Abstract
Variable lymphocyte receptors (VLRs) are leucine-rich repeat proteins in jawless vertebrates that function similarly to Ig antibodies. However, VLRs possess a distinct crescent-shaped structure and modularity that results in a concave binding interface that contrasts significantly with Ig antibodies. Antigen binding interactions result in specific, high affinity VLR binding interactions with both proteins and glycans. The natural sourcing of VLRs allows for immunization strategies, while the modularity enables a whole host of protein engineering approaches including consensus scaffolds, designed libraries and directed evolution with display technologies. VLR technologies have been recently deployed for applications in cell-specific targeting, drug delivery, tumor diagnostics and even protein stabilization. It is anticipated that the VLR field will continue to emerge to provide unique solutions for targeting glycans, evolutionarily conserved proteins and cellular specificity.
Visual Abstract
Introduction
Antibodies represent a rapidly growing therapeutic class given that their high affinity selective binding can provide neutralizing or cytotoxic therapy for many diseases. However, the antibody field has recognized that certain applications could benefit from binding scaffolds that have enhanced properties such as tissue penetration, stability or production. As such, alternative binding scaffolds have been developed, including DARPINS, affibodies, adnectins, anticalins, and cysteine knot proteins, among others [1–6]. This review will focus on a relatively new antibody alternative based on a class of natural antigen binders found in jawless vertebrates known as variable lymphocyte receptors (VLRs) and describe recent engineering and therapeutic applications.
VLR biology and structure
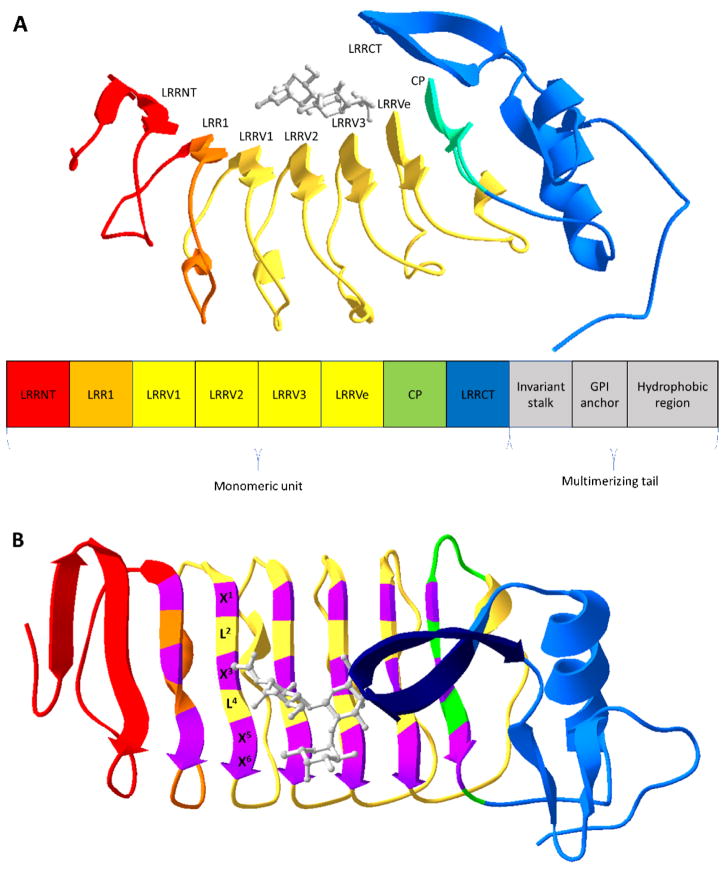
VLRs are part of the adaptive immune system of jawless vertebrates. Early evidence indicated that lamprey and hagfish had an adaptive immune system; and while they had cells that resemble mammalian lymphocytes, no immunoglobulin, T cell receptor, or major histocompatibility complex genes could be identified [7–9]. Instead, Pancer and colleagues identified VLRs as the antigen receptors in activated lamprey lymphocytes [10]. In contrast to the mammalian immunoglobulin fold, VLRs are leucine rich repeat (LRR) proteins that generate diversity by a combination of differential repeat number and sequence variability [10,11] (Figure 1). In a bit more detail, three different VLR genes, VLRA, VLRB and VLRC have been identified in jawless vertebrates, with VLRA and VLRC being expressed in lymphocytes that resemble T cells and VLRB expressed in lymphocytes resembling B cells [12,13]. Since VLRB is expressed in a soluble format by B cell-like lymphocytes which respond to an adaptive challenge, VLRB is the most widely used form for VLR applications. VLR diversity is generated by gene conversion-type mechanisms [10,11] which yield VLRs comprising an N-terminal cap (LRRNT), the first LRR (LRR1), up to seven variable LRR (LRRVs), an end LRR (LRRVe), a connecting peptide (CP), a C-terminal cap (LRRCT), an invariant stalk, a