Abstract
Vascularization of micro-tissues in vitro has enabled formation of tissues larger than those limited by diffusion with appropriate nutrient/gas exchange as well as waste elimination. Furthermore, angiocrine signaling from the vasculature may be essential in mimicking organ-level functions in these micro-tissues. In drug screening applications, the presence of an appropriate blood-organ barrier in the form of a vasculature and its supporting cells (pericytes, appropriate stromal cells) may be essential to reproducing organ-scale drug delivery pharmacokinetics. Cutting-edge techniques including 3D bioprinting and in vitro angiogenesis and vasculogenesis could be applied to vascularize a range of tissues and organoids. Herein, we describe the latest developments in vascularization and prevascularization of micro-tissues and provide an outlook on potential future strategies.
Graphical abstract
Introduction
In vitro micro-technologies to engineer microscale versions of various organs and tissues have attracted significant attention recently. These micro-tissues have been developed for transplantations of cornea [1], retina [2], bladder [3], or heart [4] [5], to recapitulate organ level function for drug screening [6] [7], drug discovery, personalized medicine and disease models, as well as for basic scientific understanding of in vivo like cell-cell interactions. In spite of growing demand for these systems, however, current ones are mostly limited to thin or avascular tissues, and for these there have been successful clinical trials [8].
Most major tissue engineered solid organs, though, lack perfusable microvascular networks. Vascularization of micro-tissues is necessary for a variety of reasons: 1) replicate angiocrine signaling [9] for the formation of micro-tissues mimicking developmental processes, 2) construct tissues of macroscopic scale, larger than can be maintained by diffusion alone with appropriate nutrient delivery and waste elimination, 3) mimic blood tissue or organ barrier such as the blood-brain barrier. The latter is especially relevant in immune cell interactions as well as tumor cell transmigration which are significantly influenced the organ specific barrier. We focus this paper on state-of-the-art developments in the vascularization of these micro-tissues, the limitations of current systems and potential future strategies to overcome them.
In vitro vascularization
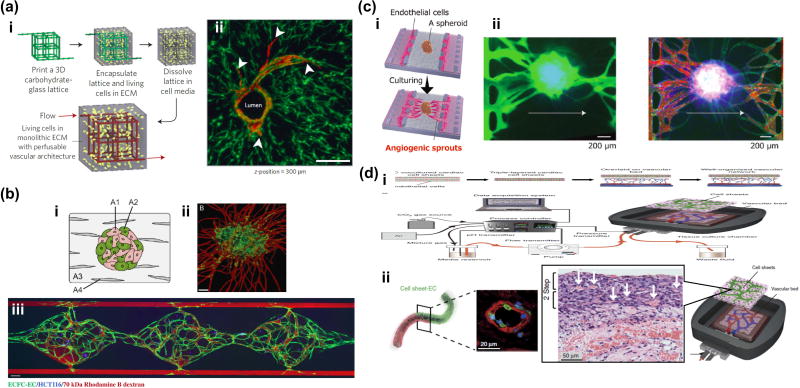
There are several techniques that could be used to vascularize tissues in vitro. For instance, Miller et al. used 3D printing to mold dissolvable vascular channels around which any engineered hydrogel based tissue could be grown. Subsequently, the molds were dissolved and endothelialized. This technique allows building large scale vascularized tissues with tunable vascular geometries (Fig. 2A) [10]••. These robust perfusable vascular networks could be cultured with other parenchymal cells and micro-tissues. However, with the current 3D printing technology, engineering capillary structures is limited to those larger than ~100 µm in diameter. However, one can engineer sub-100 µm capillary structures (even down to 10 µm) using the other major strategies of vascularization through angiogenesis [11] or vasculogenesis [12] [13] [14] [15] (Fig. 2B) which draws upon the emergent behavior of endothelial cells. Using angiogenesis or vasculogenesis techniques, it is possible to achieve sub-100 µm capillary structures even down to 10 µm. In particular, Nashimoto et al. recently induced angiogenesis in vitro producing vascularized and perfusable tissues in a microfluidic device containing prevascularized spheroids (Fig. 2C) [16]••. This work demonstrated a prevascularized spheroid with lung fibroblasts and endothelial cells that was placed in the microfluidic device in a collagen gel. Then, other endothelial cells that were adhered to the wall of the gel migrate and sprout towards the spheroid, resulting in anastomoses between the vascular networks. Lastly, Sekine et al. demonstrated long-term culture of prevascularized skin-like micro-tissues by using an