in the placebo group (relative risk, 1.08; 95% CI, 0.95-1.22; P = .24) (Table 2). Although there was a 6.0% difference in rates, this was not statistically significant. Similarly, there was no difference between treatment groups for any of the secondary outcomes, including the number of participants who crossed over to open-label tamsulosin, the proportion of participants who returned to work within 28 days of enrollment, rate of surgery, rate of hospitalization, or return to the emergency department for urinary stones (Table 2). The time to urinary stone passage and the length of time the patient required analgesia were also not significantly different between the treatment groups (P = .92 and P = .17, respectively). Self-reported adherence to study medication was 82.9% and 72.9% at days 15 and 29 of follow-up, respectively.
Table 2. Primary and Secondary Outcomes by Treatment Group Through 28 Daysa.
| Outcome | No./Total No. (%) | Relative Risk (95% CI) | P Value | |
|---|---|---|---|---|
| Tamsulosin | Placebo | |||
| Primary outcome | ||||
| Stone passed by patient report | 128/258 (49.6) | 113/239 (47.3) | 1.05 (0.87-1.27)b | .60 |
| Secondary outcomes | ||||
| Stone passed on follow-up computed tomographyc | 102/122 (83.6) | 90/116 (77.6) | 1.08 (0.95-1.22) | .24 |
| Crossover to open label tamsulosinc | 15/214 (7.0) | 14/189 (7.4) | 0.95 (0.47-1.91) | .88 |
| Surgery for urinary stoned | 14/214 (6.5) | 13/189 (6.9) | 0.95 (0.46-1.97) | .89 |
| Hospitalization(s) due to stone | 2/226 (0.9) | 1/210 (0.5) | 1.88 (0.17-20.34) | >.99e |
| Return to work (if employed) | 202/204 (99.0) | 185/188 (98.2) | 1.00 (0.98-1.03) | .67e |
| Return emergency department visit(s) due to stone | 5/226 (2.2) | 5/210 (2.4) | 0.93 (0.27-3.16) | >.99e |
Data presented as n/N (%) or median (interquartile range).
95.8% CI adjusted for age and clinical center.
Phase 2 participants with follow-up computed tomographic scans.
Phase 2 only.
Fisher exact test.
Table 3. Adverse Effects by Treatment Assignment by 28 Days in Phase 2 Participantsa.
| Adverse Effect | No. (%) | |
|---|---|---|
| Tamsulosin (n = 212) | Placebo (n = 187) | |
| Stomach upset, nausea, or vomiting | 50 (23.6) | 46 (24.6) |
| Headache | 51 (24.1) | 43 (23.0) |
| Abdominal pain or stomach ulcer | 31 (14.6) | 36 (19.3) |
| Dizziness when standing up | 25 (11.8) | 18 (9.6) |
| Dizziness at rest | 25 (11.8) | 16 (8.6) |
| Facial flushing | 5 (2.4) | 5 (2.7) |
| Tachycardia or fast heart rate | 4 (1.9) | 7 (3.7) |
| Urinary tract infection(s) | 3 (1.4) | 4 (2.1) |
| Bloody and/or black stool or bloody vomiting | 3 (1.4) | 3 (1.6) |
| Abnormalities of ejaculation (males only)b | 28 of 154 (18.2) | 10 of 135 (7.4) |
For phase 2 participants with any contact after randomization.
P = .007.
In the preplanned exploratory subgroup analyses of the primary outcome, there was no significant interaction between treatment and the following subgroups (Table 4): sex (males, 53.4% in the tamsulosin group vs 51.5% in the placebo group; P = .93), age (18-39 years; 54.1% in the tamsulosin group vs 48.8% in the placebo group; P = .57), history of previous urinary stone (99.0%; 48.7% in the tamsulosin group vs 52.6% in the placebo group; P = .35), urinary stone size (<5 mm; 56.4% in the tamsulosin group vs 51.7% in the placebo group; P = .45), and urinary stone location (upper ureter, 41.8% in the tamsulosin group vs 29.4% in the placebo group; P = .17) (99.0% in the tamsulosin group vs 98.2% in the placebo group; P = .67). In a post hoc analysis combining urinary stone size and location, no significant interaction with treatment was found, although there was low power for all the statistical tests of interaction. Surgery rates were 6.5% in the tamsulosin group vs 6.9% in the placebo group (P = .89). The hospitalization rate was 0.9% in the tamsulosin group vs 0.5% in the placebo group (P > .99). Emergency room use rates were 2.2% in the tamsulosin group vs 2.4% in the placebo group (P > .99).
