Abstract
Increasing evidence suggests that regular consumption of coffee, tea and dark chocolate (cacao) can promote brain health and may reduce the risk of age-related neurodegenerative disorders. However, the complex array of phytochemicals in coffee and cacao beans and tea leaves has hindered a clear understanding of the component(s) that affect neuronal plasticity and resilience. One class of phytochemicals present in relatively high amounts in coffee, tea and cacao are methylxanthines. Among such methylxanthines, caffeine has been the most widely studied and has clear effects on neuronal network activity, promotes sustained cognitive performance and can protect neurons against dysfunction and death in animal models of stroke, Alzheimer’s disease and Parkinson’s disease. Caffeine’s mechanism of action relies on antagonism of various subclasses of adenosine receptors. Downstream xanthine metabolites, such as theobromine and theophylline, may also contribute to the beneficial effects of coffee, tea and cacao on brain health.
INTRODUCTION
Coffee, tea and chocolate are among the most commonly consumed substances in the world [1]. The use of the seeds from the cacao tree (Theobroma cacao) to create beverages dates to the early formative period of Mesoamerican history (2000–1000 B.C.). In Mayan and Aztecan societies, cacao beans were so valued they were not only used for food and medicinal purposes, but also as a currency. The explorer Hernán Cortés is credited with understanding cacao’s potential after drinking xocoatl at the Aztec emperor Montezuma’s court. In 1528, Cortés brought the beans and tools necessary to recreate “the drink that builds up resistance and fights fatigue” back to the Spanish court [2]. Mixed with honey, sugar and spices, dark chocolate (cacao) soon became a favorite of the Spanish nobility and, less than a 100 years later, of Europe.
The origins of coffee usage are less clear. The first substantiated evidence of coffee drinking dates to the 15th century in Yemenite Sufi monasteries, where monks used the brew to keep themselves awake during nightly prayers. The invigorating properties of the beverage soon spread through other Arabic countries and the Ottoman Empire, where Venetian merchants discovered it and began introducing caffe’, from the Turkish word kahveh, in Italy around 1570. It is estimated that over 2.3 billion cups of coffee are now consumed daily throughout the world [3].
In 1902 the chemist Emil Fischer was awarded the Nobel prize for his work on purine and sugar metabolism, including the discovery that caffeine is a purinergic component of coffee [4]. Indeed, during the century following Fischer’s discovery, studies of the effects of caffeine on the nervous system established it as a psychostimulant and have elucidated its cellular and molecular mechanisms of action on nerve cells. Together with the fact that caffeine is a major psychoactive component of coffee and tea, it has been concluded that caffeine is the most commonly consumed psychoactive chemical throughout the world [5]. While caffeine is present in relatively high concentrations in coffee and tea, several other purine metabolites are also present in lower amounts including theobromine, theophylline and paraxanthine. On the other hand, theobromine and theophylline are present in high concentrations in cacao.
Plants likely evolved enzymatic pathways to produce caffeine and related methylxanthines as a mechanism to protect themselves against consumption by insects and herbivorous and omnivorous animals [6]. As evidence, caffeine has a very bitter taste, is concentrated in vulnerable regions of the plants (seeds and leaves) and is considered a natural pesticide [6, 7]. Some species of carnivores including canines (which presumably did not consume cacao during their evolution) can be killed by doses of theobromine well below doses readily tolerated by herbivores and omnivores including humans [8]. As we shall see later in this article, methylxanthines may affect signaling pathways that enable neurons to overcome dysfunction and degeneration. This possibility is consistent with literature in the field of hormesis, a general process by which low levels of an environmental challenge increase the ability of cells and organisms to resist more severe stress and disease [6]. Indeed, emerging evidence suggests that many of the chemicals present in plants that can be beneficial for health are noxious agents/toxins from an evolutionary perspective [6, 9].
In the present short review article, we focus on the neurobiological actions of caffeine, theophylline, theobromine in the contexts of neuroplasticity, cognition and vulnerability to age-related neurological disorders. Some, but not all, epidemiological studies have found a negative association between moderate consumption of coffee and the risk of age-related cognitive disorders and Parkinson’s disease (PD) [10 – 13]. However, the influence of caffeine and other methylxanthines present in these beverages and cacao on brain aging and disease risk remain to be determined.
THE PURINE CHEMISTRY OF COFFEE AND CACAO
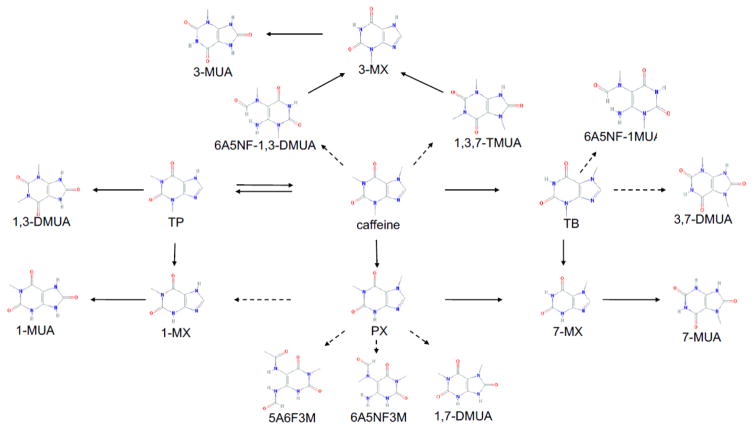
Coffee and cacao contain different ratios of 1,3,7-trimethylxanthine (caffeine) and 3,7-dimethylxanthine (theobromine) and traces of 1,3-dimethylxanthine (theophylline). In humans, within 45 minutes of oral ingestion, 99% of consumed caffeine is absorbed by the small intestine and stomach [14, 15], with less than 2% of caffeine being excreted untransformed in urine [16]. The majority of caffeine is metabolized in the liver via cytochrome P450 enzymes to form mono- and dimethylxanthines, and methylated uracil-derivatives (Figure 1) [17, 18]. CYP1A2 accounts for more than 95% of the primary metabolism of caffeine [19]. Although the main metabolic pathways are similar between humans and rodents, there are substantial quantitative differences in their metabolic profiles. In humans, caffeine is primarily metabolized via N-3 demethylation into paraxanthine (1,7-dimethylxanthine) (~80%), over theobromine (3,7-dimethylxanthine) (~12%), and theophylline (1,3-dimethylxanthine) (~4%), with C-8 hydroxylation to 1,3,7-trimethyluric acid accounting for less than 6% [20] (Figure 1). Consequently, the main urinary metabolites in humans are paraxanthine and its derivatives 1-methyluric acid, 1-methylxanthine, 1,7-dimethyluric acid, and 6-amino-5-(N-formylmethylamino)-3-methyluracil. In rodents, on the other hand, about 40% of caffeine undergoes C-8 hydroxylation to generate trimethyl derivatives, with the primary demethylation products being N-1 theobromine and N-7 theophylline [1].
Figure 1.
Caffeine metabolites in humans. Solid arrows indicate a direct pathway. Dashed arrows indicate the presence of an unknown intermediate. Abbreviations from top left to bottom right: 3-MUA (3-Methyluric Acid); 3-MX (3-Methylxanthine); 6A5NF-1,3-DMUA (6-Amino-5-(N-formylmethylamino)-1,3-dimethyluracil); 1,3,7-TMUA (1,3,7-Trimethyluric Acid); 6A5NF-1MUA (6-Amino-5-(N-formylmethylamino)-1-methyluracil); 1,3-DMUA (1,3-Dimethyluric Acid); TP (Theophylline); CA (Caffeine); TB (Theobromine); 3,7-DMUA (3,7-Dimethyluric Acid); 1-MUA (1-Methyluric Acid); 1-MX (1-Methylxanthine); PX (Paraxanthine); 7-MX (7-Methylxanthine); 7MUA (7-Methyluric Acid); 5A6F3M (5-Acetylamino-6-formylamino-3-methyluracil); 6A5NF3M (6-Amino-5-(N-formylmethylamino)-3-methyluracil); 1,7-DMUA (1,7-Dimethyluric Acid).
While paraxanthine is not present in plant extracts, in humans its concentrations in the blood reach levels comparable to or higher than those of caffeine [18]. Paraxanthine should thus be considered when investigating the physiological effects of caffeine, especially under chronic caffeine intake conditions [21].
Like caffeine, theophylline undergoes extensive hepatic biotransformation. Approximately 10% of theophylline is excreted unchanged in the urine, 6% is methylated to generate caffeine [22], 50% is converted to 1,3-dimethyluric acid, and the remaining portion is demethylated to 1- and 3-methylxanthine (Figure 1). Fewer studies are available on theobromine metabolism; however, it has been shown that it is converted to 3- and 7-methylxanthine, and, based on the reducing status of the cell, to 3,7-dimethyluric acid or 6-amino-5-(N-formylmethylamino)-1-uracil [23, 24].
