Abstract
Introduction
The etiology of chronic rhinosinusitis (CRS)-associated olfactory loss is unclear, but may result from inflammatory changes in the olfactory epithelium that result in signaling dysfunction or loss of olfactory neurons. Several pro-inflammatory cytokines have been associated with CRS but their expression within the olfactory cleft microenvironment and association with olfactory function is unknown.
Level of evidence
NA
Methods
Mucus was collected from the olfactory cleft and middle meatus of 31 CRS without nasal polyp (CRSsNP) subjects, 36 CRS with nasal polyps (CRSwNP) subjects, and 12 healthy controls. Olfactory function was assessed using the validated Smell Identification Test (SIT). Site-specific levels of 14 cytokines/chemokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17A, TNF-α, Eotaxin, RANTES) were assessed using a multiplex flow cytometric bead assay and correlated with SIT scores.
Results
Mucus cytokine levels in the olfactory cleft were strongly or moderately correlated with levels in the middle meatus for all but one measured inflammatory mediators. SIT scores were inversely correlated with levels of IL-2 (p=0.006), IL-5 (p<0.0001), IL-6 (p=0.0009), IL-10 (p<0.0001), and IL-13 (p<0.0001), with significance largely driven by CRSwNP patients.
Conclusions
The inflammatory microenvironment within the olfactory cleft mirrors that within the middle meatus. Elevated levels of IL-2, IL-5, IL-6, IL-10, and IL-13 in olfactory cleft mucus are associated with reduced olfactory identification scores in CRS patients. Altered levels of select olfactory mucus cytokines could potentially have deleterious effects on olfactory neuron function and turnover.
Keywords: rhinosinusitis, sinusitis, olfaction, smell, cytokine, anosmia, polyps
INTRODUCTION
Chronic rhinosinusitis (CRS) is among the most common chronic inflammatory diseases with a prevalence of up to 12% of the U.S. population, at an estimated annual direct cost of more than 11 billion dollars1–3. Olfactory dysfunction is a frequent complaint of CRS patients, particularly those with nasal polyposis, with an estimated prevalence of between 30 and 80% 4–6. It is also a major contributor to poor quality of life and reduced productivity in this patient population 7–9. Despite the common association between CRS and loss of smell, very little is known about the mechanisms that mediate CRS-specific olfactory dysfunction 10. Most evidence suggests that hyposmia or anosmia in CRS patients is due to a combination of 1) conductive olfactory loss, and 2) sensorineural olfactory loss. Conductive loss has traditionally been attributed to nasal polyps, which can potentially obstruct the ability of odorants to reach the olfactory cleft. However, a minority of CRS patients have nasal polyps, and many prior studies suggest that olfactory dysfunction is largely independent of nasal airflow and nasal obstruction 11,12.
Recent evidence suggests that sensorineural olfactory loss may be the primary cause of hyposmia in CRS patients 13–15. This mechanism is linked to chronic inflammation that mediates a loss of functional olfactory neurons and replacement of the olfactory epithelium with respiratory epithelium. In support of this hypothesis, Yee et al. reported that anosmic CRS patients, when compared to healthy normosmic controls, display histopathologic changes to the olfactory epithelium, including reduced olfactory neurons, squamous metaplasia, olfactory epithelial erosion, and goblet cell hyperplasia 14.
Inflammation in CRS is mediated largely by pro-inflammatory cytokines that circulate locally in sinonasal tissue. Several cytokines have neurotoxic potential16–19, and it is conceivable that the olfactory dysfunction in CRS patients may be mediated in part by their effects on olfactory neuronal cell function, apoptosis, differentiation, and proliferation. Recent evidence from transgenic mouse models have supported this ‘olfactory inflammation’ hypothesis, with local overexpression of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) resulting in inhibition of olfactory neuron function, turnover, or survival 20–23. Likewise, a recent study in patients with CRS found that quantitative olfactory function may be inversely associated with olfactory mucus levels of IL-5 24.
Despite the putative importance of pro-inflammatory cytokines in olfactory function, studies to clarify the role of cytokines in human olfactory tissue have been limited. This is largely due to the inherent difficulty associated with establishing olfactory neuronal cultures, and technical challenges associated with obtaining human olfactory tissue. We hypothesized that the human olfactory cleft may represent a unique inflammatory microenvironment and that measurement of mucus cytokine levels could be used as a reflection of olfactory inflammation and function. The goal of this study was to 1) evaluate mucus cytokine levels in the human olfactory cleft, 2) compare the inflammatory microenvironment within the olfactory cleft to the remainder of the middle meatus, and 3) identify potential associations between individual cytokines and objective olfactory function.