Table 4. Subgroup Analyses for Primary Outcome of Passage Rate.
| Characteristic | No. (%) | P Valuea | |
|---|---|---|---|
| Tamsulosin (n = 267) | Placebo (n = 245) | ||
| Sex | .93 | ||
| Male | 102 (53.4) | 89 (51.5) | |
| Female | 27 (39.1) | 24 (36.4) | |
| Age, y | .57 | ||
| 18-39 | 66 (54.1) | 63 (48.8) | |
| ≥40 | 62 (45.6) | 50 (45.4) | |
| History of kidney stones | .35 | ||
| Any prior episodes | 36 (48.7) | 40 (52.6) | |
| No prior episodes | 92 (50.0) | 73 (44.8) | |
| Size of symptomatic stone, mm | .45 | ||
| 0 to <5 | 106 (56.4) | 92 (51.7) | |
| ≥5 | 22 (31.4) | 21 (34.4) | |
| Location of symptomatic stone | .17 | ||
| Upper ureter | 33 (41.8) | 20 (29.4) | |
| Lower ureter | 95 (53.1) | 90 (53.6) | |
Breslow-Day test for homogeneity of odds ratio.
Discussion
The prevalence of urinary stone disease has been steadily increasing in the United States over the past several decades, and many patients present to the emergency department for initial treatment.1,20 A treatment that promotes the passage of urinary stones without the need for surgery could reduce both patient morbidity and health care costs associated with this condition. Prior published meta-analyses, as well as the current American Urological Association Guidelines on Surgical Management of Stones, provide a strong recommendation that patients with ureteral stones 10 mm or less in diameter be offered α-blockers to promote stone passage.8,21 The most recent systematic review and meta-analysis suggest that the benefit of tamsulosin may be limited to larger stones in the ureter.10,22
Use of medical expulsive therapy for urinary stone disease in the setting of the emergency department is common, varying between about 15% and 55%.23,24,25 Recent clinical trials of medical expulsive therapy for urinary stones with different primary outcomes have found no benefit of drug treatment (the α-blocker tamsulosin alone in one trial, and tamsulosin and the calcium channel blocker nifedipine in the other) compared with placebo.13,14,15 These findings have called into question current treatment guidelines, which were based primarily on evidence from meta-analyses of clinical trials of variable reporting quality.23,26 Meta-analyses have inherent limitations, which may explain the discrepant conclusions from recent clinical trials. In a recent meta-analysis, which concluded that there was a benefit of α-blockers as medical expulsive therapy for urinary stone disease, a large number of randomized clinical trials (n = 55) were considered. Only one-fourth of these trials were placebo-controlled, however; few (5%) had a low risk of bias, and most (95%) were small (<100 participants per arm).10 One possible strength of meta-analyses is to increase the sample size and statistical power of subgroup analyses. When only studies such as placebo-controlled trials and other trials of higher methodological quality are considered, meta-analysis has demonstrated a significant but more attenuated effect size for medical expulsive therapy for urinary stone disease.27
Our study, the largest clinical trial of medical expulsive therapy in the United States to our knowledge, found no difference in the overall 28-day urinary stone passage rate between participants who were treated with tamsulosin and those who received placebo. We also observed no benefit of drug treatment for any of our secondary outcomes, including the duration of use of analgesic medication, the time to return to work, and the need for surgery.
Our findings agree with those of 2 recent large multisite clinical trials conducted in the United Kingdom15 and Australia14 but are at odds with findings of one conducted in China. In a pragmatic clinical trial conducted in the United Kingdom, Spontaneous Urinary Stone Passage Enabled by Drugs (SUSPEND), 1167 patients with ureteral stones less than 10 mm in diameter were randomized to treatment with either tamsulosin, placebo, or the calcium channel blocker nifedipine.15 No difference was detected between the 3 treatment groups in the proportion of participants who required further treatment to achieve urinary stone passage by 4 weeks. In the Australian study, 403 patients with distal ureteral stones 10 mm or less in diameter were enrolled in 5 emergency departments and randomized to either tamsulosin or placebo.14 No difference was detected between treatment groups in the overall rate of urinary stone passage after 28 days of therapy. Although the designs of our study and those conducted in the United Kingdom and Australia differ somewhat, in each study tamsulosin did not result in a significantly higher rate of overall urinary stone passage compared with placebo. Thus, the evidence from these 2 clinical trials and our study indicates that this drug is not useful as medical expulsive therapy for urinary stones frequently encountered in the emergency department. In contrast, a recent large clinical trial (n = 3450 participants) conducted in China demonstrated a higher overall rate of stone passage among persons treated with tamsulosin over a 28-day period compared with placebo (86% vs 79%, respectively; P < .001).16 This trial, however, included only participants with stones 4 to 7 mm in diameter that were located in the lower ureter, as confirmed by plain radiography, ultrasonography, or CT scan.