glycosylphosphatidylinositol (GPI) anchor, and a cysteine rich hydrophobic region that can drive VLR multimerization [14,15] (Figure 1A). These modules combine to form a crescent-shaped solenoid structure with a concave surface consisting of parallel β-sheets which forms the antigen binding site [16,17] (Figure 1). Within the antigen binding site, there exist highly variable residues with each interfacial LRRV β-sheet having 6 residues of the general form X1LX3LX5X6 [18,19]. The leucine residues (L) form the hydrophobic core and the variable amino acids (X) generate the unique antigen binding interface (Figure 1B). Soluble VLRs also multimerize by forming disulfide bonds via a cysteine-rich hydrophobic region [14], but VLRs can also be used in monomeric forms for biotechnology purposes by expression without the hydrophobic region. Thus, VLRs represent an alternative antigen receptor, with many parallels to mammalian antibodies, that can combine with antibody engineering platforms to address both traditional and unique challenges.
Figure 1.
Overview of VLR structure. (A) Ribbon structure of a monomeric VLR in complex with an H-trisaccharide (gray) (PDB code 326J [16]). Below the ribbon structure is a modular rendition of a full-length VLR consisting an N-terminal cap (LRRNT), the first leucine-rich repeat (LRR) module (LRR1), three variable LRR (LRRVs), an end variable LRR (LRRVe), a connecting peptide (CP), a C-terminal cap (LRRCT), an invariant stalk, a glycosylphosphatidylinositol (GPI) anchor, and a hydrophobic region. (B) The VLR structure is rotated 90° with highly variable residues of the 6-residue motif (X1LX3LX5X6) in purple and the variable sized insert of the LRRCT in dark blue. X represents highly variable amino acids and L, leucine.
Design and selection of VLR binders
For selection of VLRs that bind the antigen of interest, nonimmune, immune or designed libraries can be employed. Since VLRs are natural antigen binders, they have evolved to have a potential diversity of 1014 [15]; and thus, the nonimmune VLR repertoire can be used to identify specific antigen binders [19,20]. Another option is lamprey or hagfish immunization with a desired antigen to produce a diverse immune library. As a result of substantial evolutionary distance from mammals, VLRs may be advantageous for recognition of conserved mammalian epitopes or glycans that may be troublesome for Ig-based systems because of tolerance mechanisms, particularly those relying on immunization techniques. Particulates and cells work well for lamprey or hagfish immunizations; however, soluble proteins often require an adjuvant or be displayed on a cell surface to elicit a robust immune response [12,21]. Using these strategies, immunized libraries have also been used successfully to identify antigen binders [12,14,21,22]. Typically, the VLRB cDNA which encodes for the soluble form of VLRs is recovered from the lymphocytes and cloned into a surface-display platform for screening. To date, most studies have employed the monomeric VLR form spanning from LRRNT to the LRRCT (Figure 1). After VLR identification, the multimerizing tail can be reintroduced to increase the avidity for lower affinity clones.
