Abstract
Despite a marked improvement in 10-year survival for SLE patients over the past five decades, mortality rates from SLE remain high compared to those in the general population. SLE was also among the leading causes of death in young women in the United States during 2000–2015. However, it is encouraging that SLE mortality rates and the ratios of SLE mortality rates to non-SLE mortality rates have decreased every year since the late-1990s. Despite this improvement, disparities in SLE mortality persist according to sex, race, age and place of residence. Furthermore, demographic and geographic variables seem to modify the effect of each other in influencing SLE mortality, leading to interactions between sex/race/ethnicity-associated factors and geographic differences. In other words, persons of the same sex/race/ethnicity had differences in SLE mortality depending on where they lived. These observations highlight SLE as an important public health issue. The recognition of SLE as a leading cause of death in general population might spur targeted public health programs and research funding to address the high lupus mortality.
In the late 1940s, over 40% patients with systemic lupus erythematous (SLE) died within 3 years of onset of first symptom(s).1 Since then, the 5-year and 10-year survival of SLE patients has remarkably improved to over 90%.2 In order to estimate the true burden of SLE mortality in the general population and to reflect changes in SLE incidence over time in mortality estimates, we used a national mortality database and census data to analyze temporal trends and demographic and regional differences in SLE mortality over five decades.3 From 1968 to 2013, SLE was recorded as the underlying cause in 50,249 deaths in the entire United States (U.S.). There were 100,851,288 deaths from all other causes (non-SLE) during the same 46-year period. In an additional 12,463 deaths, SLE was recorded as a contributing causes of death during 1999 to 2013. We calculated mortality rates for SLE and compared these rates with mortality rates for non-SLE causes.
To calculate the number of expected deaths as compared to the number of observed deaths, two methods of standardization are commonly used in epidemiological studies. An indirect method of adjustment has been used to compute the standardized mortality ratio in previous studies on SLE mortality.4–7 These studies have shown that the standardized mortality ratios in cohorts of patients with SLE are 1.3 to 5.3 times that of age-matched controls from the general population of the same region.4–7 The indirect method of adjustment depends on the age structure of the study population (SLE in this case).8 However, the age structure may vary between different study cohorts, and across populations of different regions and countries.9 Thus, the standardized mortality ratios computed for one population may not represent SLE mortality in another population. Therefore, we performed a direct method of adjustment using a standard population to calculate the age-standardized mortality rate (ASMR) for each year for both SLE and non-SLE causes.9 We then computed the ratio of the SLE ASMR to the non-SLE ASMR for each year.
The ASMR for SLE was 4.5 (95% CI, 4.2 to 4.8) per million persons in 1968 and 3.4 (95% CI, 3.2 to 3.6) per million persons in 2013. However, this reduction in mortality attributed to SLE (24.4%) over 46 years was smaller than the reduction in mortality due to non-SLE causes (43.9%) during the same period. Importantly, the ratio of SLE to non-SLE mortality was about 35 percent higher in 2013 than in 1968.
In order to identify natural trends in SLE mortality over five decades, we conducted joinpoint trend analysis of annual ASMRs for SLE and non-SLE causes by sex, race/ethnicity, and geographic region. The joinpoint method identifies the year when mortality began to increase, stabilize, or decrease, and the degree to which these changes occurred.3 We found that after an initial decrease between 1968 and 1975, SLE mortality increased annually for 24 years, followed by a sustained decrease for 14 years starting in 1999. Changes in lupus incidence and in treatment effectiveness and complications over time could partially explain the changing trends (Fig. 1).
Figure 1. SLE mortality trends in relation to major SLE treatment milestones and changes in incidence.
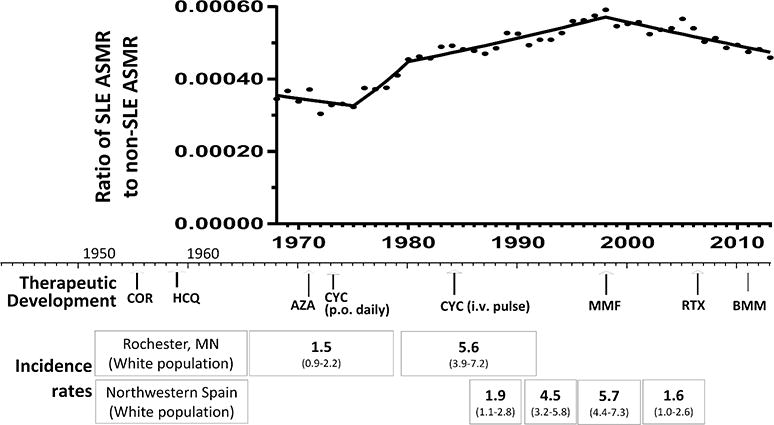
The ratio of SLE ASMR to non-SLE ASMR SLE is shown in relation to major SLE treatment milestones and changes in incidence rates over time. Corticosteroids and hydroxychloroquine were introduced to treat patients with SLE in the 1950s.17, 18 Immunosuppressive drugs, including azathioprine19 and daily oral cyclophosphamide,20 were introduced in the 1970s and were associated with increased drug toxicities that were reduced to some extent with the introduction of intravenous pulse cyclophosphamide in the 1980s.21, 22 Subsequent use of mycophenolate23 and combination therapy with induction and maintenance phases in the 1990s24 led to further reductions in drug-associated complications and greater efficacy. Rituximab has been used since the mid-2000s, and belimumab was approved by the U.S. Food and Drug Administration to treat SLE in 2011.24 The pattern of changes in mortality that we observed may reflect these therapeutic advances and potential benefits or complications of treatment. In addition, changes in SLE incidence over time could partially explain the observed changing trends in SLE mortality. In Minnesota, incidence of SLE tripled between 1950 and 1992,25 and in Spain, incidence increased from 1.9 cases per 100 000 persons in 1987 to 1991 to 4.5 cases per 100 000 persons in 1992 to 1996 before decreasing to 1.6 cases per 100 000 persons in 2002 to 2006.26 ASMR, age-standardized mortality rate; AZA, azathioprine; BMM, belimumab; COR, corticosteroids; CYC, cyclophosphamide; HCQ, hydroxylchloroquine; i.v., intravenous; MMF, mycophenolate mofetil; p.o., oral; RTX, rituximab.
Similar rise-and-decline trends in the SLE mortality were seen in both sexes, black persons, and in all four geographic regions. However, statistically significant increases in the SLE ASMR did not occur among white persons over the 46-year period. Black persons, and residents of the South had higher SLE ASMRs and larger cumulative increases in the ratio of the SLE to the non-SLE ASMR (62.5%, and 58.6%, respectively) than other racial/ethnic groups, and residents of other census regions of the U.S., respectively. In contrast to this changing trend for the SLE mortality, the non-SLE mortality decreased or stayed stable throughout the study period in all sexes, races and geographic regions.
Multiple logistic regression showed independent associations of sex, race, age, and geographic region with SLE mortality risk.3 After adjustment for age, sex, geographic region, and calendar period, the risk for SLE death was significantly higher risk in persons of racial/ethnic minorities (black, Hispanic, and others [Asian, Pacific Islanders, American Indians, and Alaska Natives; combined due to small numbers]) relative to white persons (Fig. 2, upper panel). The SLE death risk was also higher in residents of the South or West relative to the Northeast census region of the U.S. (Fig. 2, middle panel). Females had significantly higher SLE mortality risk than males, as did persons aged 65 years or older relative to those aged 0 to 64 years.3
Figure 2. SLE mortality associations with race/ethnicity and place of residence.
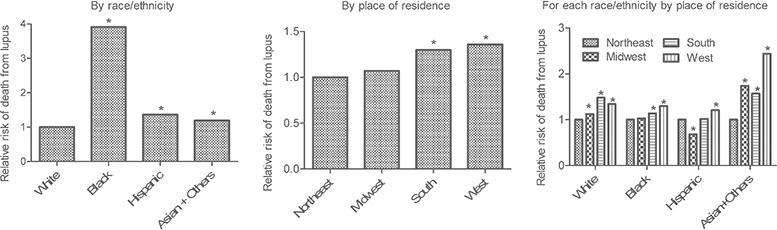
SLE mortality associations with demographic and geographic variables were determined using multiple logistic regression analysis.3 The period of 1999–2013 was chosen for this analysis, since information on Hispanic ethnicity was not available prior to 1999. Total number of deaths in sample, 18,866. *Statistically significant relative to the reference group: White in the left panel Northeast census region of the United States in the middle panel, and Northeast for each of the four subpanels in the right panel.
(Left and Middle Panels) Main effects. The adjusted odds ratios for race/ethnicities and geographic regions are shown, as predicted by the model, integrated across all other demographic/region/time characteristics. For example, the left panel shows the SLE mortality risk in the three non-white groups relative to white persons, integrated over all men and women, all age groups, all calendar periods, and all geographical regions. The middle panel shows the SLE mortality risk in the Midwest, South and West geographic regions relative to the Northeast, integrated over all men and women, all age groups, all calendar periods, and all race/ethnicities.
(Right Panel) SLE mortality association with geographical regions stratified by race/ethnicity. Multiple logistic regression models included interaction terms. For each racial/ethnic group, SLE mortality risk was greater in all other regions relative to the Northeast census region of the United States, except for non-Hispanic black persons in the Midwest and Hispanic persons in the South and Midwest. Hispanic persons in the Midwest had significantly lower SLE mortality than those in the Northeast. In non-Hispanic white persons, residence in the South conferred the highest SLE mortality risk, but in the other 3 racial/ethnic groups, residents of the West had the highest risk. The largest regional disparity in SLE mortality risk was in the Asian/PI/AI/AN group in the West versus the Northeast; the next largest regional disparity was in Hispanic persons in the West versus the Midwest.
In order to determine if the demographic and geographic variables modify the effect of each other, we included interaction terms in the multiple logistic regression model. This analyses revealed significant interactions between race/ethnicity and both sex and geographic region, indicating that race/ethnicity modified the relationship among sex, geographic region, and SLE mortality. In other words, persons of the same race / ethnicity had differences in lupus mortality, depending on where they lived. In non-Hispanic white persons, residence in the South conferred the highest SLE mortality risk, but in the other racial/ethnic groups, residents of the West had the highest risk (Fig. 2, lower panel). A previous study identified clusters of elevated SLE mortality in Alabama, Arkansas, Louisiana and New Mexico and clusters of low mortality in Minnesota, Vermont, Virginia and Washington.10 The elevated mortality areas had higher poverty rates and/or greater concentrations of Hispanics than the lower mortality areas.10 Further, a study in non-whites suggested a greater impact of poverty than race/ethnicity on SLE mortality.11 Geographic differences in the quality of care for lupus nephritis patients has also been reported, with more Northeast U.S. patients receiving standard-of-care medications.12 Interactions between race/ethnicity-associated genetic and non-genetic factors and geographic differences in environment such as increased sunlight exposure, socioeconomic factors, and access to medical care might also influence SLE mortality.
To address the possibility that SLE may not be listed as the underlying cause on death certificates of some patients who died of SLE complications and that this coding error may differ across different subpopulations, we repeated the multiple logistic regression analyses for cases where SLE was recorded as a contributing cause of death. Findings were similar to trends observed in cases where SLE was recorded as the underlying cause of death.3 This suggests that secular changes over time in physicians’ reporting/attributing SLE as underlying cause in death did not substantially influence SLE mortality trend estimates. Although, it is less likely that SLE would be recorded as a cause of death on death certificates of cases that do not have SLE, underreporting of SLE on death certificates is not uncommon, especially in older individuals and those without health insurance and with low education levels.13, 14 Such underreporting of SLE on death certificates may occur, because many SLE patients die of complications such as infections and cardiovascular disease under care of primary care physicians and hospitalists. A Swedish study reported that 86% of 2,314 SLE deaths occurred in hospital units other than rheumatology,15 which underscores the importance of increasing awareness among primary care physicians and hospitalists to recognize SLE as an important cause of death. Nevertheless, the large differences we found in SLE mortality by age, sex, race, and region are unlikely to be artifacts from misclassification of cause of death, because greater underreporting of SLE as the cause of mortality in underprivileged groups13, 14 would lead to greater underestimation of SLE mortality in the groups we found the risk to be larger; e.g., females, black persons, Hispanics, and residents of the South census region of the U.S.
In conclusion, despite improving trends in mortality, mortality rates from SLE remain high compared to those in the general population, and disparities persist between subpopulations and geographic regions. Furthermore, SLE was among the leading causes of death in young women in the U.S. between 2000 and 2015.16 In women ages 15–24 years, SLE was the number one cause of death among chronic inflammatory diseases, ranking higher than diabetes mellitus, HIV, chronic lower respiratory disease, nephritis, pneumonitis and liver disease.16 However, it is encouraging that SLE mortality rate has decreased 2.7% every year since 1999 and the ratio of SLE to non-SLE mortality has decreased 1.2% every year since 1998. Additional research using prospective population-based data collection could help to identify any potentially modifiable risk factors that might inform targeted research and public health programs to address the disparities in lupus mortality rates.
Acknowledgments
The authors thank Dr. Magda Shaheen, Ms. Jennifer Woo, Dr. Ning Li, Dr. Deborah McCurdy, Dr Arun Karlamangla, and the UCLA Institute for Digital Research and Education for statistical assistance and helpful suggestions.
Financial Support: This work was supported in part by the National Institutes of Health (R01-AI080778, R01-AR056465), the Lupus Foundation of America, and the Rheumatology Research Foundation. Dr. Yen was supported by the National Institutes of Health (T32-DK-07789 and 5T32-HD-007512), the UCLA Children’s Discovery and Innovation Institute, and a Mallinckrodt Research Fellowship Award.
Footnotes
Disclosures: Authors have disclosed no conflicts of interest.
References
- 1.Jessar RA, Lamont-Havers RW, Ragan C. Natural history of lupus erythematosus disseminatus. Ann Intern Med. 1953;38:717–31. doi: 10.7326/0003-4819-38-4-717. [DOI] [PubMed] [Google Scholar]
- 2.Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 2012;41:830–9. doi: 10.1016/j.semarthrit.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Yen EY, Shaheen M, Woo JMP, et al. 46-Year Trends in Systemic Lupus Erythematosus Mortality in the United States, 1968 to 2013: A Nationwide Population-Based Study. Ann Intern Med. 2017 doi: 10.7326/M17-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727–34. doi: 10.1177/0961203315627202. [DOI] [PubMed] [Google Scholar]
- 6.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35:2152–8. doi: 10.3899/jrheum.080214. [DOI] [PubMed] [Google Scholar]
- 7.Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis care & research. 2014;66:608–16. doi: 10.1002/acr.22173. [DOI] [PubMed] [Google Scholar]
- 8.Schoenbach VJ, Rosamond WD. Understanding the Fundamentals of Epidemiology - An Evolving Text. Chapel Hill, NC: 2000. Standardization of rates and ratios; pp. 129–51. [Google Scholar]
- 9.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age Standardization of Rates. World Health Organization; [Google Scholar]
- 10.Walsh SJ, DeChello LM. Geographical variation in mortality from systemic lupus erythematosus in the United States. Lupus. 2001;10:637–46. doi: 10.1191/096120301682430230. [DOI] [PubMed] [Google Scholar]
- 11.Duran S, Apte M, Alarcon GS, Group LS Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc. 2007;99:1196–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis care & research. 2014;66:617–24. doi: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo-Alen J, Alarcon GS, Campbell R, Jr, Fernandez M, Reveille JD, Cooper GS. Lack of recording of systemic lupus erythematosus in the death certificates of lupus patients. Rheumatology (Oxford) 2005;44:1186–9. doi: 10.1093/rheumatology/keh717. [DOI] [PubMed] [Google Scholar]
- 14.Ward MM. Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheum. 2004;51:616–24. doi: 10.1002/art.20526. [DOI] [PubMed] [Google Scholar]
- 15.Bjornadal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. J Rheumatol. 2004;31:713–9. [PubMed] [Google Scholar]
- 16.Yen EY, Singh RR. Lupus - An Unrecognized Leading Cause of Death in Young Women: Population-based Study Using Nationwide Death Certificates, 2000–2015. Arthritis Rheumatol. 2018 doi: 10.1002/art.40512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullins JF, Watts FL, Wilson CJ. Plaquenil in the treatment of lupus erythematosus. J Am Med Assoc. 1956;161:879–81. doi: 10.1001/jama.1956.62970090020017k. [DOI] [PubMed] [Google Scholar]
- 18.Johnson SA, Meyer OO. The treatment of lupus erythematosus disseminatus with cortisone. Am J Med Sci. 1952;223:9–15. doi: 10.1097/00000441-195201000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Sztejnbok M, Stewart A, Diamond H, Kaplan D. Azathioprine in the treatment of systemic lupus erythematosus. A controlled study. Arthritis Rheum. 1971;14:639–45. doi: 10.1002/art.1780140511. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg AD, Kaltreider HB, Staples PJ, Goetzl EJ, Talal N, Decker JL. Cyclophosphamide in lupus nephritis: a controlled trial. Ann Intern Med. 1971;75:165–71. doi: 10.7326/0003-4819-75-2-165. [DOI] [PubMed] [Google Scholar]
- 21.Sessoms SL, Kovarsky J. Monthly intravenous cyclophosphamide in the treatment of severe systemic lupus erythematosus. Clin Exp Rheumatol. 1984;2:247–51. [PubMed] [Google Scholar]
- 22.Malaviya AN, Singh RR, Sindhwani R, et al. Intermittent intravenous pulse cyclophosphamide treatment in systemic lupus erythematosus. Indian J Med Res. 1992;96:101–8. [PubMed] [Google Scholar]
- 23.Glicklich D, Acharya A. Mycophenolate mofetil therapy for lupus nephritis refractory to intravenous cyclophosphamide. Am J Kidney Dis. 1998;32:318–22. doi: 10.1053/ajkd.1998.v32.pm9708620. [DOI] [PubMed] [Google Scholar]
- 24.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O’Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum. 1999;42:46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Alonso MD, Llorca J, Martinez-Vazquez F, et al. Systemic lupus erythematosus in northwestern Spain: a 20-year epidemiologic study. Medicine (Baltimore) 2011;90:350–8. doi: 10.1097/MD.0b013e31822edf7f. [DOI] [PubMed] [Google Scholar]


