Abstract
Zika virus (ZIKV) infection in humans has been associated with severe congenital defects (i.e. microcephaly) and pregnancy loss. Here we show that 26% of nonhuman primates infected with Asian/American ZIKV in early gestation experienced fetal demise later in pregnancy despite few clinical signs of infection. Pregnancy loss due to asymptomatic ZIKV infection may therefore be a common but under-recognized adverse outcome related to maternal ZIKV infection.
Zika virus (ZIKV) infection during pregnancy can lead to severe congenital defects collectively known as Congenital Zika Syndrome (CZS) 1,2. The spectrum of adverse outcomes ranges from hearing and ocular defects to fetal brain malformations (with and without microcephaly) and spontaneous pregnancy loss (miscarriage and stillbirth) 3. Early pregnancy losses may be clinically under-recognized as linked to ZIKV infection because miscarriages are common and ZIKV infections are often asymptomatic. Although stillbirths in late pregnancy are easily clinically recognized, they may not be counted or reported as an important public health outcome 4.
Background rates of pregnancy loss in women change as gestational age advances, with a rate of ∼25% at 4-5 weeks, 2% at 20 weeks and 0.1% after 20 weeks' gestation 5. There is limited evidence that symptomatic Zika virus infection is associated with miscarriage and stillbirth 1,3,6,7. In 2016, birth rates throughout Brazil dropped 5% with the greatest reduction in birth rates in Pernambuco, where there were 10% fewer live births in 2016 than in 2015 8. Though some of this drop can be attributed to behavioral modification, we hypothesize this could also be due in part to underreported fetal losses caused by Zika virus in hard-hit areas.
Among the experimental animal models that have been described for studying ZIKV in pregnancy, nonhuman primates (NHP) are the most physiologically similar to humans, have extensive parallels in the histology and immunology of the maternal-fetal interface 9, and recapitulate the clinical course of human ZIKV infection 10–15. In experimentally infected NHP, pregnancy loss can be assessed rigorously, as the timing, dose, route and strain of ZIKV infection is known and ultrasound frequency can be greater. Critically, many Zika virus-infected NHP do not exhibit significant clinical signs and therefore closely resemble asymptomatic human ZIKV infections.
We aggregated fetal loss data from six National Primate Research Centers collected from ZIKV-infected pregnant nonhuman primates (rhesus macaques, pigtail macaques and common marmosets) to assess the rate of fetal demise (Table 1 and Supplementary Table 1), which includes some data from previously published studies 11,12,15–18. The studies differed in viral strain, route, dose, and gestational age at infection. Data from experimental infection using five different viral isolates are included, used alone or in combination (Table 1 and Supplementary Table 1). All strains used in these studies, except one originating from Cambodia, contained the S139N substitution in the pre-membrane (prM) ZIKV gene associated with increased virulence in mice (Table 1) 19. The dose of viral inoculum in these experiments ranged from 103 to 107 plaque forming units (PFU) (Supplementary Table 1), comparable to the wide range of estimates of infectious flavivirus doses delivered by mosquito bite, from 101 to 107 PFU.
Table 1. Average rates of fetal demise among different nonhuman primate cohorts infected with ZIKV during pregnancy.