ex vivo vascular bed (Fig. 2D) [17]•. They used a rat vascular bed that was extracted from a rat’s femoral muscle tissue along with the branches of arteries and veins. Stacked cardiac sheets were placed onto this vascular bed in order to form a connection between the prevascularized stacked cardiac sheets and the rat vascular bed. This group also demonstrated that the vascularization improves the survival of tissues in vitro, after transplantation. Even though these techniques are becoming established, the development of in vitro vascularized organs using them is a field in its infancy. It is a nascent field in which the majority of vascularized engineered tissues rely on vascularization following implantation. The host response tends to induce vascularization of constructs containing a mixture of target tissue cells and endothelial cells, leading to a connected, perfusable tissue [18] [19]. In the vascularization technique described in [18], a millimeter scale tissue is implanted in an animal with surgery. This has the drawback of a having to perform a surgery for implantation. Likewise, the work in [19] requires 2 surgeries to produce a vascularized tissue, one for implantation of the target tissue that needs to be vascularized, and a second one to recover the vascular graft tissue. Both these techniques are cumbersome, and may not be as scalable and reproducible as other techniques that may involve purely in vitro vascularization. In this brief review, we will discuss the current state-of-the-art in the development of various vascularized micro-tissues for solid organs (skeletal muscle, heart, brain, liver, bone, lung, tumors) as well as potential steps for improvement.
Figure 2.
Cutting-edge technologies to vascularize tissues in vitro. (a) Engineering of 3D vascular networks in extracellular matrix. (i) 3D casting mold was fabricated with carbohydrate glass lattice by 3D printer. After dissolving the lattice, endothelial cells were seeded into the 3D microchannels. (ii) Endothelialized microchannels and angiogenic sprouting with stromal cells. Potentially, this method could be applied to vascularization of various types of tissues, even relatively large tissues. (b) Vasculogenesis based vascularization in a microfluidic device. Flow of fluorescently tagged dextrans show the perfusability and connectivity of vascular networks to supply sufficient nutrients to HCT116 cells in a gel. This strategy is promising for high-throughput drug screening of vascularized micro-tissues (c) (i) Angiogenesis based vascularization of spheroid in a microfluidic device. (ii) Perfusion of fluorescently tagged dextrans showed that of angiogenic sprouts formed anastomoses with prevascularized networks inside the spheroid, preventing necrotic cell death in the core region. This method could be applied to vascularization of various types of spheroids and organoids for drug testing. (d) Vascularization of sheet-like tissues using an ex vivo vascular bed. Rat vascular bed was extracted and connected to a perfusion system in vitro. Stacked cell sheet with epithelial cells and endothelial cells were placed on this vascular bed. This ex vivo system has promise for the extension of culture time which is currently limited by the lack of vasculature (ii).
Brain
Neurospheroids, derived from neural stem cells or neural progenitor cells, are versatile cell aggregates that model forebrain and cerebellar development [20][21]. This model could be utilized to study several brain pathologies including synaptopathy and epilepsies. A significant advance in in vitro brain models occurred several years ago with the first reports of cerebral organoids [22]. These organoids which are typically produced by the emergent behavior of human stem cells such as ES cells and induced pluripotent stem (iPS) cells in a bioreactor represent a complex human brain model that may be useful for drug screening. Recently, fused forebrain-like organoids were formed by placing two spheroids next to each other in order to more closely mimic the forebrain physiology [23] (Fig. 1a(i)).
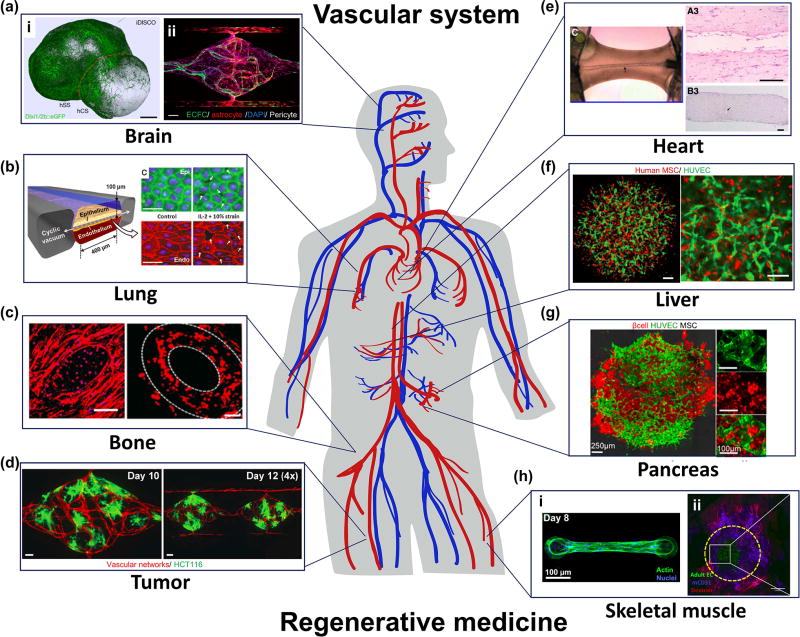
Figure 1.