Consensus VLR scaffolds represent another emerging option for deploying VLR technology. Scaffolds known as dVLR and repebody represent two examples that are based on a VLR consensus sequence. First, a designed VLR (dVLR) was developed by choosing the most frequently encountered amino acid at each position of aligned lamprey VLRB sequences, and the dVLR was designed with just 1 LRRV module since the average usage in lamprey VLRBs is 1.3 LRRVs [23,24]. This scaffold was produced solubly in bacteria and showed excellent stability over a broad range of pH range temperature. A combinatorial library was produced by randomizing 11 highly variable residues in the contact interface of LRR1, LRRV and LRRVe and screened to identify VLRs capable of binding lysozyme and S100A7 protein [24]. The repebody is another consensus VLR-based scaffold comprising 5 consensus LRRV modules, based on the number of LRRVs in known antigen binding VLRs, and with an internalin B cap replacing the LRRNT module for better expression in bacteria [25]. After randomizing three variable residues in both LRRV1 and LRRV2, libraries were screened to identify repebodies that bind to IL-6 [25], among other targets, and as discussed in more detail below. Finally, recent computational design strategies employing Rosetta techniques have identified VLRs similar to those that are naturally occurring in addition to the designed VLRs [26]. Importantly, due to the modular nature of VLRs, the number of LRRVs can be altered, as exemplified by the two consensus VLR scaffolds, to accommodate antigens of different chemistry and sizes. Once a VLR library is created, it can be screened using a surface display platform including phage [24,25,27], and yeast display [20,21,28]. Soluble VLRs have been expressed in bacteria, mammalian cells, and even plants for further evaluation. Then, specific VLRs can undergo random or targeted mutagenesis of the concave surface to improve affinity. For example, the IL-6 repebody was subjected to affinity maturation by mutagenesis of the LRRV variable positions to yield a 63 pM binder [29].
Antigens successfully targeted by VLRs
Once VLR libraries are in hand, they can be screened against a wide variety of antigens. Like antibodies, VLRs can bind protein antigens with high affinity and specificity. Antigenic proteins have been sourced from mammalian cells (hen egg lysozyme [18] and CD38 on plasma cells [30]), bacteria (spore surface protein BclA [14] and plant pathogen protein HopM1 [28]), and viruses (avian influenza virus hemagglutinin [22]). An example of the high specificity that can be achieved with VLRs can be found in the identification of a panel of VLRs that recognized BclA on Bacillus anthracis spores but not on Bacillus cereus despite 89.5% sequence identity [14]. VLRs have also been proven adept at binding glycans such as Thomsen-Friedenreich antigen and H-trisaccharide, with high specificity and affinity [12,20]. Since jawless vertebrates elicit a strong VLR response to particulates, they can be used to identify cell-surface biomarkers. As an example, it has been difficult to identify antibodies targeting specific surface markers on plasma cells (PCs), likely a result of mammalian immune tolerance. Using the VLR repertoire from a bone marrow aspirate-immunized lamprey and clonal evaluation, a VLR targeting CD38 was identified that exclusively bound PCs [30].
Antigen recognition by VLRs
Even at this somewhat early stage in VLR applications, VLRs have been identified against a reasonable array of antigens including glycans. The broad applicability of VLR approaches could be in part due to the mode of VLR binding, which has recently been partially elucidated using the crystal structures of VLRs in complex with antigens [14,16,18,19,31,32]. Based on the available structural analyses, it appears that large antigens like proteins contact all three “ridges” formed by the variable residues in the concave interface [18,19] (Figure 1B). Interestingly, smaller antigens like glycans contact the bottom two ridges of the interface [19]. In addition to the LRRVs, a variable-sized insert in the LRRCT that can contribute to binding. This variable insert is structurally diverse forming β-hairpins or loops and in some cases, no insert is present [16]. The LRRCT insert has been shown to sandwich glycans, H-trisaccharide and Thomsen-Friedenreich antigen, against the concave binding interface [18,19] (Figure 1B). The LRRCT insert has also been shown to insert in the cleft of HEL [18], bind a flat surface of HEL [32], or lay in a shallow groove of BclA [31]. Taken together, the LRRCT exhibits an impressive contribution in terms of binding versatility and may help aid in the recognition of diverse antigen types. The structural knowledge of the binding interactions should further enable rational design of consensus VLR libraries and/or affinity maturation. However, much like antibodies, the variable contact residues are not the only factors driving binding affinity. For instance mutation of non-contact residues that promoted better shape and electrostatic complementarity caused a 13-fold affinity increase for an anti-HEL VLR [18].