| ZIKV isolate | Dose (PFU) | Route of inoculation* | Ave. inoculation gestational age (GA) | Ave. inoculation GA w/fetal demise | Fetal Demise n (%) | |
|---|---|---|---|---|---|---|
| Rhesus macaque (n=6) | Brazil SPH_2015 + PRVABC59 | 4 ×103 | IA+IV | 48 | 46 | 4 (66.7) |
| Rhesus macaque (n=4) | Brazil SPH_2015 | 2 ×105 | IA+IV | 61 | 41 | 1 (25) |
| Rhesus macaque (n=2) | FP/2013 | 1×104 | SC | 33 | N/A | 0 |
| Rhesus macaque (n=2) | FP/2013 | 1×104 | SC | 112 | N/A | 0 |
| Rhesus macaque (n=12) | PRVABC59 | 1×103 | SC | 30 | 30 | 2 (16.7) |
| Rhesus macaque (n=8) | PRVABC59 | 1×104 | SC | 45 | 48 | 2 (25) |
| Rhesus macaque (n=6) | PRVABC59 | 1×105 | SC | 49 | 54 | 1 (16.7) |
| Rhesus macaque (n=2) | PRVABC59 | 1×105 | SC | 115 | N/A | 0 |
| Rhesus macaque (n=1) | PRVABC59 | 5×107 | SC | 50 | 50 | 1 (100) |
| Pigtail macaque (n=2) | FSS 13025_Cambodia | 5×107 | SC | 101 | N/A | 0 |
| Pigtail macaque (n=3) | Brazil 2015 Fortaleza | 5×107 | SC | 61 | N/A | 0 |
| Common marmoset (n=2) | Brazil SPH_2015 | 2.5×105 | IM | 76 | 76 | 2 (100) |
IA=intra-amniotic; IV=intravenous; SC=subcutaneous; N/A=not applicable
Overall, fetal death was observed in 13 of 50 animals (26.0%), and early neonatal death in an additional 3 animals (Table 1 and Supplementary Table 1), with frequent fetal loss in animals infected with ZIKV isolated from Brazil and/or Puerto Rico (46.7% and 28.6% respectively). The average rates of fetal demise among comparably-housed healthy, ZIKV-unexposed pregnant macaques ranged from 4%-10.9% (Table 2). The numbers of pregnancies ending with fetal demise (defined here as fetal death in utero, i.e. spontaneous abortion before planned termination, or stillbirth discovered at planned termination) among healthy breeding colony rhesus macaques were collected from the California National Primate Research Center (CNPRC), Oregon National Primate Research Center (ONPRC), and Wisconsin National Primate Research Center (WNPRC) (n=2,823 pregnancies, Table 2). At these three centers, a four-fold increased odds of fetal loss was observed for ZIKV-exposed rhesus macaques (11 losses out of 40 pregnancies) compared with unexposed animals (250 losses out of 2,823 pregnancies) (Chi-squared OR=3.9; 95% CI=1.9-7.9; p<0.0001). Note that the pregnant animals included in this statistic from ONPRC were delivered by cesarean section earlier than animals from other primate centers (typically between GD 135 and 155). This is ∼30 days before the due date and therefore stillbirth that would occur in the last month of pregnancy is missed in ONPRC animals. The mean gestational age at the time of the fetal death was 96 days (range 35-166 days) (Supplementary Table 1). It is striking that more than half of the fetal loss cases occurred after mid-gestion (>GD 83), when the ratesof pregnancy loss that late in human pregnancies are very low at 0.1% (Supplementary Table 1) 5,20.
Table 2. Rates of spontaneous abortion or stillbirth in healthy breeding colony rhesus (CNPRC, ONPRC, WNPRC) and pigtail (WaNPRC) macaques.
| Institution | Breeding season | Spontaneous loss | Total pregnancies | Percent losses (%) |
|---|---|---|---|---|
| WNPRC | 2012 | 20 | 114 | 17.5 |
| 2013 | 11 | 113 | 9.7 | |
| 2014 | 10 | 142 | 7.0 | |
| 2015 | 10 | 103 | 9.7 | |
| 2016 | 12 | 124 | 9.7 | |
| 2017 (partial) | 15 | 117 | 12.8 | |
| Weighted average | 10.9 | |||
| CNPRC | 2008-2009 | 19 | 235 | 8.1 |
| 2009-2010 | 23 | 242 | 9.5 | |
| 2010-2011 | 15 | 210 | 7.14 | |
| 2011-2012 | 23 | 230 | 10.0 | |
| 2012-2013 | 24 | 173 | 13.9 | |
| 2013-2014 | 15 | 165 | 9.1 | |
| 2014-2015 | 18 | 128 | 14.1 | |
| 2015-2016 | 10 | 187 | 5.4 | |
| 2016-2017 (partial) | 11 | 192 | 5.7 | |
| Weighted average | 9.0 | |||
| ONPRC* | 2007 | 6 | 47 | 12.8 |
| 2008 | 3 | 36 | 8.3 | |
| 2009 | 0 | 37 | 0.0 | |
| 2010 | 1 | 18 | 5.6 | |
| 2011 | 1 | 11 | 9.1 | |
| 2012 | 0 | 9 | 0.0 | |
| 2013 | 0 | 6 | 0.0 | |
| 2014 | 0 | 20 | 0.0 | |
| 2015 | 1 | 39 | 2.6 | |
| 2016 | 1 | 75 | 1.3 | |
| 2017 (partial) | 1 | 50 | 2.0 | |
| Weighted average | 4.0 | |||
| WaNPRC | 2014 | 13 | 114 | 11.4 |
| 2015 | 7 | 91 | 7.7 | |
| 2016 | 15 | 122 | 12.3 | |
| 2017 (partial) | 7 | 58 | 12.1 | |
| Weighted average | 10.9 |
Included pregnancies were confirmed >GD 30 and delivered by cesarean section <GD 155, and therefore the risk of very early or late-term abortion in healthy pregnant animals is not captured in these statistics from ONPRC.