State-of-the-art of prevascularization and vascularization of micro-tissues in brain (a), lung (b), bone (c), tumor (d), heart (e), liver (f), pancreas (g) and skeletal muscle(h). (a) (i) Fabrication of multi-cellular spheroid by fusing iPS cell-derived human cortical spheroids (hCS) and human subpallium spheroids (hSS) to mimic forebrain-like tissues. (ii) A microfluidic vascularized 3D BBB model developed by co-culturing colony-forming endothelial cells, astrocytes and pericytes in a perfusable microdevice. (b) A microfluidic epithelial and endothelial interface model to simulate the lung alveolar microenvironment. (c) Vascularized bone graft by using 3D bioprinting with biocompatible scaffold. (d) Vascularization of tumor tissues in a perfusable microfluidic platform. (e) Engineering endothelialized hollow-structures in a cardiac muscle fiber in vitro. (f) Prevascularized functional liver bud by spontaneous aggregation of human iPS cell-derived hepatocytes, human umbilical vein endothelial cells (HUVEC) and mesenchymal stem cells (MSC). (g) Prevascularized islet-like tissues with pancreatic β cells, HUVECs, and MSCs. (h) (i) Representative skeletal muscle fiber bundle attached to cantilevers in a microdevice. (ii) Prevascularized skeletal muscle tissue aggregation and anastomosis of prevasculature with host vasculature after transplantation.
Although neurospheorids and cerebral organoids represent the future of human in vitro brain models there remain several important challenges to advancing these models. For example, current cerebral organoids fail to recapitulate many late brain development processes such as gliagenesis and vasculogenesis mainly due to the longer time needed [24] to obtain functional brain organoids. Furthermore, vascularization of these cerebral organoids is essential for long-term maintenance [25]. To overcome these barriers to progress, other vascularized brain models are needed, for example, Phan et al.’s co-culture model of astrocytes and pericytes with a perfusable vasculature in a microfluidic device [26]•• (Fig. 1a(ii)).
Opinion
At this time, no vascularized brain model exists, even including prevascularized models. However, the co-culture of neurosphere or organoid with endothelial cells using either molding [10]•• or vascularization techniques [16]•• could be used to develop perfusable and functional brain models in vitro.
Lung
Gas exchange in the body takes place in the alveoli where inspired air comes into intimate contact with the circulating blood. Huh et al.’s alveolar-chip incorporating alveolar epithelial and endothelial cells on an engineered, mechanically stretched substrate represents one of the most advanced alveolar models to date [27] [28]•• (Fig. 1b).
Opinion
Inflammatory responses and toxicology studies have been conducted on this chip and compared qualitatively to responses in ex vivo mouse lungs with generally impressive results. Two of the primary limitations of this system, however, are the morphology of both the endothelial channel and the airways neither of which recapitulate that of their in vivo counterparts. Capillaries in vivo are approximately 10 µm in diameter, while the endothelial lumens in the alveolar-chip are an order of magnitude larger. This may influence the adhesion of cells such as neutrophils, tumor cells etc. due to differences in the shear stress, flow velocity, or interactions with the endothelium, which could in turn affect the dynamics of extravasation of these cells. Likewise, the hierarchical structure of the in vivo airway leading to the alveoli is not recapitulated in this device, perhaps leading to exposure of the epithelial cells to a different profile of aerosol particle sizes. This is in contrast to in vivo systems in which only the smallest particles ever reach the alveoli. Future versions of this system could benefit from having a bronchial epithelial chip upstream to the alveolar chip with a hierarchical structure in between, thus correctly representing the aerosol/nanoparticle distribution in the respective compartments.
Bone
Bone is a highly vascularized tissue containing vessels categorized into two major systems, central or “Haversian” canals, and perforating or “Volkmann’s” canals [29]. Bone consists largely of a rigid, honeycomb-like matrix formed from a composite material incorporating the inorganic mineral calcium phosphate, which gives the bone its rigidity. Therefore, incorporating an extracellular matrix with calcium particles in in vitro bone models is important in order to recapitulate the mechanical strength of in vivo bone.
To vascularize bone tissues for Haversian’s canals, 3D bioprinting technology has been widely used to create a relatively large vessel. For instance, 3D bioprinting can precisely control the location of cells and extracellular matrix (ECM) for engineering complicated micro structures [30]. Cui et al. used a novel technique to engineer a vascularized bone biphasic construct using dual 3D bioprinting PLA and gelatin methacrylate hydrogel [31] (Fig. 1c). This sequential printing allows fabrication of a hierarchical bone structure with a vasculature. To create Volkmann's canal-like capillary networks in bone tissue, vasculogenesis using an encapsulation of endothelial cells and mesenchymal stem cells or osteoblasts has proved an effective strategy [32]. Correia et al. demonstrated bone grafts with micro-vascular networks by co-culturing human umbilical vein endothelial cells (HUVEC) and mesenchymal stem cells (MSC) in a decellularized bone scaffold [33]. They found that sequential application of growth factors for endothelial cells and osteoblasts helps to form microvascular networks followed by osteogenic differentiation. In vitro bone models have also been used to study diseases such as cancer. For instance, Jeon et al. developed a tumor extravasation model in a microfluidic vascularized bonelike tissue [34].
Opinion
In the future, the combination of 3D bioprinting techniques and vasculogenesis of endothelial cells with a stiffer scaffold could prove a sueful approach to model highly rigid bone with both types of vascular structures.
Tumors
The primary goal of making vascularized 3D tumor models is to represent the tumor microenvironment more accurately. Given the intimate proximity of tumors to the vasculature, it is likely that interaction between the two could influence expression of target proteins in the tumor, affect drug delivery and drug response. The vasculature is known to play a key role in cancer metastasis (both intravasation and extravasation). To address these concerns, Sobrino et al. developed a microfluidic tumor model with a range of tumor cells suspended in a 3D ECM, surrounded by a perfusable human cell based vasculature [15]• (Fig. 1d). They demonstrated that IC50 values for the effect of Oxaliplatin (a chemotherapeutic drug) on HCT116 cells is an order of magnitude larger for cells growing in the microfluidic vascularized micro-tumors, compared to 2D cultures.
Opinion
For more widespread adoption of such models, a more detailed characterization of this platform is required that may characterize the relative roles of the 3D architecture and associated paracrine effects, the direct effects of signaling with the vascular cells, and the importance of drug delivery through the vasculature versus through the tumor cells in 3D or 2D. The use of patient derived iPS cells in this platform would further personalize it for therapeutic screening. The use of organ-specific stromal/supporting cells as well as a full complement of immune cells may further improve the physiological relevance of such platforms in the modeling of specific tumors.
Heart
Heart tissue is highly vascularized in order to meet its high energetic demand. Yet the most widely used heart-on-a-chip micro-tissues are not vascularized. They primarily focus on contractility measurements with either 3D cardiomyocyte based tissues grown with flexible pillar anchors [35] or 2D cardiomyocyte sheets [36] on thin flexible cantilevers. Such systems have been used in disease models [37] such as mitochondrial cardiomyopathy in Barth’s syndrome. A fully-realistic model of heart micro-tissues, however, will require the addition of a functional vasculature that replicates in vivo like endothelial-cardiomyocyte crosstalk. In particular, nitric oxide released by endothelial cells may have a crucial cardio-protective role without which drug cardiotoxicity studies may not be representative of in vivo physiology. Attempts at solving this problem include an emergent self-assembled cardiac tissue containing endothelial networks by seeding defined tricultures of cardiac myocytes (primary human cardiac cells, human embryonic stem (hES) cell-derived, or iPS cell-derived cardiac myocytes), fibroblasts (embryonic or iPS cell-derived cardiac fibroblasts) and endothelial cells (hES cell-derived, or human coronary artery endothelial cells) either on a biodegradable polymeric scaffold [38] , or in aggregating hanging drop cultures [39]. However, neither these models contain a perfusable vasculature through cardiac tissue in a way that in vivo tissues do, despite the demonstration of structures with lumens [38]. In Chiu et al., an engineering approach is used to plate cardiac-myocytes on top of a perfusable capillary bed [40]. In this approach, appropriate endothelial-cardiac interactions should occur. Nevertheless, this system lacks the capability to measure force as in Boudou et al. [35] or Agarwal-Parker et al. [36], which is essential to assess cardiac function. Vollert et al. developed one of the most realistic cardiac models to-date with an engineered heart tissue, both with anchors that could be used as force transducers as well as with a perfusable endothelialized channel through the tissue [41] •• (Fig. 1e).