The half-lives of the various methylxanthines differ and are dose-dependent, suggesting saturable kinetics of enzymatic metabolism. Caffeine reaches peak plasma concentrations within 1–2 hours of consumption and exhibits a half-life of approximately 2.5–5 hours, with variability between individuals [17, 25]. Paraxanthine’s half-life is similar to that of caffeine (3.1–4.1 hours), whereas theophylline and theobromine have somewhat longer half-lives (6.2–7.2 hours) [26].
As for distribution throughout the body, the use of radiolabeled probes showed no bioaccumulation of the different methylxanthines [18, 27]; however, their intrinsic hydrophobic properties impact their ability to cross the blood-brain barrier. The high hydrophobicity of caffeine allows its unrestricted passage through all biological membranes, including the blood-brain barrier [28]. On the other hand, recovery of theophylline [29], theobromine [30] and paraxanthine [31] in the brain is much lower than that of caffeine.
COFFEE AND CACAO PURINES AND SYNAPTIC PLASTICITY
The arousing and energizing effects that originally led our ancestors to include coffee and cacao in their diets have been substantiated using scientific approaches. Growing evidence indicates that habitual consumption of coffee and/or chocolate results in improved cognitive performance during stressful conditions [32–34] and measurable attenuation of neurocognitive decline associated with normal aging and neurodegenerative disorders [35–37]. Acute caffeine intake improves performance on memory tasks [38, 39]. The Institute of Medicine’s Food and Nutritional Board Committee on Military Nutrition Research reported that a dose of 150 mg of caffeine enhances cognitive performance for at least 10 hours, and advised including caffeine in military rations [40]. Large longitudinal clinical studies have established an inverse relationship between coffee consumption and memory decline during normal aging [41, 42]. Similar results have been found for chocolate consumption. In controlled studies, 8 weeks of daily chocolate drink intake resulted in improved cognitive performance in patients with mild cognitive impairment [43], as well as in cognitively intact elderly [44].
While caution is warranted when extrapolating the results of studies conducted in rodents to humans [45], the mechanisms of action of coffee and cacao methylxanthines have begun to emerge from animal studies. Most studies have focused on caffeine, yet there is evidence that its metabolites share some of the same historically reported cellular actions, including adenosine receptor antagonism at physiologically relevant doses [1], and non-selective inhibition of cyclic nucleotide phosphodiesterases [30, 46, 47] and stimulation of intracellular calcium release [48] at higher concentrations that are toxic in vivo. Concentrations of caffeine in the millimolar range are necessary to activate calcium release from ryanodine receptors and neurotransmitter exocytosis [49–52]. Relatively high concentrations (100 μM - 1 mM), are also required to inhibit cyclic nucleotide phosphodiesterases, thereby elevating cyclic AMP levels and its downstream signaling pathways [53]. The concentrations that can induce such responses are more than ten-fold higher than the biological levels found in plasma and brain after ingestion of food containing methylxanthines. It is therefore implausible that such mechanisms contribute to the effects of methylxanthines on neurological functions [54]. Instead, at physiologically relevant doses, the neurological actions of caffeine, paraxanthine and theophylline actions are mediated by non-selective adenosine receptor antagonism [55, 1] (Figure 2).
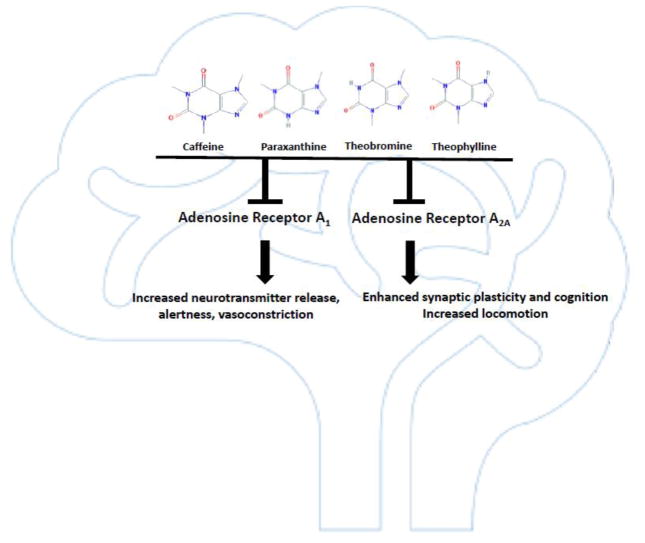
Figure 2.
Impact of antagonism of neuronal adenosine A1 and A2A receptors by caffeine, theobromine, paraxanthine and theophylline on brain physiology and behavior.
Adenosine receptors are G protein-coupled receptors expressed in a variety of organs including heart, colon, lungs, bladder, skeletal muscles and brain [56]. In the central nervous system, adenosine receptors regulate sleep/wakefulness, synaptic plasticity, motor function and neuronal signaling [57–63]. Methylxanthines have been shown to bind with different affinities and specificities to A1 and A2A, the adenosine receptor subtypes found in the brain [62, 64]. Compared to caffeine, paraxanthine and theophylline have an overall higher binding affinity, while theobromine is a low affinity ligand and a weaker adenosine receptor antagonist [1, 65]. Studies using adenosine receptor knock-out mice have shown that adenosine receptor A2A regulates sleep and motor activity, whereas A1 and A2A influence heart rate, body temperature and oxygen consumption [66].
The selective antagonism of adenosine receptors A1 and A2A can also modulate hippocampal long-term potentiation (LTP) [62, 67] a type of synaptic plasticity strongly associated with learning and memory. A1Rs are highly expressed in the CA2 region of the hippocampus [68], and their antagonism enhances the induction and stabilization of activity-dependent LTP [61, 69]. Under basal conditions Shaffer collateral synapses in the CA2 fail to elicit activity-dependent LTP due to the higher calcium buffering and extrusion capacity of CA2 neurons and the expression of the inhibitory protein regulator of G protein signaling 14 [70–72]. However, oral administration of caffeine in vivo, as well as short-term application of caffeine to hippocampal slices, causes a persistent increase in synaptic responses in CA2 neurons [73]. The ability of low doses of caffeine to facilitate basal synaptic transmission, possibly via A1R antagonism, is also observed in CA1 following acute in vitro application [74]. This effect is not only achieved with physiologically relevant concentrations of caffeine, but appears to be age-independent [74].
In the brain A2A receptors are present at high density in the ventral and dorsal striatum and, to a lower extent, in the cortex and hippocampus [56]. While in the striatum they are found predominantly in post-synaptic neurons, in the hippocampus A2ARs are most abundant in the pre-synaptic active zone of the nerve terminals [75]. The role played by A2A receptors in modulation of synaptic plasticity is quite limited under basal conditions [76, 77], but is fundamental in CA1 and CA3 areas under high frequency stimulation [74, 78]. A2A receptors essentially act as controllers, switching presynaptic modulation from inhibitory to facilitatory [67]. Optogenetic studies have shown that activation of A2A receptor signaling in the hippocampus is sufficient to induce LTP in the CA1 while impairing spatial memory performance, and A2A receptor activation in the nucleus accumbens stimulates locomotor activity [79]. Similarly, selective inhibition of A2A receptor signaling in the CA3 area of an Alzheimer’s mouse model restores LTP and reverses memory deficits [80]. While the activation of A2A receptors in hippocampus is sufficient to trigger memory deficits [81], pharmacological or genetic interventions blocking A2A receptors enhance working memory [78, 82], reversal learning [83] and fear conditioning [84, 85] in normal animals, and reverse memory impairments in aged animals [86] and animal models of Parkinson’s [87] and Alzheimer’s [80, 88–90] diseases. The causal link between adenosine receptor overactivation and neurological disorders is further supported by the consistent observation of upregulated A2A receptors in conditions characterized by chronic stress and/or neurodegeneration [67, 80, 91–95]. Overall, these findings provide mechanistic insight into the nuanced beneficial effects coffee and cacao purines may have on cognition and prevention of age-related memory impairment.
While adenosine receptor antagonism can explain most of the central nervous system effects of methylxanthines, it is possible that additional yet uncharacterized mechanisms contribute as well. For example, following both acute and chronic caffeine intake, changes in local rates of cerebral energy metabolism with increased glucose utilization are found in various monoaminergic areas, motor and limbic systems, and the thalamus [96, 97]. These changes are dissociated from the effects on cerebral blood flow, as they occur under conditions of vasoconstriction and hypoperfusion [98]. Furthermore, they also involve areas of the brain not particularly enriched with adenosine receptors [96] and are insensitive to receptor-dependent desensitization [97].