Survival analysis was performed to compare outcomes in animals infected under different circumstances. Survival analyses of fetuses exposed to ZIKV isolates originating from Brazil or Puerto Rico were not statistically different based on comparison of Kaplan-Meier curves (Hazard ratio=1.17; 95% CI=0.38-3.61; log-rank test p=0.79) (Figure 1a). On the other hand, timing of infection was one of the most important predictors of fetal demise in these studies. Among the macaques studied, no (0/11) cases of fetal or neonatal death was observed in animals inoculated after GD 55 (approximately during first trimester) with any ZIKV isolate. This was less than the rate of fetal/neonatal demise among macaques inoculated on or before GD 55, which was 37.8% (n=37) (Fisher's exact test p=0.02). Survival analysis (Figure 1b) also showed a significant difference betweensurvival of fetuses whose dams were infected at ≤GD 55 compared to dams infected >GD 55 (log-rank test p=0.03), even though a hazard ratio could not be calculated because there were zero deaths in the >GD 55 group. This parallels human reports of more significant adverse outcomes in babies exposed to ZIKV during the first trimester 1,21.Sex of the fetus did not correlate with fetal demise; sex was evenly distributed in both fetal loss (5 males, 6 females) and survival cases (17 males, 17 females) (Supplementary Table 1).
Figure 1.
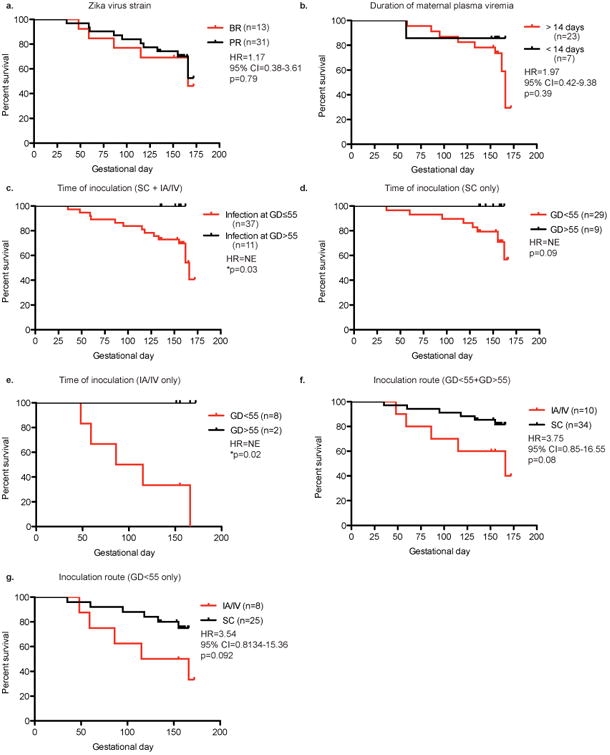
Kaplan-Meier curves comparing survival rates between macaques exposed to Zika virus under different conditions. a. Macaques exposed to Zika virus originating from Brazil (BR) (red) or Puerto Rico (PR) (black). b. Macaques exposed to Zika virus on or before gestational day 55 (red) or after gestational day 55 (black). NE-not estimable due to zero events in one group. c. Macaques exposed to Zika virus with a duration of maternal plasma viremia less than or equal to 14 days post-infection (black) or duration of viremia greater than 14 days post-infection (red) (WNPRC and CNPRC only). d. Macaques exposed to Zika virus by intraamniotic (IA) and intravenous injection (IV) (red) or subcutaneous injection (SC) (black). Hazard ratio (HR) and p-value based on log-rank test are reported.