Opinion
The main drawbacks of the Vollert model include the need for further validation in the context of several cardiac diseases, such as ischemia, as well as improved ease of fabrication and use. In the future, techniques such as 3D bioprinting could be used to further standardize such models making it more accessible, as well as more useful by combining the most attractive features of all the above platforms to produce an in vivo like heart-chip with the appropriate heterotypic cell-cell interactions and in-built instrumentation to test tissue function.
Liver
A major challenge for studying liver function in drug screening and regenerative medicine applications is that primary hepatocytes quickly lose their advanced phenotype in 2D cultures [42]. In vitro liver models have been used for numerous drug metabolism studies [43]. Liver spheroid cultures and other 3D cultures of hepatocytes offer effective strategies that help extend hepatocyte function lifetime and maintain high metabolic rates [44]. However, all these models have limited lifetime under even the best conditions (primary hepatocyte monolayer: 4–5 days, spheroid: ~2 weeks and co-culture spheroid with stellate cells: ~3 weeks). Another strategy is the use of cell lines and iPS cell and ES cell derived hepatocytes [45]. To further extend the lifetime of liver advanced metabolism in vitro [46], vascularization of liver spheroids and perfusion into micro-tissues are still needed. Timmins et al. demonstrated vascularized multi-cellular spheroid with endothelial cells and hepatocyte cell line created by the hanging-drop method [47]. They co-cultured HepG2 and human umbilical vein endothelial cells (HUVEC), resulting in the formation of a prevascularized spheroid. HUVECs are present throughout the hepatic spheroid and formed small lumen structures. However, this spheroid is not perfusable as the vasculature inside is not easily accessible.
Sasaki et al. also showed prevascularized liver tissues using a layer-by-layer fabrication method with hepatocytes and neonatal human dermal fibroblasts precoated with fibronectin and gelatin [48]. They confirmed that such prevascularized tissues could connect to the host vasculature after transplantation into mice by measuring human albumin level from mouse serum. Liu et al. created a high cell density liver model using an aggregation of many spheroids coated with endothelial cells. They demonstrated perfusion of culture medium into this dense hepatic tissue using an endothelialized alginate hydrogel fiber in vitro [49].
Takebe et al, demonstrated emergent morphogenesis of a vascularized and functional liver organoid using iPS cell-derived hepatocytes, HUVECs and MSCs [50] [51]•• (Fig. 1f). They cocultured these three types of cells in Matrigel and the cells spontaneously aggregated due to the contractile forces of MSCs and subsequently became a prevascularized liver bud. The transplantation of this prevascularized bud into mice with hepatic failure significantly improved their survival.
Opinion
Prevascularized hepatic tissues from iPS cell-derived hepatocytes are adequate for most tissue engineering applications. Many groups have shown that such prevascularized tissues rapidly connect to host vasculature and support liver function. However, systems that meet the requirements for applications in drug development, vascularization and bile-duct-genesis of hepatic spheroids and organoids to maintain advanced hepatic function and metabolism in vitro are still a major unmet need.
Skeletal muscle
Widely used 3D skeletal muscle models include engineered muscle tissues formed by suspending myoblasts in a 3D ECM such as collagen or fibrin, and anchored to velcro or PDMS micro-pillars to measure force output [52] [35] [53] [54] (Fig. 1h(i)). Such models have been used to elucidate disease mechanisms. For instance, Lee et al. used a bioengineered 3D skeletal muscle model using primary mouse myoblasts in a collagen/matrigel based tissue to show that skeletal muscle atrophy results from length shortening [52]. The next generation of skeletal muscle models also incorporate neuro-muscular junctions [55] [56] for instance with a motor neuron spheroid in co-culture with a 3D skeletal muscle tissue in a microfluidic compartmentalized device [56]. Nevertheless, the lack of a functional perfusable vasculature in these in vitro models also limits the size and density of engineered skeletal muscle tissues. Furthermore, this may lead to under-representation of key vasculature-associated signaling mechanisms as there is a high capillary density in skeletal muscle in vivo. This could contribute to the neonatal sarcomeric protein isoforms in in vitro muscle [57], and also account for the small size of each muscle cell because of key missing angiocrine signaling.
Prevascularized in vitro skeletal muscle tissues when implanted into mice abdomen or legs become vascularized by the host [18] [19] [58] (Fig. 1h(ii)). These could serve as potential vascularized skeletal muscle models but are quite tedious and may not have the same level of control as purely in vitro engineered tissues. Dacha et al. have shown the first engineered skeletal muscle with endothelial cell networks, by co-culturing human skeletal muscle progenitor cells with human umbilical vein endothelial cells. However, these networks are little more than a system of connected cells [59]; perfusability of this network was not demonstrated.
Opinion
A fully vascularized and perfusable skeletal muscle tissue has not yet been engineered in in vitro systems to date. The use of next generation technologies such as 3D bioprinting, molding techniques to incorporate vascular tubes within engineered muscle tissue similar to Vollert et al. [41]••, angiogenesis/vasculogenesis, vascular beds with prevascularized cell sheets have potential to solve the remaining issues for skeletal muscle models.
Future outlook
We have summarized the state-of-art in vascularized micro-tissues for many major organs. Some, such as pancreatic islets, have very few reports in spite of important applications such as insulin regulation and insulinomas. Takebe et al., demonstrated prevascularized tissues with β-cells and mesenchymal cells [60] (Fig. 1g). This method was adapted from the same method used to create a liver bud as discussed earlier. Recently, there has also been progress in bioprinting of a vascularized thyroid gland [61]. Overall, there has been rapid progress in this field, and future advances in technologies such as 3D bioprinting and novel biomaterials are likely to further accelerate the work in terms of reproducing the cellular heterogeneity and complex 3D structure of in vivo tissues in in vitro micro-fluidic platforms. The combination of fundamental technologies for vascularization [10,16] [17,62] and organoid engineering [20, 21, 60, 63] could promise to obtain functional vascularized tissues. Furthermore, cutting edge genome engineering techniques will be transformative in creating genetic disease models using these in vitro micro-tissue platforms. With rapid progress in iPS cell differentiation technologies and manufacturing processes, we will start to see more personalized medicine applications of such chips, particularly in areas such as drug screening for patient-specific tumor (to replace time consuming patient-derived xenograft, or PDX, models).
Highlights.
Several viable methods currently exist to create vascularized in vitro tissues.
Progress has been made in developing systems that mimic specific organs or tissues.
New technologies such as 3D printing have great potential to advance the field.
A major challenge is to vascularize organoids grown from human cells.
Applications include drug screening or growing human implants.
Acknowledgments
This work was supported by National Science Foundation for a Science and Technology Center on Emergent Behaviors of Integrated Cellular Systems (grant number CBET-0939511). T.O. was also supported by JSPS (Japan Society for the Promotion of Science) research fellow overseas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. New England Journal of Medicine. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 2.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. New England Journal of Medicine. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 3.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. The Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 4.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. The Journal of Thoracic and Cardiovascular Surgery. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa S, Domae K, Yoshikawa Y, Fukushima S, Nakamura T, Saito A, Sakata Y, Hamada S, Toda K, Pak K, et al. Phase I Clinical Trial of Autologous Stem Cell–Sheet Transplantation Therapy for Treating Cardiomyopathy. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.116.003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue H, Yamanaka S. The Use of Induced Pluripotent Stem Cells in Drug Development. Clinical Pharmacology & Therapeutics. 2011;89:655–661. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- 7.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nature reviews. Drug discovery. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouwkema J, Khademhosseini A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends in Biotechnology. 2016;34:733–745. doi: 10.1016/j.tibtech.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. General technology for 3D patterning of perfusable vascular structures. This technology could be used to scale up the size of any tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab on a Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 12.Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CCW, George SC. A threedimensional in vitro model of tumor cell intravasation. Integrative Biology. 2014;6:603–610. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Phan DTT, Sobrino A, George SC, Hughes CCW, Lee AP. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab on a Chip. 2016;16:282–290. doi: 10.1039/c5lc01050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MB, Whisler JA, Frose J, Yu C, Shin Y, Kamm RD. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protocols. 2017;12:865–880. doi: 10.1038/nprot.2017.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Sobrino A, Phan DTT, Datta R, Wang X, Hachey SJ, Romero-López M, Gratton E, Lee AP, George SC, Hughes CCW. 3D microtumors in vitro supported by perfused vascular networks. Scientific Reports. 2016;6:31589. doi: 10.1038/srep31589. Microfluidic tumor model with tumor cells in a 3D ECM and perfusable vascular networks. Demonstrated the difference in IC50 for a chemotherapeutic drug between 3D vascularized tumor culture and 2D culture of tumor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Nashimoto Y, Hayashi T, Kunita I, Nakamasu A, Torisawa Y-s, Nakayama M, Takigawa-Imamura H, Kotera H, Nishiyama K, Miura T, et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integrative Biology. 2017;9:506–518. doi: 10.1039/c7ib00024c. Demonstrates vascularization of multi-cellular spheroids with lung fibroblasts and endothelial cells. This spheroid was placed in the microfluidic device in a collagen gel. Other endothelial cells adherent to the wall of the gel migrate and sprout towards the spheroid, resulting in anastomoses between the vascular networks. [DOI] [PubMed] [Google Scholar]
- 17•.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. In vitro vascularization of cardiac cell sheet. Rat vascular beds were extracted from rat femoral muscle tissue, including branches of arteries and veins. Connection between prevascularized and stacked cardiac sheets and rat vascular bed improves the survival of tissues in vitro, after transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juhas M, Engelmayr GC, Fontanella AN, Palmer GM, Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5508–5513. doi: 10.1073/pnas.1402723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shandalov Y, Egozi D, Koffler J, Dado-Rosenfeld D, Ben-Shimol D, Freiman A, Shor E, Kabala A, Levenberg S. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci U S A. 2014;111:6010–6015. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park J-Y, O'Rourke NA, Nguyen KD, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Meth. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C-T, Bendriem RM, Wu WW, Shen R-F. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. Journal of Biomedical Science. 2017;24:59. doi: 10.1186/s12929-017-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelava I, Lancaster MA. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Developmental Biology. 2016;420:199–209. doi: 10.1016/j.ydbio.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Phan DT, Bender RH, Andrejecsk JW, Sobrino A, Hachey SJ, George SC, Hughes CC. Blood-brain barrier-on-a-chip: Microphysiological systems that capture the complexity of the blood-central nervous system interface. Exp Biol Med (Maywood) 2017:1535370217694100. doi: 10.1177/1535370217694100. 3D BBB model was formed by co-culturing endothelial cells, astrocytes and pericytes in the perfused microfluidic device. Multi-cellular interactions between astrocytes and endothelial cells have been shown, in which astrocytes increase expression of endothelial tight junction genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. 3D lung on a chip model with epithelial and endothelial interface in a microfluidic device. Stretching of membrane by cyclic vacuum simulates respiration. This model could be applied to diseases such as pulmonary edema which was induced by IL-2 exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke B. Normal Bone Anatomy and Physiology. Clinical Journal of the American Society of Nephrology. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien CM, Holmes B, Faucett S, Zhang LG. Three-Dimensional Printing of Nanomaterial Scaffolds for Complex Tissue Regeneration. Tissue Engineering Part B: Reviews. 2014;21:103–114. doi: 10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui H, Zhu W, Nowicki M, Zhou X, Khademhosseini A, Zhang LG. Hierarchical Fabrication of Engineered Vascularized Bone Biphasic Constructs via Dual 3D Bioprinting: Integrating Regional Bioactive Factors into Architectural Design. Advanced Healthcare Materials. 2016;5:2174–2181. doi: 10.1002/adhm.201600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G. In Vitro Model of Vascularized Bone: Synergizing Vascular Development and Osteogenesis. PLOS ONE. 2011;6:e28352. doi: 10.1371/journal.pone.0028352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proceedings of the National Academy of Sciences. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18:910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 39.Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, Gentile C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. 2017;7:7005. doi: 10.1038/s41598-017-06385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A. 2012;109:E3414–3423. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Vollert I, Seiffert M, Bachmair J, Sander M, Eder A, Conradi L, Vogelsang A, Schulze T, Uebeler J, Holnthoner W, et al. In vitro perfusion of engineered heart tissue through endothelialized channels. Tissue Eng Part A. 2014;20:854–863. doi: 10.1089/ten.TEA.2013.0214. An endothelialized hollow structure was formed inside the cardiac muscle fiber bundle with an anchoring silicone rack. Perfusion was achieved by connecting the hollow posts to a tubing system in the culture dish. The perfusion culture improves cell viability in the cardiac tissues and enables toxicity studies. [DOI] [PubMed] [Google Scholar]