Although both SUSPEND and the Australian study failed to show an overall benefit of tamsulosin to promote stone passage, subgroup analyses suggested a higher passage rate of stones with a mean diameter greater than 5 mm and those located in the distal ureter or ureterovesical junction.14,15 In the Chinese trial, the overall benefit of tamsulosin was the result of a significantly higher passage rate observed among the subgroup of persons with larger stones (>5 mm in diameter); there was no beneficial effect among persons with smaller stones (≤5 mm).16 Our study was not designed to detect a treatment effect of tamsulosin in subgroups based on stone size. However, we found no significant interaction based on stone size and location, alone or in combination, in both preplanned and post hoc exploratory analyses.
An important shortcoming of the SUSPEND trial was the absence of a CT scan to confirm stone passage. We sought to address this concern by performing a CT scan at the end of the 28-day treatment period. The follow-up CT measure was added at the start of phase 2. Overall, 59.1% of those participants received a follow-up CT scan, and the passage rate based on CT scan was not significantly different between treatment groups. The use of tamsulosin as medical expulsive therapy over a 28-day period resulted in similar rates of treatment-related adverse effects in the 2 treatment groups, with the exception of higher rates of abnormal ejaculation in male participants in the tamsulosin group. These findings are consistent with the treatment-related adverse effects profile of this drug from prior clinical trials of tamsulosin for benign prostatic hyperplasia associated with lower urinary tract symptoms.28
Strengths and Limitations
Our study has several strengths. First, we recruited from emergency departments, where many patients first present for treatment for symptomatic urinary stones. Second, overall we had a diverse sample with respect to race (110 of 482 [22.8%] were nonwhite) and ethnicity (35 of 509 [6.9%] were Hispanic), making our results more generalizable. Third, we achieved a high rate of ascertainment of the primary outcome, having contacted 97.1% of study participants to ask whether they had visualized or captured a stone by 28 days. In addition, we observed a high rate of adherence to the study medication. These features are notable because acceptable participation in a clinical trial is often challenging when enrolling in the emergency department and obtaining follow-up information by telephone.17 Fourth, we assessed a broad range of secondary outcomes to adequately characterize the potential beneficial effects of treatment. Fifth, we obtained a follow-up CT scan in most of our phase 2 participants as a secondary, more definitive measure of stone passage. Finally, unlike prior clinical trials of medical expulsive therapy, which have generally limited enrollment to participants with distal ureteral stones, we included all patients who had stones in any part of the ureter to increase the generalizability of our study.
Our findings should be considered with a number of the study’s design features in mind. Study participants were required to have a urinary stone confirmed by CT scan prior to randomization. This eligibility requirement allowed us to make the most accurate determination of the size and location of each participant’s stone or stones. This requirement differs from current clinical practice, in which not every patient presenting to the emergency department with a suspected ureteral stone receives a CT scan.6 Consequently, we may have preferentially enrolled persons in whom the diagnosis of a symptomatic urinary stone was more severe or less certain by clinical assessment. In addition, we may have been more likely to enroll persons in whom radiation exposure was of less concern—for example, older patients, men, or patients who had not had a prior CT scan.23 Other limitations of the study include the potential lack of generalizability of our findings to patients seen outside of a US tertiary care center that allows for easy access to urologists and high rates of surgery for larger stones. In addition, a high proportion of our participants had stones smaller than 5 mm in diameter.
Conclusions
We found that compared with placebo, 28-day treatment with tamsulosin did not increase the overall stone passage rate or improve a wide range of secondary outcomes in patients who presented to the emergency department with symptomatic ureteral stones less than 9 mm in diameter. In addition, an exploratory analysis did not suggest a beneficial effect based on stone location, size, or the combination. Although tamsulosin may still play a role in medical expulsive therapy for larger stones, guidelines that recommend tamsulosin for ureteral stones may need to be revised.