All but one animal challenged with ZIKV had detectable plasma viremia after infection (Supplementary Table 1). Maternal plasma viremia was detectable in many pregnant animals beyond the typical 10-14 days seen in non-pregnant macaques (Supplementary Table 1) 10,14,22. In animals from WNPRC and CNPRC, survival of fetuses or neonates was marginally, but not significantly, better if the duration of viremia was ≤14 days than if the duration of maternal viremia was >14 days (Figure 1c) (HR=1.97, 95% CI=0.42-9.38, log-rank test p=0.39). Lastly, route of infection was compared between subcutaneous (SC) inoculation and intra-amniotic/intravenous (IA/IV) inoculation in macaques (Figure 1d). While the hazard ratio of IA/IV inoculation was 3.75 (95% CI=0.85-16.55; log-rank test p=0.08) the difference in survival among animals infected IA/IV and SC was not statistically significant.
At the time of cesarean section, comprehensive necropsies were performed on maternal reproductive/fetal extraembryonic (MR/FE) tissues and fetal tissues. All fetal and neonatal loss cases tested at the time of this writing had detectable ZIKV RNA in the MR/FE tissues and/or in multiple fetal tissues (Supplementary Table 2). All but one fetal and neonatal loss cases with available data also showed evidence of inflammation in one or more MR/FE tissues with other pathologies including frequent placentitis, trophoblastic necrosis and infarction in the placenta and villi as described in Supplementary Table 3. It should be noted that necrosis may be the result of fetal death hours or days prior to tissue collection and may not be part of the etiology caused directly by ZIKV infection. While many of the fetal tissues were autolyzed due to death of the fetus hours or days prior to cesarean section, tissues that could be assessed for histopathology revealed major ocular pathologies (i.e. choroidal coloboma, retinal dysplasia, hemorrhage in retina), lung pathologies (i.e. neutrophil, lymphocyte, and erythrocyte infiltration, amniotic fluid aspiration), and, in one animal, a mild ventricular hemorrhage and mild loss of ependyma in the lateral ventricle of the brain (Supplementary Table 3). Two fetal demise cases were associated with preterm premature rupture of membranes (PPROM) and one case of neonatal death was associated with preterm cervical dilation (Supplementary Table 1). Altogether, this data provides evidence that ZIKV was present at the MR/FE tissues and in many of the fetuses with adverse outcomes. Fetal demise cases also have evidence of histopathology in the MR/FE tissues that may have contributed to their demise, though histopathology does not necessarily mean dysfunction. A recently published paper characterized placental dysfunction following ZIKV infection in rhesus macaques 17, and while this has not been found in the few human case reports with extensive placental characterization 23, that study along with the histopathology shown here suggests that the role of placental dysfunction in ZIKV-related fetal loss should be carefully studied.
To monitor the health and growth of the fetuses, many animals underwent weekly ultrasounds. Of animals with complete analysis of ultrasound data (Supplementary Table 3), the most common finding was increased placental calcification. One animal showed a slightly lower biparietal diameter and head circumference measurement (Supplementary Table 3), however few other significant ultrasound findings shedding light on fetal loss were observed by the ultrasound imaging.
Fetal death also occurred in common marmosets. The average rate of late term (88-142 days of gestation) fetal loss in healthy marmosets has been reported to be 4.4% (26 of 596)24, whereas 2 of 2 ZIKV-infected marmoset pregnancies ended in fetal demise. In these cases, ZIKV RNA was detectable in both MR/FE and fetal tissues (Supplementary Table 2). Minimal histopathology was noted in the MR/FE or fetal tissues, but one fetus showed evidence of disorganization of the cortical neurons which may signal a disruption of development and neuronal migration patterns of the cerebral cortex (Supplementary Table 3) 18.
A limitation of our study is the modest number of experiments associated with each species and experimental condition. In addition, given the urgency of understanding ZIKV impact during pregnancy in a relevant animal model and the need to identify both common and rare outcomes, resources were devoted to infecting pregnant macaques and using historical controls rather than mock-infecting pregnant animals. However, mock-infected animals are critical and it is important to note that 9/9 mock infected pregnant rhesus macaques (3 from ONPRC, 3 from CNPRC, and 3 from WaNPRC) did not show any adverse fetal outcomes, suggesting that experimental procedures, (i.e., frequent blood draws under sedation, weekly ultrasound imaging; regular amniocentesis) or the stress associated with them, were not sufficient to lead to the fetal loss rate seen in the ZIKV-exposed animals (Supplementary Table 1). As shown in Supplementary Table 1, the frequency of blood draws and ultrasounds for the mock-infected animals was comparable or identical to the frequencies of procedures used in fetal loss cases, suggesting that factors beyond the experimental procedures are contributing to increased rates of fetal demise. Amniocentesis was performed in some studies to detect infection in the fetal compartment (Supplementary Table 1). Although amniocentesis is known to carry a small risk for fetal loss (miscarriage or stillbirth), fetal loss occurred in animals that did (7/29=24.1%) and did not receive amniocentesis (6/21=28.6%) with no statistical difference in the number of fetal losses between these two groups (Fisher's exact test; p=0.75) (Supplementary Table 1). Of animals with amniocentesis, animals that survived to term had more weekly amniocentesis (ave.= 11, range 1-18, n=22) than those that ended with a fetal demise (ave.=6, range 2-18, n=7), further suggesting that the amniocentesis procedure did not contribute to the observed increased fetal demise.
The reported 6% rate of stillbirth in the Rio de Janeiro cohort of women with symptomatic ZIKV infection is among the highest known for any teratogenic virus. The actual incidence of miscarriage and stillbirth in women infected with ZIKV during pregnancy is unknown and likely dependent upon a number of factors, including gestational age at time of infection, inoculum, viral strain, maternal age and other non-ZIKV coinfections/comorbidities 1. The high rates of fetal loss among ZIKV infected nonhuman primate pregnancies reported here (26%) raises concern that ZIKV-associated pregnancy loss in humans may be more frequent than currently thought. In nonhuman primates, there was evidence of vertical transmission in all fetal demise cases including vRNA and/or histopathology found in fetal and MR/FE tissues. Importantly, fetal demise was more common in animals infected during the first trimester and in animals with a longer duration of detectable maternal plasma viremia. That fetal demise occurred across multiple centers, multiple ZIKV isolates, and multiple ZIKV doses, suggests that the observation of fetal loss is robust and that ZIKV may contribute to fetal loss under a variety of conditions seen in nature.
These results, along with recent reports of increased fetal loss in human studies and reduced live birth rates in Brazil, suggests that careful monitoring of fetal loss and stillbirth are warranted. Specifically, nucleic acid testing of samples from pregnancy loss in women from ZIKV-endemic areas, those who may have had travel-related or sexual exposure, or those enrolled in ongoing cohort studies looking at Congenital Zika Syndrome, is an important step to understanding this important outcome.
Methods
Care and use of nonhuman primates
All macaque monkeys used in this study were cared for by staff at their respective primate centers in accordance with the regulations and guidelines outlined in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Each study received approval by their institution's Animal Care and Use Committee:
WNPRC
University of Wisconsin-Madison College of Letters and Sciences and Vice Chancellor for Research and Graduate Education CentersInstitutional Animal Care and Use Committee (Animal Care and Use Protocol Numbers G005401 and G005246).
CNPRC
University of California-Davis IACUC protocol numbers 19211 and 19695.
ONPRC
Oregon National Primate Research Center Institutional Animal Care and Use Committee (IACUC #0887-05 and #0300).
TNPRC
Tulane National Primate Research Center Institutional Animal Care and Use Committee (IACUC #P0336)
WaNPRC
University of Washington Institutional Animal Care and Use Committee (4165-02 and 4202-02).
SNPRC
Texas Biomedical Research Institute Animal Care and Use Committee (protocol 1528CJ)
Additional details on the animals in this study are summarized in Supplementary Table 1.
Zika virus infections
WNPRC
ZIKV strain H/PF/2013 (GenBank: KJ776791), originally isolated from a 51-year-old female in France returning from French Polynesia with a single round of amplification on Vero cells, was obtained from Xavier de Lamballerie (European Virus Archive, Marseille France). ZIKV strain PRVABC59 (ZIKV-PR; GenBank:KU501215), originally isolated from a traveler to Puerto Rico with three rounds of amplification on Vero cells, was obtained from Brandy Russell (CDC, Ft. Collins, CO). For each inoculation, the stock was thawed, diluted in PBS to the appropriate concentration for each challenge, and loaded into a 1 mL syringe that was kept on ice until challenge. 1 mL of inocula was administered subcutaneously over the cranial dorsum. At the conclusion of the procedure, animals were closely monitored by veterinary and animal care staff for adverse reactions and signs of disease.
CNPRC
ZIKV strain Zika virus/H.sapiens-tc/BRA/2015/Brazil_SPH2015 was isolated from Brazil in 2015; (GenBank accession number KU321639.1), as described earlier 22. ZIKV strain PRVABC59 was obtained from WNPRC. Aliquots were stored in liquid nitrogen thawed right before each inoculation procedure. The inoculum was adjusted to the proper PFU with RPMI1640 medium to 0.5 to 1 ml of final volume for administration to the pregnant dam by IA, IV or SC route. For animals that received both IA and IV, the PFU value listed in Table 1 represents the total dose, divided in half for each route; when viral mixtures were used, equal amounts of each virus (in PFU) were used.
ONPRC
Viral preparation and inoculation was the same as previously described 14,14: ZIKV PRVABC59 was obtained from the CDC, and passaged twice in C6/36 cells (American Type Culture Collection, ATCC). Infected C6/36 tissue culture supernatant was concentrated through a 20% sorbitol cushion and titrated in Vero cells (ATCC) through a focus-formation assay. Animals were subcutaneously inoculated in the hand, wrist and forearm. Each animal received a total inoculum of 1×105 PFU diluted in PBS to a volume of 1 mL.
TNPRC
ZIKV PRVABC59 was obtained from WNPRC and was prepared as described above. All animals were challenged via subcutaneous inoculation with 10,000 PFU of virus.
WaNPRC
We used the following isolates: ZIKV strain isolated in Cambodia (FSS13025, 2010, GenBank Accession Number: KU955593) and ZIKV strain isolated in Fortaleza Brazil (Brazil 2015 (Fortaleza), GenBank Accession Number: KX811222. ZIKV was inoculated subcutaneously at five separate locations on the forearms, each with 107 plaque-forming units (PFU).
SNPRC
Pregnant marmoset dams were infected intramuscularly with 2 inoculums of ZIKV strain Zika virus/H.sapiens-tc/BRA/2015/Brazil_SPH2015 (GenBank accession number KU321639.1) (Cunha, 2016, Genome Announcements), each inoculum containing 2.5×105 PFU of P3 (passage 3 in Vero cells) virus diluted in PBS to a volume of 1 mL. The 2 inoculums were administered on gestational days 79 and 83 for dam 1 (human equivalent = 14 weeks) or days 68 and 72 days for dam 2 (human equivalent=9 weeks). The P3 viral inoculum was deep sequenced to >100X coverage on an Illumina MiSeq instrument and the assembled consensus sequence was identical to the reference sequence in GenBank.
Details on Zika virus infections are also summarized in Supplementary Table 1.
Statistics
Baseline fetal loss data was collected from multiple primate centers based on time-mated breeding colony data. Chi-squared and Fisher's Exact Test statistics with additional odds ratios for fetal loss, as well as Kaplan-Meier curves with hazard ratios and log-rank tests to compare survival curves were calculated using Graphpad Prism 5 software for Mac OS X (Version 5.0a), Graphpad Software, La Jolla California USA and reported along with the corresponding 95% confidence intervals. All reported P-values are two-sided and P<0.05 was used to define statistical significance.
Data availability
The authors declare that all data supporting the findings in this study are available within the article or from the corresponding authors upon request.
Supplementary Material
Acknowledgments
We thank the Veterinary Services, Colony Management, Scientific Protocol Implementation, and the Pathology Services staff at the Wisconsin National Primate Research Center (WNPRC) for their contribution to this study. We thank Michelle Harke, Kevin Brunner, Tammie Frost, Michele Schotzko, Wendy Newton, Kathleen Antony, Sarah Kohn, Heather Simmons, Andres Mejia, Christina Newman, Laurel Stewart, Michelle Koenig, Meghan Breitbach, and Mariel Mohns for technical assistance with monitoring the ZIKV-infected pregnant animals at WNPRC. We thank Jens Eickhoff for help with statistical analysis. Studies at WNPRC were supported by DHHS/PHS/NIH R01Al116382-01A1 to D.H.O. We thank Esper Kallas for providing information on 2016 live birth rates in Brazil. This work was also supported in part by the Office of Research Infrastructure Programs/OD (P51OD011106) awarded to the WNPRC, Madison-Wisconsin. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grants RR15459-01 and RR020141-01. The studies at CNPRC were supported by a CNPRC pilot research grant, 1R21AI129479-01, the Office of Research Infrastructure Programs/OD (P51OD011107), FDA contract #HHSF223201610542P and start-up funds from the Pathology, Microbiology and Immunology Department to L. Coffey. We thank V. Bakula, I. Cazares, M. Christensen, C. Cruzen, A. Gibbons, D. Lemos, A. Singapuri, J. Usachenko, W. von Morgenland, J. Watanabe, J. Yee and the staff of Clinical Labs, Veterinary and Colony Research Services for technical assistance. Work at the Oregon National Primate Research Center (ONPRC) was supported by the ONPRC P51OD011092 NIH Core Grant and R21HD091032 (D.N.S.). We thank Lauren Drew Martin, Brandy Dozier and Nikki Sternberger for their veterinary expertise, and the dedicated staff in the ONPRC Department of Comparative Medicine for assistance in animal care. The studies at the TNPRC were supported by NIH grant R01 AI099795-05 and by theP51OD011104NIH Core Grant. Thanks to Faith Schiroand Robert Blair for technical assistance. At the Washington National Primate Research Center (WaNPRC), the work was primarily supported by generous private philanthropic gifts including five donors from Florida, who wish to remain anonymous. Further support was obtained from the University of Washington Department of Obstetrics & Gynecology, Seattle Children's Research Institute and the National Institutes of Health, Grant # R01AI100989 (L.R. and K. A. W), AI083019 (M.G. Jr.), and AI104002 (M.G. Jr.). The National Institutes of Health Office of Research Infrastructure Programs (P51 OD010425) also supported this project. We thank Jason Ogle, Wonsok Lee and Jason Thiel for technical assistance with monitoring the animals. Work at SNPRC was supported by NIH grants R01HL105704 and R21AI129455 to C.Y.C. andSNPRC grant P51 ODOD011133 and by the Texas Biomedical Research Institute. We would like to thank Donna Layne-Colon for marmoset pregnancy management, ultrasonography, and collection and processing of placental and fetal material. We would also like to thank Stephanie Davis for technical support and Melissa Suter for guidance of the placental studies and analysis.
Footnotes
Author Contributions: D.M.D, D.H.O., T.C.F., E.L.M., K.K.V.R., L.L.C, P.L.G., D.N.S, A.J.H., J.L.P, S.T., M.S., K.M.A., C.Y.C., X.W., R.S.V., K.M.A.W., M.G.Jr., L.R., N.J.M., A.T.P., M.H.G., and R.P.B., designed experiments. D.M.D., K.K.V.R., L.L.C, A.A., P.L.G., R.J.S., D.N.S., A.J.H., J.L.P., M.T., M.S., K.M.A., C.S-S.M., C.Y.C., X.W., K.M.A.W., M.G.Jr., L.R., R.S.V., N.J.M., and A.T.P., analyzed data. D.M.D., E.L.M., T.G.G., T.C.F., S.V.C., K.K.V.R., L.L.C., R.J.S., M.S., S.T., K.M.A., C.Y.C., K.M.A.W., M.G.Jr., and A.T.P., drafted the manuscript. S.V.C., R.P.M., T.H., M.H.G., and R.P.B. are part of the veterinary staff who monitored the nonhuman primates. S.V.C., T.H., and C.E.H. collected data from breeding colony records. R.I.K. provided pathology assistance. E.L.M., A.A., and R.J.S. managed projects. The publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Competing Financial interest statement: Competing financial interests: The authors declare no competing financial interests.
References
- 1.Brasil P, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Campo M, et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet A. 2017;173:841–857. doi: 10.1002/ajmg.a.38170. [DOI] [PubMed] [Google Scholar]
- 3.Schaub B, et al. Late miscarriage: another Zika concern. Eur J Obstet Gynecol Reprod Biol. 2016;207:240–241. doi: 10.1016/j.ejogrb.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Lawn JE, et al. Stillbirths: Where? When? Why? How to make the data count. Lancet. 2011;377:1448–1463. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox AJ, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 6.van der Eijk AA, et al. Miscarriage Associated with Zika Virus Infection. N Engl J Med. 2016;375:1002–1004. doi: 10.1056/NEJMc1605898. [DOI] [PubMed] [Google Scholar]
- 7.Esposito DL, et al. Abortion rate is much higher than microcephaly rate in zika virus infections occurring during the first trimester of pregnancy. American Society of Tropical Medicine and Hygiene. 2017 [Google Scholar]
- 8.Alvarenga D. Number of births in Brazil falls for the first time since 2010, says IBGE. 2017 [Google Scholar]
- 9.Carter AM, Pijnenborg R. Evolution of invasive placentation with special reference to non-human primates. Best Pract Res Clin Obstet Gynaecol. 2011;25:249–257. doi: 10.1016/j.bpobgyn.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Dudley DM, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen SM, et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13:e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams Waldorf KM, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu CY, et al. Experimental Zika virus inoculation in a New World monkey model reproduces key features of the human infection. Scientific Reports. 2017 doi: 10.1038/s41598-017-17067-w. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch AJ, et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13:e1006219. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldorf KMA, et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med. 2018 doi: 10.1038/nm.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr EL, et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS One. 2018;13:e0190617. doi: 10.1371/journal.pone.0190617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch AJ, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun. 2018;9:263. doi: 10.1038/s41467-017-02499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seferovic M, S-S MC, Tardif SD, Rutherford J, Castro ECC, Li T, Hodara VL, Parodi LM, Giavedoni L, Layne-Colon D, Tamhankar M, Yagi S, Martyn C, Reyes K, Suter M, Aagaard KM, Chiu CY, Patterson JL. Experimental Zika Virus Infection in the Pregnant Common Marmoset Induces Spontaneous Fetal Loss and Neurodevelopmental Abnormalities. BioRxiv preprint server. 2018 doi: 10.1038/s41598-018-25205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan L, et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science. 2017;358:933–936. doi: 10.1126/science.aam7120. [DOI] [PubMed] [Google Scholar]
- 20.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 21.van der Linden V, et al. Description of 13 Infants Born During October 2015 - January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth - Brazil. MMWR Morb Mortal Wkly Rep. 2016;65:1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 22.Coffey LL, et al. Zika Virus Tissue and Blood Compartmentalization in Acute Infection of Rhesus Macaques. PLoS One. 2017;12:e0171148. doi: 10.1371/journal.pone.0171148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch Pathol Lab Med. 2017;141:43–48. doi: 10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- 24.Heger W, Merker HJ, Neubert D. Non-Human Primates-Developmental Biology and Toxicology. Ueberreuter Wissenshaft; Berlin: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings in this study are available within the article or from the corresponding authors upon request.