- 42.Ng SS, Xiong A, Nguyen K, Masek M, No DY, Elazar M, Shteyer E, Winters MA, Voedisch A, Shaw K, et al. Long-term culture of human liver tissue with advanced hepatic functions. JCI Insight. 2017;2:e90853. doi: 10.1172/jci.insight.90853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnology and Bioengineering. 2007;97:1340–1346. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Fisher JE, Lillegard JB, Rodysill B, Amiot B, Nyberg SL. Cell therapies for liver diseases. Liver Transplantation. 2012;18:9–21. doi: 10.1002/lt.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK, et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials. 2013;34:1781–1789. doi: 10.1016/j.biomaterials.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Riccalton-Banks L, Liew C, Bhandari R, Fry J, Shakesheff K. Long-term culture of functional liver tissue: three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003;9:401–410. doi: 10.1089/107632703322066589. [DOI] [PubMed] [Google Scholar]
- 47.Timmins N, Dietmair S, Nielsen L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis. 2004;7:97–103. doi: 10.1007/s10456-004-8911-7. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki K, Akagi T, Asaoka T, Eguchi H, Fukuda Y, Iwagami Y, Yamada D, Noda T, Wada H, Gotoh K, et al. Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials. 2017;133:263–274. doi: 10.1016/j.biomaterials.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Sakai S, Taya M. Engineering tissues with a perfusable vessel-like network using endothelialized alginate hydrogel fiber and spheroid-enclosing microcapsules. Heliyon. 2016;2:e00067. doi: 10.1016/j.heliyon.2016.e00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. For translational therapy using liver buds in vitro, vascularized liver tissues were fabricated. After transplantation of these tissues into a mouse model of liver failure, anastomoses between tissues and host vasculature occurred, resulting in the perfusion of blood to the liver bud. This transplantation supported liver metabolism and incresed the survival rate of these mice. [DOI] [PubMed] [Google Scholar]
- 51.Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 52.Lee PH, Vandenburgh HH. Skeletal muscle atrophy in bioengineered skeletal muscle: a new model system. Tissue Eng Part A. 2013;19:2147–2155. doi: 10.1089/ten.TEA.2012.0597. [DOI] [PubMed] [Google Scholar]
- 53.Neal D, Sakar MS, Ong LL, Harry Asada H. Formation of elongated fascicle-inspired 3D tissues consisting of high-density, aligned cells using sacrificial outer molding. Lab Chip. 2014;14:1907–1916. doi: 10.1039/c4lc00023d. [DOI] [PubMed] [Google Scholar]
- 54.Kalman B, Picart C, Boudou T. Quick and easy microfabrication of T-shaped cantilevers to generate arrays of microtissues. Biomedical Microdevices. 2016;18:43. doi: 10.1007/s10544-016-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morimoto Y, Kato-Negishi M, Onoe H, Takeuchi S. Three-dimensional neuron-muscle constructs with neuromuscular junctions. Biomaterials. 2013;34:9413–9419. doi: 10.1016/j.biomaterials.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 56.Uzel SG, Platt RJ, Subramanian V, Pearl TM, Rowlands CJ, Chan V, Boyer LA, So PT, Kamm RD. Microfluidic device for the formation of optically excitable, threedimensional, compartmentalized motor units. Sci Adv. 2016;2:e1501429. doi: 10.1126/sciadv.1501429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Shansky J, Vandenburgh H. Induced formation and maturation of acetylcholine receptor clusters in a defined 3D bio-artificial muscle. Mol Neurobiol. 2013;48:397–403. doi: 10.1007/s12035-013-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perry L, Flugelman MY, Levenberg S. Elderly Patient-Derived Endothelial Cells for Vascularization of Engineered Muscle. Mol Ther. 2017;25:935–948. doi: 10.1016/j.ymthe.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gholobova D, Decroix L, Van Muylder V, Desender L, Gerard M, Carpentier G, Vandenburgh H, Thorrez L. Endothelial Network Formation Within Human Tissue-Engineered Skeletal Muscle. Tissue Engineering Part A. 2015;21:2548–2558. doi: 10.1089/ten.tea.2015.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Bulanova EA, Koudan EV, Degosserie J, Heymans C, Pereira FD, Parfenov VA, Sun Y, Wang Q, Akhmedova SA, Sviridova IK, et al. Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication. 2017;9:034105. doi: 10.1088/1758-5090/aa7fdd. [DOI] [PubMed] [Google Scholar]
- 62.Phan DTT, Wang X, Craver BM, Sobrino A, Zhao D, Chen JC, Lee LYN, George SC, Lee AP, Hughes CCW. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab on a Chip. 2017;17:511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]