ADENOSINE RECEPTOR SIGNALING IN SYNAPTIC PLASTICITY
Emerging findings suggest that a general mechanism by which some chemicals in vegetables, fruits, coffee and tea provide health benefits is by inducing adaptive cellular responses [8,9]. From an evolutionary perspective, such phytochemicals might function to dissuade insects and herbivorous and omnivorous animals from eating plants. It is conceivable that animals, in turn, evolved adaptations not only enabling them to consume limited amounts of phytochemicals without toxic effects but to potentially benefit from their consumption [6, 9]. For example, it was recently shown that while honeybees are repulsed by high doses of caffeine, the low amounts present in the nectar of coffee and citrus species facilitate associative learning and memory acting via adenosine receptor antagonism [99]. Moreover, the consumption of sugar syrup supplemented with low doses of caffeine significantly increased the resistance to parasite infestation and lifespan of worker honeybees [100]. As mentioned in previous sections, converging evidence suggests that at physiologically relevant doses methylxanthines act as non-selective adenosine receptor antagonists. Here we describe the signaling pathways elicited by adenosine receptors A1 and A2A that may be functional targets of caffeine and its metabolites, with a focus on modulation of synaptic plasticity.
The best characterized mechanism of signal transduction elicited by activation of the adenosine receptors is the modulation of adenylate cyclase activity [101, 102]. Adenosine receptors A1 and A2A are indeed respectively coupled to inhibitory (Gi) and stimulatory (Gs) GTP-binding proteins which inhibit and stimulate adenylate cyclase (Figure 3). Inhibition of adenylate cyclase by A1Rs promotes the activation of potassium channels and phospholipase C as well as the inactivation of N, P, and Q-type calcium channels [103]. Presynaptically, A1R activation depresses the release of neurotransmitters including glutamate, gamma-aminobutyric acid (GABA), norepinephrine, and dopamine [61], with a particularly prominent effect on glutamatergic excitatory transmission [104]. Post- and extra-synaptic A1R activation influences the response to excitatory stimuli by hyperpolarizing the resting membrane potential via activation of inward rectifying potassium channels [105] and controlling the N-type channels and N-methyl-D-aspartate (NMDA) receptors [106, 107]. Dose and time-dependent phosphorylation of extracellular signal-regulated kinases (ERKs) 1/2 by A1R activation has also been reported [108] (Figure 3a). Impairment of paired-pulse facilitation at Shaffer collateral-CA1 synapses is observed in A1R knockout mice, without overall changes in LTP and LTD [109]. Both genetic and pharmacological approaches suggest that under physiological conditions A1Rs are not essential for plasticity at mossy fiber synapses [110]. On the other hand, studies using genetic A1R ablation, specific A1R antagonists or removal of adenosine via adenosine deaminase have shown a selective augmentation of mossy fiber basal transmission in the hippocampus, but decreased short-term plasticity (i.e. paired pulse facilitation) and LTP at this synapse [111]. Furthermore, under non-physiological conditions such as in sleep deprivation [112] or chronic morphine administration [113], the activation of A1Rs normalizes CA3-CA1 LTP in animals. The possibility that the role of A1Rs in learning and memory varies under physiological and pathological conditions is supported by the fact that A1Rs knockout animals can normally acquire and retain spatial reference and working memory [109, 114, 115]; whereas pharmacological interventions implicate A1Rs in preventing spatial and working memory impairments induced by morphine [113] or scopolamine [116].
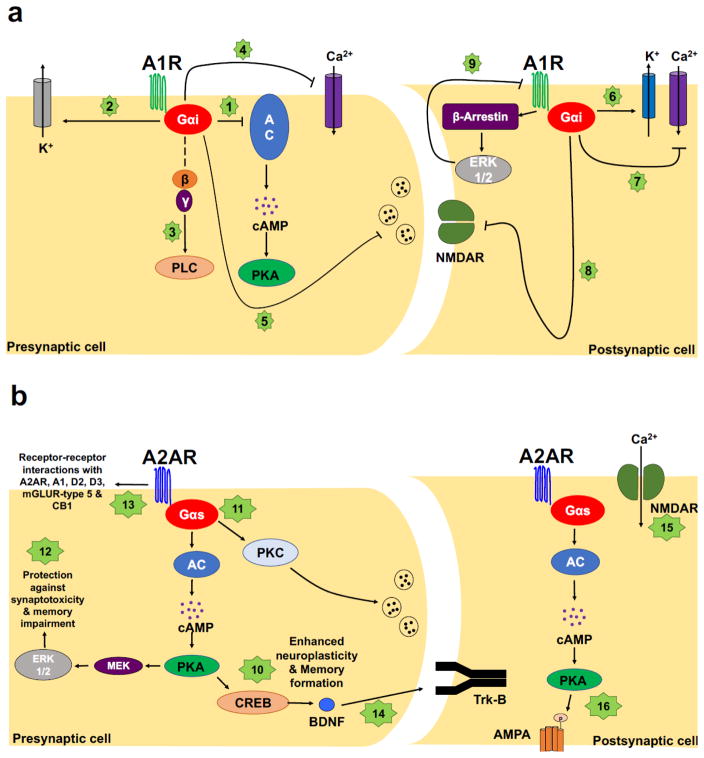
Figure 3.
Signaling pathways associated with A1R and A2A activation. a) Inhibition of adenylate cyclase by A1Rs (1) promotes the activation of potassium channels (2) and phospholipase C (3), as well as the inactivation of N, P, and Q-type calcium channels (4). Presynaptically, A1R activation depresses the release of almost every classical neurotransmitter (i.e. glutamate, gamma-aminobutyric acid, norepinephrine, dopamine, etc.) (5), with a most prominent effect on glutamatergic excitatory transmission. Post- and extra-synaptic A1R activation influences the response to excitatory stimuli by hyperpolarizing the resting membrane potential via activation of inward rectifying potassium channels (6) and controlling the N-type channels (7) and N-methyl-D-aspartate (NMDA) receptors (8). Dose and time-dependent phosphorylation of extracellular signal-regulated kinases (ERK) 1/2 by A1R activation has also been reported (9). b) Agonism of A2ARs coupled with Gs or, in the striatum, with Golf, leads to an increase in cAMP and activation of protein kinase A and downstream signaling pathways (10). It is indeed well established that activation of A2AR controls the recruitment of cAMP response element binding protein (CREB), a transcription factor involved in memory formation. It has also been shown that A2AR modulation of neurotransmitter release is dependent on protein kinase C activity (11), and that the recruitment of mitogen activated protein kinases (12) underlies the ability of A2AR to prevent synaptotoxicity and memory impairment in Alzheimer’s mouse models. A2AR can dimerize with themselves, A1R, D2 dopamine receptors, D3 dopamine receptors, metabotropic glutamate type 5 receptors and the cannabinoid CB1 receptor in a synergistic or antagonistic fashion (13). Activation of A2ARs facilitates the release of BDNF, BDNF-mediated synaptic transmission and hippocampal LTP (14). A2ARs can influence hippocampal synaptic plasticity by modifying post-synaptic calcium responses. At hippocampal mossy fiber-CA3 synapses A2AR activation mediates LTP elicited by (15) NMDA and mGluR5 -dependent calcium increases. A2AR-dependent activation of PKA regulates the phosphorylation of GluR1, its insertion in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, thus AMPA-evoked LTP in CA1 pyramidal neurons as well as the potentiation of LTP at CA3-CA1 synapses (16).
Agonism of A2ARs coupled to Gs or, in the striatum, to Golf [117], leads to an increase in cAMP and activation of protein kinase A and downstream signaling pathways [107]. It is indeed well established that activation of A2AR controls the recruitment of cAMP response element binding protein (CREB), a transcription factor involved in memory formation [118–120]. It has also been shown that A2ARs modulation of neurotransmitter release is dependent on protein kinase C activity [121–123], and that the recruitment of mitogen activated protein kinases underlies the ability of A2AR to prevent synaptotoxicity and memory impairment in Alzheimer’s mouse models [90]. The understanding of A2AR signaling mechanisms is further complicated by the fact that they can dimerize with themselves [124], A1R [125], D2 dopamine receptors [126], D3 dopamine receptors [127], metabotropic glutamate type 5 receptors [128] and the cannabinoid CB1 receptor [129] in a synergistic or antagonistic fashion [107]. A2ARs can also interact and transactivate receptor tyrosine kinases in the absence of neurothrophins [130–132]. Brain-derived neurotrophic factor (BDNF) is a critical modulator of hippocampal synaptic plasticity under physiological and pathological conditions [133]. Activation of A2ARs facilitates the release of BDNF, BDNF-mediated synaptic transmission [134] and hippocampal LTP [135]. Pharmacological inhibition as well as genetic knockout of A2ARs results in decreased levels of BDNF in the brain [136]. In addition to modulation of BDNF signaling, A2ARs can influence hippocampal synaptic plasticity by modifying post-synaptic calcium responses. At hippocampal mossy fiber-CA3 synapses A2AR activation modulates LTP elicited by NMDA- and mGluR5- dependent calcium increases [78]. A2AR-dependent activation of PKA regulates the phosphorylation of GluR1, its incorporation into α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and thus AMPA-evoked LTP in CA1 pyramidal neurons as well as the potentiation of LTP at CA3-CA1 synapses [137] (Figure 3b).