Study Protocol
References
- 1.Litwin M, Saigal C. Urologic Diseases in America. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. NIH Pub 12-7865. [Google Scholar]
- 2.Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project . Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasian GE, Kabarriti AE, Kalmus A, Furth SL. Kidney stone recurrence among children and adolescents. J Urol. 2017;197(1):246-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. 2014;66(4):724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong IG, Kang T, Bang JK, et al. . Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis. 2011;58(3):383-388. [DOI] [PubMed] [Google Scholar]
- 6.Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits, use of imaging, and drugs for urolithiasis have increased in the United States. Kidney Int. 2013;83(3):479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preminger GM, Tiselius HG, Assimos DG, et al. ; EAU/AUA Nephrolithiasis Guideline Panel . 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418-2434. [DOI] [PubMed] [Google Scholar]
- 8.Assimos D, Krambeck A, Miller NL, et al. . Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. J Urol. 2016;196(4):1153-1160. [DOI] [PubMed] [Google Scholar]
- 9.Hollingsworth JM, Rogers MA, Kaufman SR, et al. . Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet. 2006;368(9542):1171-1179. [DOI] [PubMed] [Google Scholar]
- 10.Hollingsworth JM, Canales BK, Rogers MA, et al. . Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4(4):CD008509. [DOI] [PubMed] [Google Scholar]
- 12.Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U. Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol. 2009;56(3):455-471. [DOI] [PubMed] [Google Scholar]
- 13.Hermanns T, Sauermann P, Rufibach K, Frauenfelder T, Sulser T, Strebel RT. Is there a role for tamsulosin in the treatment of distal ureteral stones of 7 mm or less? results of a randomised, double-blind, placebo-controlled trial. Eur Urol. 2009;56(3):407-412. [DOI] [PubMed] [Google Scholar]
- 14.Furyk JS, Chu K, Banks C, et al. . Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial. Ann Emerg Med. 2016;67(1):86-95.e2. [DOI] [PubMed] [Google Scholar]
- 15.Pickard R, Starr K, MacLennan G, et al. . Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet. 2015;386(9991):341-349. [DOI] [PubMed] [Google Scholar]
- 16.Ye Z, Zeng G, Yang H, et al. . Efficacy and safety of tamsulosin in medical expulsive therapy for distal ureteral stones with renal colic: a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2017;38(17):30972-30977. [DOI] [PubMed] [Google Scholar]
- 17.Burrows PK, Hollander JE, Wolfson AB, et al. ; STONE Study Investigators . Design and challenges of a randomized clinical trial of medical expulsive therapy (tamsulosin) for urolithiasis in the emergency department. Contemp Clin Trials. 2017;52:91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9(4):345-364. [DOI] [PubMed] [Google Scholar]
- 19.Gordon Lan KK, Demets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659-663. [Google Scholar]
- 20.Scales CD Jr, Lin L, Saigal CS, et al. ; NIDDK Urologic Diseases in America Project . Emergency department revisits for patients with kidney stones in California. Acad Emerg Med. 2015;22(4):468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Türk C, Petřík A, Sarica K, et al. . EAU Guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474. [DOI] [PubMed] [Google Scholar]
- 22.Wang RC, Smith-Bindman R, Whitaker E, et al. . Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.e3. [DOI] [PubMed] [Google Scholar]
- 23.Wang RC. Managing urolithiasis. Ann Emerg Med. 2016;67(4):449-454. [DOI] [PubMed] [Google Scholar]
- 24.Scales CD Jr, Bergman J, Carter S, Jack G, Saigal CS, Litwin MS; NIDDK Urologic Diseases in America Project . Quality of acute care for patients with urinary stones in the United States. Urology. 2015;86(5):914-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesan V, Loftus CJ, Hinck B, et al. . Clinical predictors of 30-day emergency department revisits for patients with ureteral stones. J Urol. 2016;196(5):1467-1470. [DOI] [PubMed] [Google Scholar]
- 26.Skolarikos A, Ghani KR, Seitz C, Van Asseldonk B, Bultitude MF. Medical expulsive therapy in urolithiasis: a review of the quality of the current evidence. Eur Urol Focus. 2017;3(1):27-45. [DOI] [PubMed] [Google Scholar]
- 27.Wang RC, Addo N, Chi T, et al. . Medical expulsive therapy use in emergency department patients diagnosed with ureteral stones. Am J Emerg Med. 2017;35(8):1069-1074. [DOI] [PubMed] [Google Scholar]
- 28.Yu YD, Kang MH, Choi CI, Shin HS, Oh JJ, Park DS. Clinical efficacy of combination therapy with an alpha blocker and low-dose sildenafil on post-therapy lower urinary tract symptoms after low-dose-rate brachytherapy for prostate cancer. World J Urol. 2016;34(9):1269-1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol