Abstract
The advent of human pluripotent stem cells (hPSCs) has benefited many fields, from regenerative medicine to disease modeling, with an especially profound effect in cardiac research. Coupled with other novel technologies in genome engineering, hPSCs offer a great opportunity to delineate human cardiac lineages, investigate inherited cardiovascular diseases, and assess the safety and efficacy of cell-based therapies. In this review, we provide an overview of methods for generating genetically engineered hPSC reporters and a succinct synopsis of a variety of hPSC reporters, with a particular focus on their applications in cardiac stem cell biology.
Graphical abstract
Introduction
From the first successful isolation of human embryonic stem cells (hESCs) two decades ago to the more recent breakthrough in generation of patient-specific induced pluripotent stem cells (iPSCs), human pluripotent stem cells (hPSCs) have revolutionized the ways in which scientists study developmental biology, investigate disease mechanisms, and explore new drugs in many fields [1–4]. In particular, scientists have made significant progress in cardiovascular research by developing de novo heart tissue as means of achieving cardiac repair [5] and understanding causes of genetic cardiac diseases using the model of “heart in a dish” [6]. Additionally, the advent of hPSCs has ushered into an exciting new era for drug discovery and safety testing [7]. These broad applications of hPSCs in the cardiovascular field have been further enhanced by the burgeoning field of genetic engineering. With the aid of efficient and precise gene targeting, genes can be readily modified to study their physiological functions and monitor their expression in hPSC-derived progeny. Hence, the marriage of hPSC biology and genetic engineering technology is enabling cardiovascular investigators to better understand the complex mechanisms of cardiac diseases and develop potential cell-based therapies. In this review, we aim to briefly summarize the genome-editing techniques commonly used for generating hPSC reporters (Table 1). Furthermore, we highlight the currently available hPSC reporters in the ever-growing tool chest and draw particular attention to the utilization of these tools to address biological questions (Figure 1).
Table 1.
Summary of methods for genetic engineering and typical examples of application in cardiac field.
| Targeting methods | Overall Advantages | Overall Disadvantages | Applications | |
|---|---|---|---|---|
| Random integration | Bacterial artificial chromosome (BAC) |
|
||
| Lentivirus | Rapid and easy operation | |||
| Transposons | ||||
| Plasmids | ||||
|
|
||||
| Targeted integration | Zinc-finger nucleases (ZFNs) |
|
|
|
| Transcription activator-like effector nucleases (TALENs) | ||||
| Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 | ||||
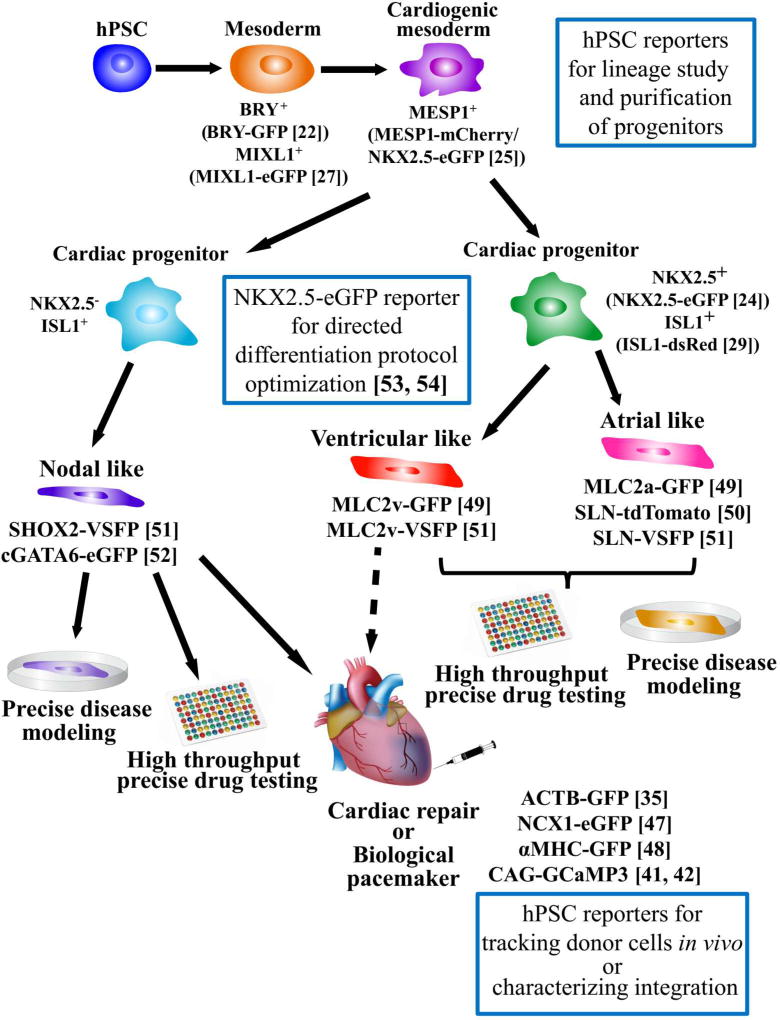
Figure 1. Schematic summary of applications of genetically engineered hPSC reporters in the cardiac research.
A variety of hPSC reporters have been generated either using virus-mediated or homologous recombination-mediated methods. These hPSC reporters have been used for delineation of human cardiac lineages in vitro, development of directed differentiation protocol for myocyte subtypes, tracking of engrafted hPSC-CMs, and precise disease modeling and drug testing. Dashed arrow indicates the potential application. hPSC: human pluripotent stem cell; BRY: Brachyury; MIXL1: Mix Paired-Like Homeobox; MESP1: Mesoderm Posterior BHLH Transcription Factor 1; NKX2.5: NK2 Homeobox 5; ISL1: ISL LIM Homeobox 1; SHOX2: Short Stature Homeobox 2; cGATA6: chicken GATA Binding Protein 6; MLC2a: Myosin Light Chain 2 atrial isoform; MLC2v: Myosin Light Chain 2 ventricular isoform; SLN: Sarcolipin; ACTB: Actin Beta; NCX: Na+/Ca2+ Exchanger; αMHC: α-Myosin Heavy Chain; GFP: green fluorescent protein; eGFP: enhanced green fluorescent protein; VSFP: voltage sensitive fluorescent protein; GCaMP3: GFP-based Ca2+ indicator.
Methods of generating genetically engineered hPSC reporters
Direct manipulation of an organism’s genes to generate transgenic animal models is an established approach to understand gene function in specific tissues. Off-the-shelf or custom-engineered gene targeting technologies are also emerging as powerful tools to generate reporter lines or correct mutations. For a detailed discussion on genome-editing in hPSCs, we refer readers to several excellent review articles [8–10]. Here, we briefly summarize the commonly used tools for generating genetically engineered hPSC reporters (Table 1).
Both random and targeted gene integration methods can be used to generate hPSC reporter lines. A reporter gene (e.g., fluorescent protein, luciferase, or antibiotic resistance gene) driven by the promoter of a gene of interest can be delivered into the hPSC genome by Bacterial artificial chromosome (BAC), lentivirus, transposons or plasmids. However, integration events are random, and the reporter may not faithfully reflect endogenous activation of the relevant gene. Moreover, the expression of inserted reporter genes can be affected by nearby gene elements or vice versa. Therefore, an effective approach to precisely insert reporter genes into the desired genomic loci is needed.
Homologous recombination-mediated gene integration allows reporter genes to share the same epigenetic conditions and chromosomal geometry with the endogenous gene of interest, allowing it to more faithfully reflect the activity of the target gene promoter. To facilitate homologous recombination, gene targeting constructs usually comprise 5’ and 3’ homology arms flanking the reporter gene and selection markers. Recombination is facilitated by introducing FLP/FRT or Cre/loxP sites into the DNA target site [11]. However, there is always a short FRT or loxP sequence left in the targeted genome, which is not “footprint” free. This limitation can be overcome by the piggyBac transposon system, which allows rapid, precise and seamless directed gene editing [12]. It should be noted that the homologous recombination is inherently inefficient. Therefore, to enhance its frequency, a double-strand break at the DNA target site is usually introduced, mediated by engineered nucleases including zinc-finger nucleases (ZFNs) [13], transcription activator-like effector nucleases (TALENs) [14], and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology [15]. CRISPR/Cas9, considered as the most efficient genome-editing approach thus far, is becoming a popular tool to generate hPSC reporter lines [16,17], as it only requires a single guide RNA and a donor vector containing the reporter gene sequence.
In summary, it is possible to insert reporter genes into any locus of the genome with innovative genome-editing tools, but several aspects of genome-editing must be considered. Extensive analysis is necessary to scrutinize the potential risk of off-target effects for nuclease, potential mutagenesis near the target site, and abnormality of karyotyping.
Applications of hPSC reporters
1. Study of cardiac lineages
In vivo heart development (cardiogenesis) is orchestrated by an intricate network of transcription factors that tightly regulate proliferation, differentiation and migration of specialized cells derived from multiple lineages. Cardiac developmental biologists have garnered comprehensive knowledge of cardiogenesis using lineage tracing approaches, particularly transgenic animal models bearing LacZ or fluorescent reporters at desired genetic loci [18]. Additionally, In vitro differentiation of hPSCs can recapitulate many cellular aspects of cardiogenesis to reveal genetic networks and signalling pathways involved in cell lineage determination and terminal differentiation [19]. Despite various cardiac differentiation approaches have been developed [20], generation of hPSC-derived cardiomyocytes (hPSC-CMs) has common sequential stages: mesoderm induction and subsequent cardiac mesoderm determination, followed by cardiac lineage specification and terminal differentiation. This developmental hierarchy of cardiac lineages is established by a set of stage-specific biomarkers (see recent review [21]). Moreover, several fluorescent hPSC reporter lines have also been generated to identify, purify, and characterize these specialized cells during this multistep cardiac differentiation process (Figure 1).
Upon mesoderm induction, hPSC-derived cells enter a transitory state, in which a multipotent population is marked by the expression of TBX transcription factor Brachyury/T (BRY), Mix Paired-Like Homeobox (MIXL1) or Kinase Insert Domain Receptor (KDR). Tracing cardiogenic lineages back to this intermediate population has led to the concept that similar to hematopoietic lineages, different cardiovascular cells such as cardiomyocytes, endothelial cells, and vascular smooth muscle cells are derived from a common cardiovascular progenitor population. Indeed, in vitro differentiation of hPSCs has demonstrated that BRY-GFP+ cells have cardiac potential [22], and KDR+ (low expression) cardiovascular progenitors hold the capacity to differentiate into cardiomyocytes, endothelial cells, and vascular smooth muscle cells [23]. It should also be noted that the BRY+/KDR+ (high expression) population possesses hematopoietic potential [22], suggesting that early lineage diversification is associated with the expression level of key players in the mesoderm specification. As cardiac development is a dynamic process, it is not surprising that gene expression levels display dynamic patterns to determine the cell fate. As demonstrated using a NK2 Homeobox 5 (NKX2.5, a cardiac lineage marker)-eGFP hESC reporter line, a purified eGFP+ population can further differentiate into eGFP+ cardiomyocytes and eGFP− endothelial and smooth muscle cells [24]. More recently, by tagging mCherry to a cardiac mesoderm marker, Mesoderm Posterior BHLH Transcription Factor 1 (MESP1), a MESP1mCherry/w/NKX2.5eGFP/w hESC dual reporter line was generated, which demonstrated a transient expression of MESP1-mCherry followed by the NKX2.5-eGFP expression [25]. This MESP1-mCherry+ population is defined by a distinct set of biomarkers that include PDGFRα, CD13, ROR2, and LGR4 [25,26]. Using a different hPSC reporter line of Mix Paired-Like Homeobox (MIXL1)-eGFP, Skelton et al. further corroborated that CD13 and ROR2 can be used to isolate cardiac mesoderm progenitors [27].
During cardiogenesis, commitment of nascent cardiac mesoderm progenitors towards a more specified lineage fate involves lineage segregation: one group of cardiac progenitor cells (CPCs) known as the first heart field (FHF), contributes to the left ventricle and atria, whereas a second pool of CPCs known as the second heart field (SHF) develops into right ventricle, outflow tract and atria [28]. In human fetal hearts at 11 and 18 weeks of gestation, ISL LIM Homeobox 1 (ISL1) positive cells are found in a pattern that matches the SHF-derived structure in murine fetal hearts, suggesting that ISL1 is a potential marker for the SHF [29]. Isolated ISL1+ cells using a ISL1-DsRed hPSC reporter are multipotent and can differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells [29]. More recently, a study reported that using the NKX2.5eGFP/w hESC reporter line with regulated expression of MYC (a transcription factor that plays a role in cell cycle progression), expandable hPSC-derived CPCs were generated for the first time [30]. The authors further demonstrated that by modulating FGF and BMP4 signalling pathways, these expandable hPSC-derived CPCs proceeded to the early heart field development stage characterized by unique gene expression signatures [30].
In summary, although lineage tracing mice have been widely used for understanding cardiogenesis, there are significant differences in the heart between human and mice, making it impossible for animal models to fully recapitulate the developmental process of the human heart. As alternatives, a variety of fluorescent hPSC reporter lines have been created and validated as tools more capable of faithfully recapitulating human cardiac lineages.
2. Application in cell transplantation
Because human adult cardiomyocytes lack a robust capacity to proliferate, the initial cardiomyocyte death associated with myocardial infarction can trigger cardiac remodeling and eventually lead to scarring of the heart. There has long been an urgent need to develop an effective cell-based therapy to replace these dead cardiomyocytes; to that end, hPSCs have emerged as an appealing source for cell replacement therapies for myocardial infarction. The potential and challenges of using hPSC-derived cells for cardiac repair are thoroughly reviewed elsewhere [31–33]. In this section, we focus on the application of hPSC reporters in pre-clinical studies.
To evaluate the efficacy of cell therapy, it is critical to monitor cell survival, proliferation, migration and functional integration into the host myocardium. Genetically encoded fluorescent reporters have been commonly used for long-term tracking of the grafted hPSC-CMs in vivo [34–36]. Stable expression of fluorescent reporter genes has advantages over transient cell labelling with fluorescent probes, because factors such as labelling efficiency, cell toxicity, and cell division-induced dilution effect may affect the interpretation of results [37]. Interestingly, despite the low survival rate of transplanted cells, some recent studies have reported that engrafted hPSC-CMs can partially remuscularize the infarcted areas and improve myocardial function in small animal models [38–41]. Furthermore, using a genetically encoded Ca2+ indicator, GCaMP3, two reports showed that donor hPSC-CMs can electrically couple with host myocardium in both small animals [41] and non-human primates [42]. However, in contrast to small animals, these primates experienced ventricular arrhythmias and the cardiac function showed no significant improvement after transplantation. Taken together, fluorescent reporter lines either expressing a fluorescent protein or a Ca2+ indicator have shown promise in tracking hPSC-CMs and investigating functional integration of engrafted cells after transplantation. However, significant hurdles remain before these approaches can move forward toward clinical trials. One limiting factor in previous studies is the use of a heterogeneous pool of hPSC-CMs, which carries the potential risk of arrhythmias and compromises the engraftment efficiency. Therefore, transplantation of lineage-specific cardiomyocytes or ventricular cardiomyocytes purified with genetically engineered hPSC reporter lines is currently being investigated by several groups.
3. Application in purification of myocyte subtypes, disease modeling, and drug screening
Over the past few years, in parallel with development of robust protocols to efficiently differentiate hPSC-CMs (see review [20]), transgenic approaches have also been used to enrich cardiomyocytes [43–48]. However, the resulting yield is still a heterogeneous pool of nodal, atrial, and ventricular subtypes, which hinders precise disease modeling and drug testing. To overcome this hurdle, sarcolipin (SLN), atrial myosin light chain 2 (MLC-2a), and ventricular myosin light chain 2 (MLC-2v) are increasingly being used as chamber-specific markers to drive the expression of fluorescent reporter genes [44,49–51], allowing identification and purification of chamber-specific subtypes. Additionally, using a cGATA6-eGFP lentiviral vector, Zhu and colleagues were able to identify 95% of eGFP+ cells with pacemaker action potential (AP) morphology, suggesting that activation of GATA6 is necessary for differentiating nodal cells [52]. Moreover, by taking advantage of NKX2.5eGFP/w hESCs, a directed differentiation protocol to generate nodal cells was developed [53]. These hPSC-derived nodal cells not only display the genetic signature and functional features of pacemaker cells, but also have the capacity to function as biological pacemakers when transplanted into rat hearts. More recently, the same group used NKX2.5eGFP/w hESCs to identify ventricular and atrial lineages marked by CD235a and RALDH2, respectively, which diverged during the mesoderm developmental stage [54]. These studies demonstrate the broad applications of genetically engineered hPSC reporters, which not only facilitate optimization of differentiation protocols, but also help to identify novel biomarkers for cardiac lineages.
Subtype phenotyping of hPSC-CMs heavily relies on the use of single cell patch-clamp. Despite being the “gold standard”, the low throughput and invasive nature of this technique has limited its application in areas such as drug screening. Sinnecker’s group constructed a series of lentiviral vectors encoding voltage-sensitive fluorescent protein (VSFP) driven by the promoters of Short Stature Homeobox 2 (SHOX2; essential for directing sinoatrial node differentiation [55]), SLN, and MLC-2v to identify nodal, atrial and ventricular subtypes, respectively [51]. When hiPSC-CMs from a long QT patient were transfected with these vectors, only atrial and ventricular, but not nodal cells, exhibited the prolonged AP duration by optical recording. However, when cardiomyocytes were subjected to ivabradine, a “funny” current inhibitor, only nodal cells responded. This study demonstrates the feasibility of precise disease modeling and drug screening using optical imaging in genetically modified hiPSC-CMs. Indeed, with the significant advances in the field of optogenetics, the concept of “all-optical electrophysiology” for high throughput drug screening in the cardiac field is emerging [56–59]. In combination with hiPSCs, all-optical imaging is a promising approach to address the Comprehensive in vitro Proarrhythmia Assay (CiPA) mandate [60].
Conclusions and future perspectives
Genetically engineered hPSC reporters are a powerful tool with far-reaching applications in cardiovascular research and medicine. In this review, we summarized recent advances in studying cardiac lineages, cardiac regenerative medicine, precise disease modeling, and drug testing using hPSC reporters. Cardiogenesis is a complex process in which multiple specialized cells interact and migrate, leading to a 3D structure. However, the current lineage study model using hPSC reporters is predominantly based on a 2D monolayer, which does not fully represent cardiogenesis in vivo. Therefore, it is critical to build a more complex 3D organoid heart using hPSC reporters to gain a deeper understanding of cardiogenesis. In addition, multi-color reporter constructs and mosaic analysis, which have shed light on the complex development of the intestine and cerebellar cortex [61], may be useful in future studies of cardiogenesis. Multi-color hPSC reporters are expected to be available in the near future to monitor the activation state of intracellular signalling pathways, cell turnover, and motility of different cell types derived either from the same or different lineages. Furthermore, with recent advances in microfluidics, single-cell analysis paired with hPSC reporters will be able to reveal cell heterogeneity and build a precise roadmap of lineage hierarchy in cardiogenesis.
A better understanding of human cardiac lineages is also essential for identifying and isolating suitable hPSC-CPCs for regenerative medicine. Transplantation of hPSC-CPCs may have advantages over hPSC-CMs, as CPCs are multipotent and able to differentiate into all types of cardiac cells, which is essential for maintaining proper function of engraftments. Moreover, using functional reporters such as genetically encoded Ca2+ indicators or voltage sensors would not only allow the donor cells to be tracked, but may also improve our ability to study electrical coupling between donor and host cells. To these ends, optical sensors with a favourable combination of brightness, dynamic range, and speed are necessary.
A central goal of Precision Medicine Initiative is to make precise disease modeling and drug testing a reality. Recently, hPSC reporters have played an important role in developing directed differentiation protocols that make scalable production of hPSC-derived myocyte subtypes possible, taking us closer to precise disease modeling and drug testing. The future of applying hPSC reporters in different disciplines of biomedical research is bright. Combined with other novel technologies (e.g., single cell analysis), hPSC reporters will have even broader applications and contribute to our growing insights into the mysteries and complexities of cardiac stem cell biology.
Human pluripotent stem cells (hPSCs) revolutionize cardiac research.
HPSC reporters are commonly generated by lentivirus and homologous recombination.
A variety of hPSC reporters are used to delineate cardiac lineages in vitro.
HPSC reporters facilitate to track engraftments in animals.
HPSC reporters provide a platform for precise disease modeling and drug screening.
Acknowledgments
The authors gratefully acknowledge Blake Wu for critical reading of the manuscript. This publication was supported in part by research grants from the National Institutes of Health (NIH) R01 HL128170, R01 HL123968, NIH R01 HL126527, and California Institute of Regenerative Medicine RT3-07798, TRAN4-09884 and DR2A-05394 (J.C.W.). Because of space constraints, the authors apologize in advance for not including all of the relevant citations on the subject matter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016;17:170–182. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- 2••.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. This review provides a comprehensive view of the applications of hiPSCs in different fields. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12:656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Nelson TJ, Martinez-Fernandez A, Terzic A. Induced pluripotent stem cells: developmental biology to regenerative medicine. Nat. Rev. Cardiol. 2010;7:700–710. doi: 10.1038/nrcardio.2010.159. [DOI] [PubMed] [Google Scholar]
- 5.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013;14:529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat. Rev. Cardiol. 2016;13:333–349. doi: 10.1038/nrcardio.2016.36. This review provides a systematic discussion of Precision Medicine Initiative in the cardiac field using hiPSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Gintant G, Sager PT, Stockbridge N. Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discov. 2016;15:457–471. doi: 10.1038/nrd.2015.34. This review provides a detailed discussion of the three main preclinical elements of CiPA initiative: 1) drug effects on human cardiac ion channels in vitro; 2) in silico reconstruction of human ventricular electrophysiology; and 3) confirmation using hPSCs. [DOI] [PubMed] [Google Scholar]
- 8•.Hendriks William T, Warren Curtis R, Cowan Chad A. Genome Editing in Human Pluripotent Stem Cells: Approaches, Pitfalls, and Solutions. Cell Stem Cell. 2016;18:53–65. doi: 10.1016/j.stem.2015.12.002. This review summarizes the advantages and disadvantages of genome-editing tools, and highlights the theoretical and technical considerations when applied to hPSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaj T, Gersbach CA, Barbas CF., III ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hockemeyer D, Jaenisch R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell. 2016;18:573–586. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolden L, Edenhofer F, Haupt S, Koch P, Wunderlich FT, Siemen H, Brustle O. Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nat. Methods. 2006;3:461–467. doi: 10.1038/nmeth884. [DOI] [PubMed] [Google Scholar]
- 12•.Termglinchan V, Seeger T, Chen C, Wu JC, Karakikes I. Efficient Genome Editing in Induced Pluripotent Stem Cells with Engineered Nucleases In Vitro. Methods Mol. Biol. 2017;1521:55–68. doi: 10.1007/978-1-4939-6588-5_4. The authors provide a step-by-step protocol to generate genome-edited hPSCs using TALEN or CRISPR/Cas in coupled with piggybac transposon system. [DOI] [PubMed] [Google Scholar]
- 13.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 14.Zu Y, Tong X, Wang Z, Liu D, Pan R, Li Z, Hu Y, Luo Z, Huang P, Wu Q, et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat. Methods. 2013;10:329–331. doi: 10.1038/nmeth.2374. [DOI] [PubMed] [Google Scholar]
- 15.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas-Fernandez A, Herhaus L, Macartney T, Lachaud C, Hay RT, Sapkota GP. Rapid generation of endogenously driven transcriptional reporters in cells through CRISPR/Cas9. Sci. Rep. 2015;5 doi: 10.1038/srep09811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Hunt SD, Xue H, Liu Y, Darabi R. Generation and Characterization of a MYF5 Reporter Human iPS Cell Line Using CRISPR/Cas9 Mediated Homologous Recombination. Sci. Rep. 2016;6 doi: 10.1038/srep18759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong JJH, Forte E, Harvey RP. Developmental origins and lineage descendants of endogenous adult cardiac progenitor cells. Stem Cell Res. 2014;13:592–614. doi: 10.1016/j.scr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 19•.Burridge PW, Sharma A, Wu JC. Genetic and Epigenetic Regulation of Human Cardiac Reprogramming and Differentiation in Regenerative Medicine. Annu. Rev. Genet. 2015;49:461–484. doi: 10.1146/annurev-genet-112414-054911. This review provides an overview of the genetic and epigenetic control of cardiogenesis and cardiac differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Skelton RJP, Kamp TJ, Elliott DA, Ardehali R. Biomarkers of Human Pluripotent Stem Cell-Derived Cardiac Lineages. Trends. Mol Med. 2017;23:651–668. doi: 10.1016/j.molmed.2017.05.001. This review summarizes the genetic makers and cell surface markers in hPSC-derived cardiac lineages and discusses how these biomarkers help to understand the cardiac lineage hierarchy and isolate lineage-specific hPSC-derived cells. [DOI] [PubMed] [Google Scholar]
- 22.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc. Natl. Acad. Sci. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 24.Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods. 2011;8:1037–1040. doi: 10.1038/nmeth.1740. [DOI] [PubMed] [Google Scholar]
- 25•.Den Hartogh SC, Schreurs C, Monshouwer-Kloots JJ, Davis RP, Elliott DA, Mummery CL, Passier R. Dual reporter MESP1 mCherry/w-NKX2-5 eGFP/w hESCs enable studying early human cardiac differentiation. Stem Cells. 2015;33:56–67. doi: 10.1002/stem.1842. This is the first hPSC dual reporter line used for delineating human cardiac lineages and isolate cardiac progenitors. [DOI] [PubMed] [Google Scholar]
- 26.den Hartogh SC, Wolstencroft K, Mummery CL, Passier R. A comprehensive gene expression analysis at sequential stages of in vitro cardiac differentiation from isolated MESP1-expressing-mesoderm progenitors. Sci. Rep. 2016;6:19386. doi: 10.1038/srep19386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelton RJ, Brady B, Khoja S, Sahoo D, Engel J, Arasaratnam D, Saleh KK, Abilez OJ, Zhao P, Stanley EG, et al. CD13 and ROR2 Permit Isolation of Highly Enriched Cardiac Mesoderm from Differentiating Human Embryonic Stem Cells. Stem Cell Reports. 2016;6:95–108. doi: 10.1016/j.stemcr.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005;6:826–837. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 29.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 30••.Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat. Biotechnol. 2015;33:970–979. doi: 10.1038/nbt.3271. Using NKX2.5eGFP/w hESC reporter line and the regulated expression of MYC gene, these authors generate expandable hPSC-derived cardiac progenitors. This is the first study to generate human cardiovascular progenitors and systematically characterize these cells and their progenies. [DOI] [PubMed] [Google Scholar]
- 31.Jackman CP, Shadrin IY, Carlson AL, Bursac N. Human Cardiac Tissue Engineering: From Pluripotent Stem Cells to Heart Repair. Curr. Opin. Chem. Eng. 2015;7:57–64. doi: 10.1016/j.coche.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Ruiz I. Stem cells: A step closer to cardiac repair therapies. Nat. Rev. Cardiol. 2016;13:695–695. doi: 10.1038/nrcardio.2016.178. [DOI] [PubMed] [Google Scholar]
- 34.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, Graichen RE, Kay GL, Jyrala AJ, Colman A, et al. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J. Mol. Cell. Cardiol. 2007;43:504–516. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Laake LW, Passier R, den Ouden K, Schreurs C, Monshouwer-Kloots J, Ward-van Oostwaard D, van Echteld CJ, Doevendans PA, Mummery CL. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res. 2009;3:106–112. doi: 10.1016/j.scr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Fu Y, Kraitchman DL. Stem cell labeling for noninvasive delivery and tracking in cardiovascular regenerative therapy. Expert Rev. Cardiovasc. Ther. 2010;8:1149–1160. doi: 10.1586/erc.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J. Am. Coll. Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 39.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K, Watt SM. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21:977–986. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol. Ther. 2007;15:2027–2036. doi: 10.1038/sj.mt.6300303. [DOI] [PubMed] [Google Scholar]
- 44.Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A, Habib M, Yankelson L, Kehat I, Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 2007;21:2551–2563. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- 45.Kita-Matsuo H, Barcova M, Prigozhina N, Salomonis N, Wei K, Jacot JG, Nelson B, Spiering S, Haverslag R, Kim C. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PloS One. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu XQ, Zweigerdt R, Soo SY, Ngoh ZX, Tham SC, Wang ST, Graichen R, Davidson B, Colman A, Sun W. Highly enriched cardiomyocytes from human embryonic stem cells. Cytotherapy. 2008;10:376–389. doi: 10.1080/14653240802105307. [DOI] [PubMed] [Google Scholar]
- 47.Ovchinnikov DA, Hidalgo A, Yang S-K, Zhang X, Hudson J, Mazzone SB, Chen C, Cooper-White JJ, Wolvetang EJ. Isolation of contractile cardiomyocytes from human pluripotent stem-cell-derived cardiomyogenic cultures using a human NCX1-EGFP reporter. Stem Cells Dev. 2014;24:11–20. doi: 10.1089/scd.2014.0195. [DOI] [PubMed] [Google Scholar]
- 48.Ritner C, Wong SS, King FW, Mihardja SS, Liszewski W, Erle DJ, Lee RJ, Bernstein HS. An engineered cardiac reporter cell line identifies human embryonic stem cell-derived myocardial precursors. PLoS One. 2011;6:e16004. doi: 10.1371/journal.pone.0016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bizy A, Guerrero-Serna G, Hu B, Ponce-Balbuena D, Willis BC, Zarzoso M, Ramirez RJ, Sener MF, Mundada LV, Klos M, et al. Myosin light chain 2-based selection of human iPSC-derived early ventricular cardiac myocytes. Stem Cell Res. 2013;11:1335–1347. doi: 10.1016/j.scr.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Josowitz R, Lu J, Falce C, D'Souza SL, Wu M, Cohen N, Dubois NC, Zhao Y, Sobie EA, Fishman GI, et al. Identification and purification of human induced pluripotent stem cell-derived atrial-like cardiomyocytes based on sarcolipin expression. PLoS One. 2014;9:e101316. doi: 10.1371/journal.pone.0101316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Chen Z, Xian W, Bellin M, Dorn T, Tian Q, Goedel A, Dreizehnter L, Schneider CM, Ward-van Oostwaard D, Ng JKM, et al. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur. Heart J. 2016 doi: 10.1093/eurheartj/ehw189. Using specific promoters, these authors identify cardiomyocyte subtypes in a heterogeneous pool of hPSC-CMs. These authors then apply specific myocyte subtypes to precisely mode long QT and test drugs for the first time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ. Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017;35:56–68. doi: 10.1038/nbt.3745. This study develops an approach to differentiate hPSC-derived nodal cells using NKX2.5eGFP/w hESC reporter line. The authors demonstrate that hPSC-derived nodal cells have the capacity to function as biological pacemakers. [DOI] [PubMed] [Google Scholar]
- 54••.Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM. Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell. 2017;21:179–194.e174. doi: 10.1016/j.stem.2017.07.003. This study reveals that hPSC-derived ventricular and atrial myocytes diverge at mesoderm developmental stage. CD235a and RALDH2 can be used as biomarkers to identify these two specific mesoderm populations which can differentiate into ventricular and atrial myocytes, respectively. [DOI] [PubMed] [Google Scholar]
- 55.Espinoza-Lewis RA, Yu L, He F, Liu H, Tang R, Shi J, Sun X, Martin JF, Wang D, Yang J, et al. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev. Biol. 2009;327:376–385. doi: 10.1016/j.ydbio.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dempsey GT, Chaudhary KW, Atwater N, Nguyen C, Brown BS, McNeish JD, Cohen AE, Kralj JM. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Entcheva E, Bub G. All-optical control of cardiac excitation: combined high-resolution optogenetic actuation and optical mapping. J. Physiol. 2016;594:2503–2510. doi: 10.1113/JP271559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Klimas A, Ambrosi CM, Yu J, Williams JC, Bien H, Entcheva E. OptoDyCE as an automated system for high-throughput all-optical dynamic cardiac electrophysiology. Nat. Commun. 2016;7:11542. doi: 10.1038/ncomms11542. This study demonstrates the automated all optical imaging for drug screening in hPSC-CM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lapp H, Bruegmann T, Malan D, Friedrichs S, Kilgus C, Heidsieck A, Sasse P. Frequency-dependent drug screening using optogenetic stimulation of human iPSC-derived cardiomyocytes. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-09760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavero I, Holzgrefe H. Comprehensive in vitro Proarrhythmia Assay, a novel in vitro/in silico paradigm to detect ventricular proarrhythmic liability: a visionary 21st century initiative. Expert Opin. Drug Saf. 2014;13:745–758. doi: 10.1517/14740338.2014.915311. [DOI] [PubMed] [Google Scholar]
- 61.Woodworth MB, Girskis KM, Walsh CA. Building a lineage from single cells: genetic techniques for cell lineage tracking. Nat. Rev. Genet. 2017;18:230–244. doi: 10.1038/nrg.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]