Due to differences in expression levels of A1R and A2AR, their opposing effects on hippocampal LTP and the fact that caffeine has very similar binding affinities for each receptor, predicting the impact of caffeine on LTP/LTD remains a challenge. As mentioned previously, the data gathered over the last several decades using pharmacological and gene knockout experiments suggests that the neurological effects of caffeine, and possibly other methylxanthines, correlates well with inhibition of A2ARs [1, 62, 98]
COFFEE, AND CACAO PURINES AND NEUROLOGICAL DISORDERS
Because of the widespread distribution of adenosine receptors throughout the body and nervous system, methylxanthines have several potential therapeutic applications. Theobromine and theophylline are used as smooth muscle relaxants, vasodilators, diuretics and myocardial stimulants [138]. Caffeine can act as an adjuvant analgesic increasing the action of painkillers [139], and because of its effect on metabolism and insulin sensitivity [140–142] is included in weight loss supplements. A possible role for methylxanthines in preserving brain health is suggested by epidemiological studies evaluating the association of nutritional and lifestyle factors with neurodegenerative conditions [143–147]. Habitual intake of methylxanthines in humans is associated with a reduced risk of stroke [148], depression [149, 150] and suicide [151]. Higher caffeine levels in the cerebral spinal fluid (CSF) are correlated with better clinical outcomes in patients with traumatic brain injury [152]. Levels of theobromine in the CSF are inversely correlated with the amyloid beta 42 levels in Alzheimer’s patients [153]. Some, but not all population studies have found positive associations between coffee and tea intake and cognitive performance in older subjects [33, 154, 155]. In particular, habitual caffeine consumption appears to improve verbal memory [155], long-term memory and psychomotor speed [33].
With regard to neurodegenerative disorders, the strongest associations have been found for methylxanthine consumption and Parkinson’s disease (PD) incidence. Several meta-analyses have shown that moderate coffee intake lowers one’s risk of developing PD by 24–30% [146, 156–158]. In general, an inverse dose-response association between coffee and tea intake and PD incidence has been consistently reported in studies of men, with a maximal effect found at about 3 cups of daily coffee [156, 158]. However in women, the associations between methylxanthine consumption and PD risk is more complex with regards to dose and hormonal status [159, 160]. Data suggest that caffeine is beneficial against PD at low doses in women not receiving estrogen therapy; however, at high doses it may increase PD risk in those under hormonal replacement therapy [159]. It should be appreciated, however, that such epidemiological data cannot account for all possible confounding variables, and further experimentation is required to establish whether caffeine, theophylline and/or other methylxanthines influence symptoms and/or progression of PD.
The gender disparities suggested by epidemiological studies are substantiated by human studies showing that caffeine metabolism is inhibited in women taking estrogen either in oral contraceptives or as replacement supplements after menopause [161, 162]. Furthermore, studies in rodents have demonstrated that estrogen supplementation interferes with the protection afforded by caffeine against dopaminergic loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD [163]. Notably, in the same experimental model the caffeine metabolites paraxanthine and theophylline also conferred neuroprotection against MPTP-induced striatal dopamine loss [164]. Similar to other physiological responses, antagonism of adenosine receptors may mediate protection of dopaminergic neurons by methylxanthines. In fact, antagonism of A2A receptors with selective inhibitors or genetic knockout mimics caffeine protection in various experimental models of PD [165–167].
An inverse relationship between habitual methylxanthine consumption and risk of developing Alzheimer’s disease (AD) late in life was found in several longitudinal studies [11, 42, 168, 169]. In animal studies, caffeine intake can decrease brain amyloid burden and prevent or ameliorate memory impairment in AD transgenic mice [170–173], as well as in pharmacological models of AD [88]. Mechanisms that may mediate the benefits exerted by caffeine and derivatives in AD models include, adenosine receptor antagonism [88, 174], regulation of cerebral blood flow [175–177], increased oxygen consumption [178] and increased cerebrospinal fluid production [179, 180].
Recent genome-wide analyses studying the genetic variants that influence coffee consumption provide insights into additional mechanisms of action of caffeine, paraxanthine, theophylline and theobromine [181, 182]. Together with loci directly linked to methylxanthine metabolism, these studies discovered new loci associated with habitual caffeine intake spanning about 90 genes implicated in various metabolic and physiological functions [181, 182]. Among the various genes, some have potentially important implications for brain physiology and pathology. For example, the gene ATP-binding cassette sub-family G member 2 (ABCG2) is a xenobiotic transporter also expressed in the endothelium of the blood brain barrier, where it has been shown to regulate the clearance of amyloid beta peptides from the parenchyma [183, 184]. The locus at 2p24 includes the gene encoding glucokinase regulatory protein (GCKR) and is known to play an important role in glucose, cholesterol, triglyceride and urate metabolism [185, 186]. Similarly, 17q11.2 includes the gene encoding MLX interacting protein like (MLXIPL), which activates carbohydrate response element motifs in the promoter regions of genes implicated in glucose and lipid metabolism in a glucose-dependent manner [187].
Notably, alterations in glucose and lipid metabolism have long been associated with the incidence of different neurodegenerative disorders [188]. The locus 11p13 (associated with BDNF) and LIN7C are also relevant to neurodegenerative disorders. BDNF is a neurotrophin involved in survival, differentiation, and synaptic plasticity of several neuronal systems [133]. Alterations in BDNF levels have been found with aging and in many psychiatric and neurodegenerative diseases [133,189]. LIN7C has been shown to be instrumental for vertebrate neurulation [190] and ensures proper localization of NMDA receptor subunit 2 at post synaptic densities, as well as potassium channels (Kir2), GABA transporters and 5-hydroxytryptamine type 2C receptors [191–193]. Finally, expression of the SNP at 17q11.2 maps EF-hand calcium binding domain 5 (EFCAB5) and is negatively correlated with epigenetic age acceleration in five different brain regions [194].
CONCLUSIONS AND PERSPECTIVE
Increasing evidence suggests that regular moderate consumption of coffee, tea and cacao can enhance brain health. Purines are one class of phytochemicals that may contribute to the beneficial effects of these widely consumed plant products on the brain. Among such purines, caffeine has been the most widely studied, theobromine and theophylline less so, and other methylxanthines have been largely unexplored (Figure 1). While the neurological effects of caffeine are well-established, it is not known whether this purine is solely responsible for beneficial effects of coffee and cacao consumption on cognition and resistance to neurodegenerative disorders. Indeed, emerging evidence suggests that other classes of phytochemicals present in high amounts in coffee and cacao can enhance neuroplasticity and protect neurons against dysfunction and degeneration. Among the many non-purine phytochemicals in coffee and cacao, flavonoids such as epicatechins have been shown to promote synaptic plasticity, enhance cognition and protect neurons in experimental models of stroke and AD [195–198]. The presence of numerous neuroactive chemicals in coffee, tea and chocolate has thus far precluded the identification of the specific chemical or combination of chemicals that account for the beneficial effects of consumption of these plant materials on brain health suggested by data from epidemiological studies. Nevertheless, the literature reviewed in the present article suggests that purine metabolites are one prominent class of such neuroactive chemicals.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging.
References
- 1.Fredholm BB, Battig K, Holmen J, Nehlig A, Zxartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 2.Trivino AH. Chocolate: the story of Nahuatlism. Estudios de Cultura Nahuatl. 2013;46:37–87. [Google Scholar]
- 3.Dicum G, Luttinger N. The coffee book: anatomy of an industry from crop to the last drop. The New Press; New York: 1999. [Google Scholar]
- 4.Kunz H. Emil Fischer – unequalled classicist, master of organic chemistry research, and inspired trailblazer of biological chemistry. Agnew Chem Int Ed. 2002;41:4439–4451. doi: 10.1002/1521-3773(20021202)41:23<4439::AID-ANIE4439>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Donovan JL, DeVane CL. A primer on caffeine pharmacology and its drug interactions in clinical psychopharmacology. Psychopharmacol Bull. 2001;35:30–48. [PubMed] [Google Scholar]
- 6.Mattson MP. What doesn’t kill you…. Sci Am. 2015;313:40–45. doi: 10.1038/scientificamerican0715-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stidworthy MF, Bleakley JS, Cheeseman MT, Kelly DF. Chocolate poisoning in dogs. Vet Rec. 1997;141:28. [PubMed] [Google Scholar]
- 8.Nathanson JA. Caffeine and related methylxanthines: possible naturally occurring pesticides. Science. 1984;226:184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Jo DG, Park D, Chung HY, Mattson MP. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev. 2014;66:815–868. doi: 10.1124/pr.113.007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson-Kozlow M, Kritz-Silverstein D, Barrett-Connor E, Morton D. Coffee consumption and cognitive function among older adults. Am J Epidemiol. 2002;156:842–850. doi: 10.1093/aje/kwf119. [DOI] [PubMed] [Google Scholar]
- 11.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 12.Powers KM, Kay DM, Factor SA, Zabetian CP, Higgins DS, Samii A, Nutt JG, Griffith A, Leis B, Roberts JW, Martinez ED, Montimurro JS, Checkoway H, Payami H. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov Disord. 2008;23:88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- 13.Ma QP, Huang C, Cui QY, Yang DJ, Sun K, Chen X, Li XH. Meta-Analysis of the Association between Tea Intake and the Risk of Cognitive Disorders. PLoS One. 2016;11:e0165861. doi: 10.1371/journal.pone.0165861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchard J, Sawers SJ. The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol. 1983;24:93–98. doi: 10.1007/BF00613933. [DOI] [PubMed] [Google Scholar]
- 15.Chvasta TE, Cooke AR. Emptying and absorption of caffeine from the human stomach. Gastroenterology. 1971;61:838–843. [PubMed] [Google Scholar]
- 16.Tang-Liu DD, Williams RL, Reigelman S. Disposition of caffeine and its metabolites in man. J Pharmacol Exp Ther. 1983;224:180–185. [PubMed] [Google Scholar]
- 17.Arnaud MJ. Metabolism of caffeine and other components of coffee. In: Garattini S, editor. Caffeine, coffee and health. Raven; New York: 1993. pp. 43–95. [Google Scholar]
- 18.Arnaud MJ. Pharmocokinetics and metabolism of natural methyxanthines in animal and man. In: Fredholm BB, editor. Handbook of experimental pharmacology. Vol. 200. Springer; Berlin: 2011. pp. 33–92. [DOI] [PubMed] [Google Scholar]
- 19.Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 20.Gu L, Gonzalez FJ, Kalow W, Tang BK. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics. 1992;2:73–77. doi: 10.1097/00008571-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz NL, Jacob P, 3rd, Mayan H, Denaro C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin Pharmacol Ther. 1995;58:684–691. doi: 10.1016/0009-9236(95)90025-X. [DOI] [PubMed] [Google Scholar]
- 22.Tang-Liu DD, Riegelman S. Metabolism of theophylline to caffeine in adults. Res Commun Chem Pathol Pharmacol. 1981;34:371–80. [PubMed] [Google Scholar]
- 23.Gates S, Miners JO. Cytochrome P450 isoform selectivity in human hepatic theobromine metabolism. Br J Clin Pharmacol. 1999;47:299–305. doi: 10.1046/j.1365-2125.1999.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lelo A, Birkett DJ, Miners JO. Mechanism of formation of 6-amino-5-(N-methylformylamino)-1-methyluracil and 3,7-dimethyluric acid from theobromine in the rat in vitro: involvementof cytochrome P-450 and a cellular thiol. Xenobiotica. 1990;20:823–833. doi: 10.3109/00498259009046896. [DOI] [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer. International Agency for Research on Cancer (IARC) monographs on the evaluation of carcinogenic risks to humans: coffee, tea, mate, methylxanthines and methylglyoxal. WHO; Geneva: 1991. [PMC free article] [PubMed] [Google Scholar]
- 26.Lelo A, Birkett DJ, Robson RA, Miners JO. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Br J Clin Pharmacol. 1986;22:177–182. doi: 10.1111/j.1365-2125.1986.tb05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnaud MJ. Identification, kinetic and quantitative study of [2-14C] and [1-Me-14C] caffeine metabolites in rat’s urine by chromatographic separations. Biochem Med. 1976;16:67–76. doi: 10.1016/0006-2944(76)90010-7. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson JM, Pollard I. Accumulation of theophylline, theobromine and paraxanthine in the fetal rat brain following a single oral dose of caffeine. Brain Res Dev Brain Res. 1993;75:193–199. doi: 10.1016/0165-3806(93)90023-4. [DOI] [PubMed] [Google Scholar]
- 29.Stahle L. Drug distribution studies with microdialysis: I. Tissue dependent difference in recovery between caffeine and theophylline. Life Sci. 1991;49:1835–1842. doi: 10.1016/0024-3205(91)90486-u. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda M, Sugimoto N, Katakura M, Matsuzaki K, Tanigami H, Yachie A, Onho-Shosaku T, Shido O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J Nutr Biochem. 2017;39: 110–116. doi: 10.1016/j.jnutbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Arnaud MJ, Enslen M. The role of paraxanthine in mediating physiological effects of caffeine. 14th international conference in coffee science; San Francisco. 14–19; Paris: Proceedings ASIC; 1992. pp. 71–79. [Google Scholar]
- 32.Arnold ME, Petros TV, Beckwith BE, Coons G, Gorman N. The effects of caffeine, impulsivity, and sex on memory for word lists. Physiol Behav. 1987;41:25–30. doi: 10.1016/0031-9384(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 33.Hameleers PA, Van Boxtel MP, Hogervorst E, Riedel WJ, Houx PJ, Buntinx F, Jolles J. Habitual caffeine consumption and its relation to memory, attention, planning capacity and psychomotor performance across multiple age groups. Hum Psychopharmacol. 2000;15:573–581. doi: 10.1002/hup.218. [DOI] [PubMed] [Google Scholar]
- 34.Kelemen WL, Creeley CE. State-dependent memory effects using caffeine and placebo do not extend to metamemory. J Gen Psychol. 2003;130:70–86. doi: 10.1080/00221300309601276. [DOI] [PubMed] [Google Scholar]
- 35.Smit HJ, Gaffan EA, Rogers PJ. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology (Berl) 2004;176:412–419. doi: 10.1007/s00213-004-1898-3. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell ES, Slettenaar M, vd Meer N, Transler C, Jans L, Quadt F, Berry M. Differential contributions of theobromine and caffeine on mood, psychomotor performance and blood pressure. Physiol Behav. 2011;104:816–822. doi: 10.1016/j.physbeh.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Scholey A, Owen L. Effects of chocolate on cognitive function and mood: a systematic review. Nutr Rev. 2013;71:665–681. doi: 10.1111/nure.12065. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie K, Carrière I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 39.Corley J, Jia X, Kyle JA, Gow AJ, Brett CE, Starr JM, McNeill G, Deary IJ. Caffeine consumption and cognitive function at age 70: the Lothian Birth Cohort 1936 study. Psychosom Med. 2010;72:206–214. doi: 10.1097/PSY.0b013e3181c92a9c. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 41.van Boxtel MP, Schmitt JA, Bosma H, Jolles J. The effects of habitual caffeine use on cognitive change: a longitudinal perspective. Pharmacol Biochem Behav. 2003;75:921–927. doi: 10.1016/s0091-3057(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 42.van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE study. Eur J Clin Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 43.Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L, Bocale R, Lechiara MC, Marini C, Ferri C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The cocoa, cognition, and aging (CoCoA) study. Hypertension. 2012;60:794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 44.Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, Righetti R, Bocale R, Lechiara MC, Marini C, Ferri C, Desideri G. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the cocoa, cognition, and aging (CoCoA) study--a randomized controlled trial. Am J Clin Nutr. 2015;101:538–548. doi: 10.3945/ajcn.114.092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stavric B, Gilbert SG. Caffeine metabolism: a problem in extrapolating results from animal studies to humans. Acta Parm Jugosl. 1990;40:475–489. [Google Scholar]
- 46.Beavo JA, Rogers NL, Crofford OB, Hardman JG, Sutherland EW, Newman EV. Effects of xanthine derivatives on lipolysis and on adenosine 3′,5′-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970;6:597–603. [PubMed] [Google Scholar]
- 47.Francis SH, Sekhar KR, Ke H, Corbin JD. Inhibition of cyclic nucleotide phosphodiesterase by methylxanthines and related compounds. In: Fredholm BB, editor. Handbook of experimental pharmacology. Springer; Berlin: 2011. pp. 93–134. [DOI] [PubMed] [Google Scholar]
- 48.Rousseau E, Ladine J, Liu QY, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Meissner G. Structure-activity relationship of xanthines and skeletal muscle ryanodine receptor/Ca2+ release channel. Pharmacology. 1997;54:135–143. doi: 10.1159/000139480. [DOI] [PubMed] [Google Scholar]
- 50.Hawke TJ, Allen DG, Lindinger MI. Paraxanthine, a caffeine metabolite, dose dependently increases [Ca(2+)](i) in skeletal muscle. J Appl Physiol. 2000;89:2312–2317. doi: 10.1152/jappl.2000.89.6.2312. [DOI] [PubMed] [Google Scholar]
- 51.Pessah IN, Stambuk RA, Casida JE. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987;31:232–238. [PubMed] [Google Scholar]
- 52.Guerreiro S, Toulorge D, Hirsch E, Marien M, Sokoloff P, Michel PP. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol Pharmacol. 2008;74:980–989. doi: 10.1124/mol.108.048207. [DOI] [PubMed] [Google Scholar]
- 53.Butcher RW, Sutherland EW. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J Biol Chem. 1962;237:1244–1250. [PubMed] [Google Scholar]
- 54.Fredholm BB. On the mechanism of action of theophylline and caffeine. Acta Med Scand. 1985;217:149–153. doi: 10.1111/j.0954-6820.1985.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 55.Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 57.De Mendonca A, Ribeiro J. Adenosine and neuronal plasticity. Life Sci. 1996;60:245–251. doi: 10.1016/s0024-3205(96)00544-9. [DOI] [PubMed] [Google Scholar]
- 58.Costenla AR, de Mendonca A, Ribeiro JA. Adenosine modulates synaptic plasticity in hippocampal slices from aged rats. Brain Res. 1999;851:228–234. doi: 10.1016/s0006-8993(99)02194-0. [DOI] [PubMed] [Google Scholar]
- 59.Yacoubi ME, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Mendonca A, Ribeiro JA. Adenosine and synaptic plasticity. Drug Dev Res. 2001;52:283–290. [Google Scholar]
- 61.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 62.Costenla AR, Cunha RA, Mendonca A. Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimers Dis. 2010;20:S25–S34. doi: 10.3233/JAD-2010-091384. [DOI] [PubMed] [Google Scholar]
- 63.Reichert CF, Maire M, Schmidt C, Cajochen C. Sleep-wake regulation and its impact on working memory performance: the role of adenosine. Biology (Basel) 2016;5:1–25. doi: 10.3390/biology5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daly JW. Caffeine analogs: biomedical impact. Cell Mol Life Sci. 2007;64:2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang JN, Chen JF, Fredholm BB. Physiological roles of A1 and A2A adenosine receptors in regulating heart rate, body temperature, and locomotion as revealed using knockout mice and caffeine. Am J Physiol Heart Circ Physiol. 2009;296:H1141–H1149. doi: 10.1152/ajpheart.00754.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cunha RA. How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem. 2016;139:1019–1055. doi: 10.1111/jnc.13724. [DOI] [PubMed] [Google Scholar]
- 68.Ochiishi T, Saitoh Y, Yukawa A, Saji M, Ren Y, Shirao T, Miyamoto H, Nakata H, Sekino Y. High level of adenosine A1 receptor-like immunoreactivity in the CA2/CA3a region of the adult rat hippocampus. Neuroscience. 1999;93:955–967. doi: 10.1016/s0306-4522(99)00179-7. [DOI] [PubMed] [Google Scholar]
- 69.Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by u pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- 70.Zhao M, Choi YS, Obrietan K, Dudek SM. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. J Neurosci. 2007;27:12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, Cowan DP, Ramineni S, Yates CK, Feng Y, Smith Y, Sweatt JD, Weinshenker D, Ressler KJ, Dudek SM, Hepler JR. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci U S A. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simons SB, Escobedo Y, Yasuda R, Dudek SM. Regional differences in hippocampal calcium handling provide a cellular mechanism for limiting plasticity. Proc Natl Acad Sci U S A. 2009;106:14080–14084. doi: 10.1073/pnas.0904775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simons SB, Caruana DA, Zhao M, Dudek SM. Caffeine-induced synaptic potential in hippocampal CA2 neurons. Nat Neurosci. 2012;15:23–25. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, Agostinho PM, Ribeiro JA, Cunha RA, de Mendonça A. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci. 2011;34:12–21. doi: 10.1111/j.1460-9568.2011.07719.x. [DOI] [PubMed] [Google Scholar]
- 75.Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Lupica CR, Cass WA, Zahniser NR, Dunwiddie TV. Effects of the selective adenosine A2 receptor agonist CGS 21680 on in vitro electrophysiology, cAMP formation and dopamine release in rat hippocampus and striatum. J Pharmacol Exp Ther. 1990;252:1134–1141. [PubMed] [Google Scholar]
- 77.Cunha RA, Constantino MD, Ribeiro JA. ZM241385 is an antagonist of the facilitatory responses produced by the A2A adenosine receptor agonists CGS21680 and HENECA in the rat hippocampus. Br J Pharmacol. 1997;122:1279–1284. doi: 10.1038/sj.bjp.0701507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 79.Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X, Wang Y, van Westen GJ, Payen MP, Augusto E, Goncalves N, Tome AR, Li Z, Wu Z, Zhou Y, IJzerman A, Boyden ES, Cunha RA, Qu J, Chen JF. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry. 2015;20:1339–1349. doi: 10.1038/mp.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viana da Silva S, Habert MG, Zhang P, Bethge P, Lemos C, Goncalves N, Gorlewicz A, Malezieux M, Goncalves FQ, Grosjean N, Blanchet C, Frick A, Nagert UV, Cunha RA, Mulle C. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat Commun. 2016;7:11915. doi: 10.1038/ncomms11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pagnussat N, Almeida AS, Marques DM, Nunes F, Chenet GC, Botton PH, Mioranza S, Loss CM, Cunha RA, Porciuncula LO. Adenosine A(2A) receptors are necessary and sufficient to trigger memory impairment in adult mice. Br J Pharmacol. 2015;172:3831–3845. doi: 10.1111/bph.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, Zheng RY, Cai XH, Chen JF, He JC. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 83.Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, Chen JF. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–474. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei CJ, Augusto E, Gomes CA, Singer P, Wang Y, Boison D, Cunha RA, Yee BK, Chen JF. Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biol Psychiatry. 2014;75:855–863. doi: 10.1016/j.biopsych.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simoes AP, Machado NJ, Goncalves N, Kaster MP, Simoes AT, Nunes A, Pereira de Almeida L, Goosens KA, Rial D, Cunha RA. Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology. 2016;41:2862–2871. doi: 10.1038/npp.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 87.Kadowaki Horita T, Kobayashi M, Mori A, Jenner P, Kanda T. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology (Berl) 2013;230:345–352. doi: 10.1007/s00213-013-3158-x. [DOI] [PubMed] [Google Scholar]
- 88.Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210:776–781. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009;29:14741–51. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Batalha VL, Pego JM, Fontinha BM, Costenla AR, Valadas JS, Baqi Y, Radjainia H, Muller CE, Sebastiao AM, Lopes LV. Adenosine A2a receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol Psychiatry. 2013;18:320–331. doi: 10.1038/mp.2012.8. [DOI] [PubMed] [Google Scholar]
- 92.Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M, Baqi Y, Muller CE, Rodrigues AL, Porciuncula LO, Chen JF, Tome AR, Agostinho P, Canas Pm, Cinha AR. Caffeine acts through neuronal adenosine A2A receptors to prevent mood memory disfunction triggered by chronic stress. Proc Natl Acad Sci USA. 2015;112:7833–7838. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Albasanz JL, Perez S, Barrachina M, Ferrer I, Martin M. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathol. 2008;18:211–219. doi: 10.1111/j.1750-3639.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J, Yu GQ, Adame A, Devidze N, Dubal DB, Masliah E, Conkin BR, Mucke L. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci. 2015;18:423–434. doi: 10.1038/nn.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rebola N, Porciumcula LO, Lopes LV, Oliveira CR, Soares da Silva P, Cunha RA. Long term effect of convulsive behavior on the density of adenosine A1 and A2A receptors in the rat cerebral cortex. Epilepsia. 2005;46:159–165. doi: 10.1111/j.1528-1167.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- 96.Nehlig A, Lucignani G, Kadekaro M, Porrino LJ, Sokoloff L. Effects of acute administration of caffeine on local cerebral glucose utilization in the rat. Eur J Pharmacol. 1984;101:91–100. doi: 10.1016/0014-2999(84)90034-7. [DOI] [PubMed] [Google Scholar]
- 97.Nehlig A, Daval JL, Boyet S, Vert P. Comparative effects of acute and chronic administration of caffeine on local cerebral glucose utilization in the conscious rat. Eur J Pharmacol. 1986;129:93–103. doi: 10.1016/0014-2999(86)90340-7. [DOI] [PubMed] [Google Scholar]
- 98.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic, and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 99.Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC. Caffeine in floral nectar enhances a pollinator’s memory of reward. Science. 2013;339:1202–1204. doi: 10.1126/science.1228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strachecka A, Krauze M, Olszewski K, Borsuk G, Paleolog J, Merska M, Chobotow J, Bajda M, Grzyniwicz K. Biochemistry (Mosc) 2014;79:1192–1201. doi: 10.1134/S0006297914110066. [DOI] [PubMed] [Google Scholar]
- 101.Londos C, Cooper DM, Wolf J. Subclasses of external adenosine receptors. Proc Natl Acad Scie USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Calker D, Muller M, Hamprecht B. Adenosine regulates via two types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 103.Fredholm BB, Chen Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Dunwiddie TV, Hoffer BJ, Fredholm BB. Alkylxanthines elevate hippocampal excitability. Evidence for a role of endogenous adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:326–330. doi: 10.1007/BF00501365. [DOI] [PubMed] [Google Scholar]
- 105.Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K+ channels to A1receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990;259:H820–826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- 106.Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 107.Chen JF. Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol. 2014;119:257–307. doi: 10.1016/B978-0-12-801022-8.00012-X. [DOI] [PubMed] [Google Scholar]
- 108.Faure M, Voyno-Yasenetskaya TA, Bourne HR. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 109.Gimenez-Llort L, Masino SA, Diao L, Fernandez-Teruel A, Tobena A, Halldner L, et al. Mice lacking the adenosine A(1) receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse. 2005;57:8–16. doi: 10.1002/syn.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kukley M, Schwan M, Fredholm BB, Dietrich D. The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J Neurosci. 2005;25:2832–2837. doi: 10.1523/JNEUROSCI.4260-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Scie USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu G, Zhou QX, Kang S, Li QL, Zhai LC, Chen JD, et al. Chronic morphine treatment impaired hippocampal long-term potentiation and spatial memory via accumulation of extracellular adenosine acting on adenosine a1 receptors. J Neurosci. 2010;30:5058–5070. doi: 10.1523/JNEUROSCI.0148-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, et al. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 115.Lang UE, Lang F, Ricther K, Vallon V, Lipp HP, Schermann J, et al. Emotional instability but intact spatial cognition in adenosine receptor knock out mice. Behav Brain Res. 2003;145:179–188. doi: 10.1016/s0166-4328(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 116.Hooper N, Fraser C, Stone TW. Effects of purine analogues on spontaneous alterations in mice. Psycopharmacology. 1996;123:250–257. doi: 10.1007/BF02246579. [DOI] [PubMed] [Google Scholar]
- 117.Kull B, Svenningsson P, Fredholm BB. Adenosine A2A receptors are co-localized with and activate Golf in rat striatum. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 118.Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 119.Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 120.Kandel ER. The molecular biology of memory; cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gubitz AK, Widdowson L, Kurokawa M, Kirkpatrick KA, Richardson PJ. Dual signaling by the adenosine A2A receptor involves activation of both N- and P-type calcium channels by different G proteins and protein kinases in the same striatal nerve terminals. J Neurochem. 1996;67:374–381. doi: 10.1046/j.1471-4159.1996.67010374.x. [DOI] [PubMed] [Google Scholar]
- 122.Cunha RA, Ribeiro JA. ATP as a presynaptic modulator. Life Sci. 2000;68:119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- 123.Cunha RA, Ribeiro JA. Purinergic modulation of [3H] GABA release from rat hippocampal nerve terminals. Neuropharmacol. 2000;39:1156–1167. doi: 10.1016/s0028-3908(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 124.Vidi PA, Chemel BR, Hu CD, Watts VJ. Ligand-dependent oligomerization of dopamine D(2) and adenosine A(2A) receptors in living neuronal cells. Mol Pharmacol. 2008;74:544–551. doi: 10.1124/mol.108.047472. [DOI] [PubMed] [Google Scholar]
- 125.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 127.Torvinen M, Marcellino D, Canals M, Agnati LF, Lluis C, Franco R, Fuxe K. Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol Pharmacol. 2005;67:400–407. doi: 10.1124/mol.104.003376. [DOI] [PubMed] [Google Scholar]
- 128.Diaz-Cabiale Z, Vivo M, Del Arco A, O’Connor WT, Harte MK, Muller CE, Martinez E, Popoli P, Fuxe K, Ferre S. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett. 2002;324:154–158. doi: 10.1016/s0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 129.Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Muller C, Woods AS, Hope BT, Ciruela F, Casado V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferre S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- 130.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sebastiao AM, Ribeiro JA. Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. Br J Pharmacol. 2009;158:15–22. doi: 10.1111/j.1476-5381.2009.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sebastiao AM, Ribeiro JA. Adenosine receptors and the central nervous system. Hand Exp Pharmacol. 2009;193:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- 133.Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diogenes MJ, Fernandes CC, Sebastiao AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fontinha BM, Diogenes MJ, Ribeiro JA, Sebastiao AM. Enhancement of long-termpotentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology. 2008;54:924–933. doi: 10.1016/j.neuropharm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 136.Domenici MR, Scattoni ML, Martire A, Lastoria G, Potenza RL, Borioni A, Venerosi A, Calamandrei G, Popoli P. Behavioral and electrophysiological effects of the adenosine receptor antagonist SCH 58261 in R6/2 Huntington’s disease mice. Neurobiol Dis. 2007;28:197–205. doi: 10.1016/j.nbd.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 137.Dias RB, Ribeiro JA, Sabastiao AM. Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A2A receptors. Hippocampus. 2012;22:276–291. doi: 10.1002/hipo.20894. [DOI] [PubMed] [Google Scholar]
- 138.Fredholm BB, editor. Handbook of experimental pharmacology. Vol. 200. Springer; Berlin: 2011. [DOI] [PubMed] [Google Scholar]
- 139.Laska EM, Sunshine A, Mueller F, Elvers WB, Siegel C, Rubin A. Caffeine as an analgesic adjuvant. JAMA. 1984;251:1711–1718. [PubMed] [Google Scholar]
- 140.Fredholm BB. effect of adenosine, adenosine analogues, and drugs inhibiting adenosine inactivation on lipolysis in rat fat cells. Acta Physiol Scand. 1978;102:191–198. doi: 10.1111/j.1748-1716.1978.tb06062.x. [DOI] [PubMed] [Google Scholar]
- 141.Szkudelski T, Szkudelski K, Nogowski L. Effect of adenosine a1 receptor antagonism on lpogenesis and lipolysis in isolated rat adipocytes. Physiol Res. 2009;58:863–871. doi: 10.33549/physiolres.931467. [DOI] [PubMed] [Google Scholar]
- 142.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes; a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 143.Jiménez-Jiménez FJ, Mateo D, Giménez-Roldan S. Premorbid smoking, alcohol consumption, and coffee drinking habits in Parkinson’s disease: a case-control study. Mov Disord. 1992;7:339–344. doi: 10.1002/mds.870070407. [DOI] [PubMed] [Google Scholar]
- 144.Hellenbrand W, Seidler A, Robra B-P, et al. Smoking and Parkinson’s disease: a case control study in Germany. Int J Epidemiol. 1997;26:328–339. doi: 10.1093/ije/26.2.328. [DOI] [PubMed] [Google Scholar]
- 145.Fall P-A, Frederikson M, Axelson O, Granérus A-K. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 146.Ross GW, Abbott RD, Petrovitch H. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 147.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson’s Disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 148.Larsson SC. Coffee, tea, and cocoa and risk of stroke. Stroke. 2014;45:309–14. doi: 10.1161/STROKEAHA.113.003131. [DOI] [PubMed] [Google Scholar]
- 149.Smith AP. Caffeine, cognitive failures and health in a non-working community sample. Hum Psychopharmacol. 2009;24:29–34. doi: 10.1002/hup.991. [DOI] [PubMed] [Google Scholar]
- 150.Grosso G, Micek A, Castellano S, Pajak A, Galvano F. Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223–34. doi: 10.1002/mnfr.201500620. [DOI] [PubMed] [Google Scholar]
- 151.Kawachi I, Willett WC, Colditz GA, Stampfer MJ, Speizer FE. A prospective study of coffee drinking and suicide in women. Arch Intern Med. 1996;116:521–525. [PubMed] [Google Scholar]
- 152.Sachse KT, Jackson EK, Wisniewski SR, Gillespie DG, Puccio AM, Clark RS, Dixon CE, Kochanek PM. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab. 2008;28:395–401. doi: 10.1038/sj.jcbfm.9600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Travassos M, Santana J, Baldeiras I, Tsolaki M, Gkatzima O, Sermin G, Yener GG, et al. does caffeine consumption modify cerebrospinal fluid amyloid-b levels in patients with Alzheimer’s disease? J Alzheimers Dis. 2015;47:1069–1078. doi: 10.3233/JAD-150374. [DOI] [PubMed] [Google Scholar]
- 154.Jarvis MJ. Does caffeine intake enhance absolute levels of cognitive performance? Psychopharmacology (Berl) 1993;110:45–52. doi: 10.1007/BF02246949. [DOI] [PubMed] [Google Scholar]
- 155.van Boxtel MP, Schmitt JA, Bosma H, Jolles J. The effects of habitual caffeine use on cognitive change: a longitudinal perspective. Pharmacol Biochem Behav. 2003;75:921–927. doi: 10.1016/s0091-3057(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 156.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 157.Costa J, Lunet N, Santos C, Santos J, Vaz-Carneiro A. Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20(Suppl):221–238. doi: 10.3233/JAD-2010-091525. [DOI] [PubMed] [Google Scholar]
- 158.Qi H, Li S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson’s disease. Geriatr Gerontol Int. 2014;14:430–439. doi: 10.1111/ggi.12123. [DOI] [PubMed] [Google Scholar]
- 159.Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease. Neurology. 2003;60:790–795. doi: 10.1212/01.wnl.0000046523.05125.87. [DOI] [PubMed] [Google Scholar]
- 160.Ascherio A, Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Rodriguez C, Thun MJ. Coffee consumption, gender, and Parkinson’s disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. American Journal of Epidemiology. 2004;160:977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 161.Patwardhan RV, Desmond PV, Johnson RF, Schenker S. Impaired elimination of caffeine by oral contraceptive steroids. J Lab Clin Med. 1980;95:603–608. [PubMed] [Google Scholar]
- 162.Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur J Clin Pharmacol. 1985;28:425–8. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]
- 163.Xu K, Xu Y, Brown-Jermyn D, Chen JF, Ascherio A, Dluzen DE, Schwarzschild MA. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2006;26:535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu K, Xu YH, Chen JF, Schwarzschild MA. Neuroprotection by caffeine: time course and role of its metabolites in the MPTP model of Parkinson’s Disease. Neuroscience. 2010;167:475–481. doi: 10.1016/j.neuroscience.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen JF, Xu K, Petzer JP, Staal R, XYJ, Beilstein M, Sonsalla PK, Caatagnoli K, Castagnoli N, Schwarzschild MA. Neuroprotection by caffeine and A2a adenosine receptor inactivation in a model of Parkinson’s disease. The Journal of Neuroscience. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J Neurochem. 2002;80:262–270. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 167.Kalda A, Yu L, Oxtas E, Chen JF. Novel neuroprotection by caffeine and adenosine A2A receptor antagonists in animal models of Parkinson’s disease. Journal of Neurological Science. 2006;242:9–15. doi: 10.1016/j.jns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 168.Maia L, de Mendonça A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 169.Lindsay J, Sykes E, McDowell I, Verreault R, Laurin D. More than the epidemiology of Alzheimer’s disease: contributions of the Canadian Study of Health and Aging. Can J Psychiatry. 2004;49:83–91. doi: 10.1177/070674370404900202. [DOI] [PubMed] [Google Scholar]
- 170.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 171.Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 172.Chu YF, Chang WH, Black RM, Liu JR, Sompol P, Chen Y, Wei H, Zhao Q, Cheng IH. Crude caffeine reduces memory impairment and amyloid β(1-42) levels in an Alzheimer’s mouse model. Food Chem. 2012;135:2095–2102. doi: 10.1016/j.foodchem.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 173.Laurent C, Eddarkaoui S, Derisbourg M, Leboucher A, Demeyer D, Carrier S, Schneider M, Hamdane M, Muller CE, Bucee L, Blum D. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol of Aging. 2014;35:2079–2090. doi: 10.1016/j.neurobiolaging.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 174.Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y, Malik E, Mariciniak E, Parrot S, Van der Jeugd A, Faivre E, Flaten V, Ledent C, D’Hooge R, Sergeant N, Hamdane M, Humez S, Müller CE, Lopes LV, Buée L, Blum D. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol Psychiatry. 2016;21:97–107. doi: 10.1038/mp.2014.151. [DOI] [PubMed] [Google Scholar]
- 175.Pelligrino DA, Xu HL, Vetri F. Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis. 2010;20:S51–62. doi: 10.3233/JAD-2010-091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Klaassen EB, de Groot RH, Evers EA, Snel J, Veerman EC, Ligtenberg AJ, Jolles J, Veltman DJ. The effect of caffeine on working memory load-related brain activation in middle-aged males. Neuropharmacology. 2013;64:160–167. doi: 10.1016/j.neuropharm.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 177.Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Verius M, Haala I, Mottaghy FM, Rhomberg P, Golaszewski S, Gotwald T, Lorenz IH, Kolbitsch C, Felber S, Krause BJ. Does caffeine modulate verbal working memory processes? An fMRI study. Neuroimage. 2007;39:492–499. doi: 10.1016/j.neuroimage.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 178.Haller S, Rodriguez C, Moser D, Toma S, Hofmeister J, Sinanaj I, Van De Ville D, Giannakopoulos P, Lovblad KO. Acute caffeine administration impact on working memory-related brain activation and functional connectivity in the elderly: a BOLD and perfusion MRI study. Neuroscience. 2013:10364–10371. doi: 10.1016/j.neuroscience.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 179.Han ME, Kim HJ, Lee YS, Kim DH, Choi JT, Pan CS, Yoon S, Baek SY, Kim BS, Kim JB, Oh SO. Regulation of cerebrospinal fluid production by caffeine consumption. BMC Neurosci. 2009;30:110. doi: 10.1186/1471-2202-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wostyn P, Van Dam D, Audenaert K, De Deyn PP. Increased Cerebrospinal Fluid Production as a Possible Mechanism Underlying Caffeine’s Protective Effect against Alzheimer’s Disease. Int J Alzheimers Dis. 2011;2011:617420. doi: 10.4061/2011/617420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Coffee and Caffeine Genetics Consortium. Cornelis MC, Byrne EM, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 2015;20:647–656. doi: 10.1038/mp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cornelis MC, Kacprowski T, Menni C, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. 2016;25:5472–5482. doi: 10.1093/hmg/ddw334. [DOI] [PubMed] [Google Scholar]
- 183.Do TM, Noel-Hudson MS, Ribes S, Besengez C, Smirnova M, Cisternino S, Buyse M, Calon F, Chimini G, Chacun H, Scherrmann JM, Farinotti R, Bourasset F. ABCG2- and ABCG4-mediated efflux of amyloid-β peptide 1-40 at the mouse blood-brain barrier. J Alzheimers Dis. 2012;30:155–166. doi: 10.3233/JAD-2012-112189. [DOI] [PubMed] [Google Scholar]
- 184.Zhang W, Xiong H, Callaghan D, Liu H, Jones A, Pei K, Fatehi D, Brunette E, Stanimirovic D. Blood-brain barrier transport of amyloid beta peptides in efflux pump knock-out animals evaluated by in vivo optical imaging. Fluids Barriers CNS. 2013;10:13. doi: 10.1186/2045-8118-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Raimondo A, Rees MG, Gloyn AL. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism. Curr Opin Lipidol. 2015;26:88–95. doi: 10.1097/MOL.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Camandola S, Mattson MP. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017 Jun 1;36(11):1474–1492. doi: 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 190.Yang X, Zou J, Hyde DR, Davidson LA, Wei X. Stepwise maturation of apicobasal polarity of the neuroepithelium is essential for vertebrate neurulation. J Neurosci. 2009;29:11426–11440. doi: 10.1523/JNEUROSCI.1880-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jo K, Derin R, Li M, Bredt DS. Characterization of MALS/Velis-1, -2, and -3: a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J Neurosci. 1999;19:4189–4199. doi: 10.1523/JNEUROSCI.19-11-04189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bécamel C, Alonso G, Galéotti N, Demey E, Jouin P, Ullmer C, Dumuis A, Bockaert J, Marin P. Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J. 2002;21:2332–2342. doi: 10.1093/emboj/21.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem. 2004;279:19051–19063. doi: 10.1074/jbc.M400284200. [DOI] [PubMed] [Google Scholar]
- 194.Lu AT, Hannon E, Levine ME, Crimmins EM, Lunnon K, Mill J, Geschwind DH, Horvath S. Genetic architecture of epigenetic and neuronal ageing rates in human brain regions. Nat Commun. 2017;8:15353. doi: 10.1038/ncomms15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sokolov AN, Pavlova MA, Klosterhalfen S, Enck P. Chocolate and the brain: neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci Biobehav Rev. 2013;37:2445–2453. doi: 10.1016/j.neubiorev.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 196.Walker JM, Klakotskaia D, Ajit D, Weisman GA, Wood WG, Sun GY, Serfozo P, Simonyi A, Schachtman TR. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J Alzheimers Dis. 2015;44:561–572. doi: 10.3233/JAD-140981. [DOI] [PubMed] [Google Scholar]
- 197.Jia N, Han K, Kong JJ, Zhang XM, Sha S, Ren GR, Cao YP. (−)-Epigallocatechin-3-gallate alleviates spatial memory impairment in APP/PS1 mice by restoring IRS-1 signaling defects in the hippocampus. Mol Cell Biochem. 2013;380:211–218. doi: 10.1007/s11010-013-1675-x. [DOI] [PubMed] [Google Scholar]
- 198.Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B, Pan Y, Gowda GA, Raftery D, Arrieta-Cruz I, Sharma V, Cooper B, Lobo J, Simon JE, Zhang C, Cheng A, Qian X, Ono K, Teplow DB, Pavlides C, Dixon RA, Pasinetti GM. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J Neurosci. 2012;32:5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]