Engineering of VLRs for downstream applications
Therapeutic applications
Given their targeting capability, VLRs have been engineered for different therapeutic applications (summarized in Table 1). First, in a direct binding format, an evolved high affinity IL-6-binding repebody could inhibit IL-6 induced signaling in non-small cell lung cancer cells and suppress their growth in xenograft models [29]. As additional repebody examples, a C5a repebody could suppress proinflammatory response [27], and an anti-VEGF repebody could block neovascularization and vascular leakage in a mouse model of age-related macular degeneration [33]. Another approach is to employ a VLR as the recognition domain of a chimeric antigen receptor (CAR), and Moot and colleagues successfully created VLR-CARs that were expressed on T cells and could mediate cytotoxicity of the target cell [34]. VLRs have also been used in drug delivery applications. For example, an anti-tumor reagent was chemoenzymatically attached to the C-terminal end of the EGFR-specific repebody, and the drug conjugate elicited tumor regression in xenografted mice [35]. The same EGFR-specific repebody was also fused to a tumor apoptosis protein, apoptin, that assembled into stable nanoparticles providing anti-tumor activity in mouse xenografts [36]. Therapeutic application of VLRs is very promising; however, since VLRs are derived from jawless vertebrates, they could be immunogenic. Along these lines, preliminary evaluation of the anti-IL-6 repebody indicated negligible immunogenicity [29], although this issue warrants further attention by the field.
Table 1.
Applications of VLRs in Biomedical Research
| Application | VLR function | Result | References | |
|---|---|---|---|---|
| Therapeutic targeting | Inhibit signaling pathway | IL6 binder | Inhibits IL-6 induced signaling pathway in non-small cell lung cancer cells | [29] |
| Inhibit signaling pathway | C5a binder | Inhibits C5a pathway to suppress proinflammatory response | [27] | |
| Inhibit signaling pathway | VEGF binder | Blocks the VEGF signaling pathway for age-related macular degeneration | [33] | |
| Drug delivery | CAR-VLR | B-cells and CD5 binder | Expresses on effector cells, redirect to target cells, and activates cytotoxicity | [34] |
| VLR-drug conjugate | EGFR binder | Chemoenzymatically attaches drug for tumor suppression | [35] | |
| Nanoparticle | EGFR binder | Self-assembled nanoparticles target tumors | [36] | |
| Diagnostics/Imaging | Biomarker discovery | CD38 binder | Novel epitope that exclusively binds PCs | [30] |
| Diagnostics | TF-α binder | Stains tumor tissue with worse survival rate | [20] | |
| Diagnostics | Anti-idiotype binder | Used to monitor lymphocytic leukemia | [37] | |
| Molecular imaging | EGFR binder | In vivo tumor imaging | [38,39] | |
| Molecular imaging | mCherry binder | Tracks protein-protein interaction in real time | [40] | |
| Plant research | HopM1 binder | Expresses and binds in some plant environments | [28] | |
| Other | Stabilization | Consensus sequence | Able to crystalize LRRTM2 by altering convex surface based on VLR sequence | [41] |
| Stabilization | Stable caps | Resolves crystal structure of LRR proteins using caps comprised of VLR sequence | [42,43] | |
| Vaccine carrier | Multimerizing tail | Creates multimer with hidden VLR sequence resulting in high vaccine Ab titers | [44] |
Diagnostic applications
As discussed earlier, a VLR was identified that exclusively bound PCs via CD38 interactions. This VLR was capable of identifying healthy PCs and many multiple myelomas [30], suggesting that this VLR could be used as a diagnostic reagent. Another diagnostic demonstration employed the anti-Thomsen-Friedenreich antigen VLR to determine that non-small cell lung cancer patients that stained with the VLR had a worse survival prognosis [20]. Similarly, an anti-idiotype VLR could be used to monitor recurrence of chronic lymphocytic leukemia [37]. VLRs could also be valuable imaging reagents as demonstrated for EGFR-specific repebodies employed for in vivo imaging EGFR-expressing tumors [38,39] and mCherry-specific repebodies employed for tracking protein-protein interaction in live cells [40]. VLRs could also be used to study plant pathology and target disease forming pathogens. A VLR against a plant pathogen, HopM1, was able to be expressed and interact in planta [28].
Other applications
Non-binding VLR components have also been used in several ways. VLR residues have been used to stabilize other LRR proteins. For example, a murine neuronal adhesion molecule and LRR protein, LRRTM2, failed to form diffraction quality proteins. When LRRTM2 was stabilized using the lamprey VLR consensus sequence to mutate 33% of its amino acids, mainly located on the convex, non-binding surface, the crystal structure could be resolved [41]. Another approach to increase the stability of LRR proteins is to use the LRR hybrid technique [42]. LRRNT and LRRCT caps of hagfish VLR were grafted onto the ends of LRR toll-like receptors to solubilize and stabilize them for crystallization studies [42,43]. Another non-binding application comes from the multimerization capability of the hydrophobic region. To this end, the C-terminal multimerizing end of VLRB was fused to cancer relevant vaccine targets, creating a multimeric vaccine with minimal exposed VLR sequence. These properties resulted in a more effective vaccine with higher Ab titer against the vaccine target and lower Ab titer against the VLR component compared with other carrier strategies [44].
Conclusion
Despite their relatively recent discovery, VLRs have already been demonstrated to be a powerful antibody alternative. As described above, the crescent-shaped VLR solenoid has a highly variable concave binding surface that combines with the LRRCT insert to provide a versatile binding interface that results in highly specific binders to proteins and glycans. Like other alternative scaffolds, VLRs possess high thermal and pH stabilities and can be readily multimerized and engineered [14,23]. However, given that VLRs are bonafide antigen receptors, they are additionally compatible with immunization methods that help generate binding reagents with high specificity and affinity. Compared with mammalian antibodies, the high affinities and specificities of VLRs for glycans offer a potentially intriguing advantage, and VLRs should be explored further as novel targeting reagents that could be used to discover and/or monitor glycosylation signatures characteristic of cell types or disease. Another potential niche for VLRs versus mammalian antibodies leverages the evolutionary distance of jawless vertebrates from mammals. In particular, VLRs could be used to target highly conserved mammalian proteins, a process that is often difficult using Ig-based technologies. Finally, the multimeric structure may prove advantageous for the rapid tuning of valency and provide higher avidity for applications that require highly sensitive targeting.
Highlights.
VLRs bind proteins and glycans with high specificity and affinity.
Lamprey or hagfish immunization produces diverse VLR repertoires.
Modular engineering of VLR scaffolds is possible.
VLRs have been engineered for a variety of therapeutic applications.
Acknowledgments
This work was supported by National Institutes of Health grants NS091851 and NS099158 to E.V.S.. E.A.W. is supported by the National Human Genomes Research Institute training grant to the Genomic Sciences Training Program 5T32HG002760. Virus and spore images in the visual abstract were adapted from Dan Higgins and Janice Haney Carr of the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Plückthun A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu Rev Pharmacol Toxicol. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- 2.Ståhl S, Gräslund T, Eriksson Karlström A, Frejd FY, Nygren P-Å, Löfblom J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017;35:691–712. doi: 10.1016/j.tibtech.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Lipovšek D. Adnectins: engineered target-binding protein therapeutics. Protein Eng Des Sel. 2011;24:3–9. doi: 10.1093/protein/gzq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter A, Eggenstein E, Skerra A. Anticalins: Exploiting a non-Ig scaffold with hypervariable loops for the engineering of binding proteins. FEBS Lett. 2014;588:213–218. doi: 10.1016/j.febslet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Kintzing JR, Cochran JR. Engineered knottin peptides as diagnostics, therapeutics, and drug delivery vehicles. Curr Opin Chem Biol. 2016;34:143–150. doi: 10.1016/j.cbpa.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Škrlec K, Štrukelj B, Berlec A. Non-immunoglobulin scaffolds: a focus on their targets. Trends Biotechnol. 2015;33:408–418. doi: 10.1016/j.tibtech.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Finstad J, Good RA. The evolution of the immune response: III. immunologic responses in the lamprey. J Exp Med. 1964;120:1151. doi: 10.1084/jem.120.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linthicum DS, Hildemann WH. Immunologic Responses of Pacific Hagfish. J Immunol. 1970;105:912–918. [PubMed] [Google Scholar]
- 9.Hildemann WH, Thoenes GH. IMMUNOLOGICAL RESPONSES OF PACIFIC HAGFISH: I. Skin Transplantation Immunity1. Transplantation. 1969:7. doi: 10.1097/00007890-196906000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Pancer Z, Amemiya CT, Ehrhardt GRA, Ceitlin J, Larry Gartland G, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. [Google Scholar]
- 11.Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 12.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, Gartland GL, Boydston JA, Turnbough CL, Cooper MD. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 13.Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GRA, Boydston JA, Turnbough CL, Cooper MD. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci. 2008;105:2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and Function of Adaptive Immune Receptors in a Jawless Vertebrate. Science (80- ) 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 16.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen Recognition by Variable Lymphocyte Receptors. Science (80- ) 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HM, Oh SC, Lim KJ, Kasamatsu J, Heo JY, Park BS, Lee H, Yoo OJ, Kasahara M, Lee J-O. Structural diversity of the hagfish variable lymphocyte receptors. J Biol Chem. 2007;282:6726–6732. doi: 10.1074/jbc.M608471200. [DOI] [PubMed] [Google Scholar]
- 18.Velikovsky CA, Deng L, Tasumi S, Iyer LM, Kerzic MC, Aravind L, Pancer Z, Mariuzza RA. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol. 2009;16:725–730. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo M, Velikovsky CA, Yang X, Siddiqui MA, Hong X, Barchi JJ, Gildersleeve JC, Pancer Z, Mariuzza RA. Recognition of the Thomsen-Friedenreich pancarcinoma carbohydrate antigen by a lamprey variable lymphocyte receptor. J Biol Chem. 2013;288:23597–606. doi: 10.1074/jbc.M113.480467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong X, Ma MZ, Gildersleeve JC, Chowdhury S, Barchi JJ, Mariuzza RA, Murphy MB, Mao L, Pancer Z. Sugar-Binding Proteins from Fish: Selection of High Affinity “Lambodies” That Recognize Biomedically Relevant Glycans. ACS Chem Biol. 2013;8:152–160. doi: 10.1021/cb300399s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tasumi S, Velikovsky CA, Xu G, Gai SA, Wittrup KD, Flajnik MF, Mariuzza RA, Pancer Z. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc Natl Acad Sci. 2009;106:12891–12896. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im SP, Lee JS, Kim SW, Yu JE, Kim YR, Kim J, Lee J-H, Jung TS. Investigation of variable lymphocyte receptors in the alternative adaptive immune response of hagfish. Dev Comp Immunol. 2016;55:203–210. doi: 10.1016/j.dci.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Wezner-Ptasinska M, Krowarsch D, Otlewski J. Design and characteristics of a stable protein scaffold for specific binding based on variable lymphocyte receptor sequences. Biochim Biophys Acta - Proteins Proteomics. 2011;1814:1140–1145. doi: 10.1016/j.bbapap.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 24••.Wezner-Ptasinska M, Otlewski J. Selection of specific interactors from phage display library based on sea lamprey variable lymphocyte receptor sequences. Biochim Biophys Acta - Proteins Proteomics. 2015;1854:1833–1841. doi: 10.1016/j.bbapap.2015.09.005. A library from the previously designed dVLR scaffold was created and utilized to obtain a cancer protein binder. This study demonstrated a new strategy to create a library from a VLR scaffold and validated the library by identification of specific antigen binders. [DOI] [PubMed] [Google Scholar]
- 25.Lee S-C, Park K, Han J, Lee J, Kim HJ, Hong S, Heu W, Kim YJ, Ha J-S, Lee S-G, et al. Design of a binding scaffold based on variable lymphocyte receptors of jawless vertebrates by module engineering. Proc Natl Acad Sci. 2012;109:3299–3304. doi: 10.1073/pnas.1113193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmeggiani F, Huang P-S, Vorobiev S, Xiao R, Park K, Caprari S, Su M, Seetharaman J, Mao L, Janjua H, et al. A General Computational Approach for Repeat Protein Design. J Mol Biol. 2015;427:563–575. doi: 10.1016/j.jmb.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Hwang D-E, Choi J-M, Yang C-S, Lee J, Heu W, Jo E-K, Kim H-S. Effective suppression of C5a-induced proinflammatory response using anti-human C5a repebody. Biochem Biophys Res Commun. 2016;477:1072–1077. doi: 10.1016/j.bbrc.2016.07.041. Along with the study by Lee and colleagues [29], this study demonstrated a modular evolution strategy for affinity maturation of VLRs. The improved C5a-specific repebody was able to inhibit an immune response. [DOI] [PubMed] [Google Scholar]
- 28.Velásquez AC, Nomura K, Cooper MD, Herrin BR, He SY. Leucine-rich-repeat-containing variable lymphocyte receptors as modules to target plant-expressed proteins. Plant Methods. 2017;13:29. doi: 10.1186/s13007-017-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Kim HJ, Yang C-S, Kyeong H, Choi J, Hwang D-E, Yuk J, Park K, Kim YJ, Lee S-G, et al. A High-Affinity Protein Binder that Blocks the IL-6 / STAT3 Signaling Pathway Effectively Suppresses Non – Small Cell Lung Cancer. Mol Ther. 2014;22:1254–1265. doi: 10.1038/mt.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Yu C, Liu Y, Chan JTH, Tong J, Li Z, Shi M, Davani D, Parsons M, Khan S, Zhan W, et al. Identification of human plasma cells with a lamprey monoclonal antibody. JCI Insight. 2016;1:e84738. doi: 10.1172/jci.insight.84738. Using lamprey immunization and clonal evaluation, these authors found a VLR that exclusively bound PCs, including all healthy patient subpopulations and some multiple myelomas. This study used VLRs to identify a novel biomarker on a cell type for which Ig antibodies have not been successful. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirchdoerfer RN, Herrin BR, Han BW, Turnbough CL, Cooper MD, Wilson IA. Variable Lymphocyte Receptor Recognition of the Immunodominant Glycoprotein of Bacillus anthracis Spores. Structure. 2012;20:479–486. doi: 10.1016/j.str.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, Kerzic MC, Flajnik MF, Aravind L, Pancer Z, Mariuzza RA. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc Natl Acad Sci. 2010;107:13408–13413. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang D-E, Ryou J-H, Oh JR, Han JW, Park TK, Kim H-S. Anti-Human VEGF Repebody Effectively Suppresses Choroidal Neovascularization and Vascular Leakage. PLoS One. 2016;11:e0152522. doi: 10.1371/journal.pone.0152522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Moot R, Raikar SS, Fleischer L, Querrey M, Tylawsky DE, Nakahara H, Doering CB, Spencer HT. Genetic engineering of chimeric antigen receptors using lamprey derived variable lymphocyte receptors. Mol Ther - Oncolytics. 2016;3:16026. doi: 10.1038/mto.2016.26. VLRs were integrated into a chimeric antigen receptor platform where the VLR-CAR mediated effector cell interactions with specific target cells. This study demonstrated the ability of VLRs to be integrated into a therapeutic platform, which normally employs Ig-based antibody fragments as the antigen recognition domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Choi H-J, Yun M, Kang Y, Jung J-E, Ryu Y, Kim TY, Cha Y, Cho H-S, Min J-J, et al. Enzymatic Prenylation and Oxime Ligation for the Synthesis of Stable and Homogeneous Protein–Drug Conjugates for Targeted Therapy. Angew Chemie Int Ed. 2015;54:12020–12024. doi: 10.1002/anie.201505964. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Kang JA, Ryu Y, Han S-S, Nam YR, Rho JK, Choi DS, Kang S-W, Lee D-E, Kim H-S. Genetically engineered and self-assembled oncolytic protein nanoparticles for targeted cancer therapy. Biomaterials. 2017;120:22–31. doi: 10.1016/j.biomaterials.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Nakahara H, Herrin BR, Alder MN, Catera R, Yan X-J, Chiorazzi N, Cooper MD. Chronic Lymphocytic Leukemia Monitoring with a Lamprey Idiotope-Specific Antibody. Cancer Immunol Res. 2013;1:223–228. doi: 10.1158/2326-6066.CIR-13-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun M, Kim D-Y, Lee J-J, Kim H-S, Kim H-S, Pyo A, Ryu Y, Kim T-Y, Zheng JH, Yoo SW, et al. A High-Affinity Repebody for Molecular Imaging of EGFR-Expressing Malignant Tumors. Theranostics. 2017;7:2620–2633. doi: 10.7150/thno.18096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyo A, Yun M, Kim H-S, Kim T-Y, Lee J-J, Kim JY, Lee S, Kwon SY, Bom H-S, Kim H-S, et al. (64)Cu-Labeled Repebody Molecules for Imaging of Epidermal Growth Factor Receptor Expressing Tumors. J Nucl Med. 2017 doi: 10.2967/jnumed.117.197020. [DOI] [PubMed] [Google Scholar]
- 40.Kim H-Y, Lee J, Kim N, Do Heo W, Kim H-S. Tracking protein–protein interaction and localization in living cells using a high-affinity molecular binder. Biochem Biophys Res Commun. 2016;470:857–863. doi: 10.1016/j.bbrc.2016.01.129. [DOI] [PubMed] [Google Scholar]
- 41.Paatero A, Rosti K, Shkumatov AV, Sele C, Brunello C, Kysenius K, Singha P, Jokinen V, Huttunen H, Kajander T. Crystal Structure of an Engineered LRRTM2 Synaptic Adhesion Molecule and a Model for Neurexin Binding. Biochemistry. 2016;55:914–926. doi: 10.1021/acs.biochem.5b00971. [DOI] [PubMed] [Google Scholar]
- 42.Kim HM, Park BS, Kim J-I, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell. 2017;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Xu J-G, Huang C, Yang Z, Jin M, Fu P, Zhang N, Luo J, Li D, Liu M, Zhou Y, et al. Crystal structure of LGR4-Rspo1 complex: insights into the divergent mechanisms of ligand recognition by leucine-rich repeat G-protein-coupled receptors (LGRs) J Biol Chem. 2015;290:2455–2465. doi: 10.1074/jbc.M114.599134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saupe F, Reichel M, Huijbers EJM, Femel J, Markgren P-O, Andersson CE, Deindl S, Danielson UH, Hellman LT, Olsson A-K. Development of a novel therapeutic vaccine carrier that sustains high antibody titers against several targets simultaneously. FASEB J. 2016 doi: 10.1096/fj.201600820R. doi:10.1096/fj.201600820R. • Using the multimerizing end of a VLR as a vaccine carrier, the authors demonstrated high Ab titers of the vaccine target and low Ab titer against the VLR component. This study demonstrates a unique non-binding application of VLRs and the utility of the multimerization domain. [DOI] [PubMed] [Google Scholar]