METHODS
Subject Enrollment
Patients with CRS were recruited from the Vanderbilt Asthma, Sinus and Allergy Program (ASAP) and Otolaryngology clinics. All subjects met the criteria for CRS as defined by the European Position Paper on Rhinosinusitis and Nasal Polyps and the International Consensus Statement on Allergy and Rhinology 25,26. Patients with a history of olfactory loss due to a non-CRS etiology were excluded from the study. CRS patients offered surgery had previously failed adequate medical therapy that included two or more weeks of oral prednisone, three or more weeks of broad-spectrum or culture-directed antibiotics, and a combination of other additional therapies including oral/topical antihistamines, topical nasal steroid sprays, oral decongestants, mucolytics, or saline rinses. Patients who had received oral corticosteroids within 4 weeks of surgery were excluded from the study. Patients with a diagnosis of cystic fibrosis, Churg-Strauss syndrome, known immunodeficiency, autoimmune disease, or who were receiving any monoclonal biologic therapy were also excluded. Control patients were undergoing either endoscopic endonasal pituitary surgery, endoscopic skull base tumor resection, or endoscopic cerebrospinal fluid leak repair and had no clinical or radiographic history of CRS. Voluntary informed consent was obtained for all patients and the study protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. Sinonasal-specific quality-of-life was measured utilizing the 22-item Sinonasal Outcome Test (SNOT-22).
Computed Tomography and Histopathological Testing
All patients underwent a high-resolution computed tomography (CT) scan of the paranasal sinuses. Each scan was evaluated by 2 authors who were blinded to subject identifiers and diagnosis. A standard Lund-Mackay scoring system was used to assess overall extent of CRS. Sinonasal tissue was collected from the ethmoid bulla or adjacent ethmoid mucosa in all patients. Tissue from healthy controls was collected from either the ethmoid sinus or sphenoid face. Histopathological evaluation of excised tissue was performed by a pathologist in a blinded fashion and the mean number of eosinophils counted over 5 randomly selected HPFs was recorded.
Flow Cytometric Analysis of Mucus Cytokines
At the beginning of surgery, two 9 × 24mm polyurethane sponges (Summit Medical; St. Paul, MN) were placed, one into the middle meatus and one into the olfactory cleft of each subject under endoscopic guidance. Each sponge was removed after 5 minutes, placed in a sterile microcentrifuge tube and immediately processed. Sponges were placed into a microporous centrifugal filter device (MilliporeSigma; Billerica, MA) and centrifuged at 14,000 × g for 10 minutes to elute mucus. Samples were then gently vortexed and again centrifuged for 5 minutes to remove any cellular debris. Supernatants were removed, placed into a new microcentrifuge tube, and frozen at −80°C for later analysis.
Multiplex cytokine assays were performed using a multiplex cytokine bead assay (BD Biosciences; Franklin Lakes, NJ) according to the manufacturer’s protocol. We attempted to include all pro-inflammatory cytokines available on this particular platform, excluding only growth factors and most chemokines. Briefly, 50 μL of mucus was incubated with 50 μl of mixed capture beads for each measured inflammatory mediator and incubated for 1 hour. 50 μL of mixed detection reagent was then added to each sample and standard, and incubated for an additional 2 hours. After addition of 1 mL wash buffer, samples were centrifuged at 200 × g for 5 minutes and the supernatant was discarded. The beads were then resuspended in 300 μL wash buffer and analyzed on an LSR Fortessa flow cytometer (BD Biosciences). Data was analyzed using BD FCAP Array Software version 3.0.
Olfactory testing
Subjects enrolled in the study completed the 40-item Smell Identification Test (SIT) immediately prior to surgery. The SIT has excellent sensitivity, correlates closely with scores attained via formal threshold testing, and has the advantage of being easily administered to subjects on the day of surgical intervention 27. Raw scores were adjusted for patient age and gender by subtracting the mean normative age- and sex-appropriate SIT score from the total SIT score for each subject. Thus a negative adjusted SIT score represents reduced sense of smell compared to the mean for that subject’s age and gender. Normative SIT scores were extracted from the Smell Identification Test Administration Manual (Sensonics International; Haddon Heights, NJ).
Statistics
Mean mucus cytokine levels for each group were measured in picograms per milliliter and groups were compared using the Mann-Whitney test. Samples with individual cytokine levels that were not detectable were assigned a value of half the lowest detectable level for that particular cytokine. Correlation between olfactory cleft and middle meatus cytokine levels was measured using Spearman rank order. Interpretation of correlations was based on the r-statistic (0.20–0.39 = weak correlation; 0.40–0.59 = moderate correlation; >0.60 = strong correlation). The association between mucus cytokine levels and age- and sex-adjusted SIT scores was analyzed using Spearman rank order correlation because the data did not demonstrate parametric distribution and was not suggestive of a linear relationship. Correlation analysis was not performed for cytokines in which greater than 30% of samples were below the detection limit of the assay. A p-value < 0.05 was considered statistically significant for all comparisons. All statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software; La Jolla, CA)
RESULTS
Study Population and Demographics
A total of 31 CRSsNP, 36 CRSwNP, and 12 healthy control subjects were enrolled in the study (Table 1). The CRSsNP and CRSwNP groups were similar in age and sex distribution. As expected, subjects with CRSwNP had higher rates of comorbid asthma (p = 0.007), more severe CT scores (p < 0.0001), and worse olfactory function (p < 0.0001). CRSwNP subjects were also more likely to have had prior endoscopic sinus surgery (p = 0.05) and had more severe tissue eosinophilia on surgical pathology (p < 0.0001).
Table 1.
Demographic and clinical characteristics of study population.
| Healthy controls | CRSsNP | CRSwNP | P-value | |
|---|---|---|---|---|
|
| ||||
| No. | 12 | 31 | 36 | |
|
|
||||
| Age, years | 47.8 +/− 10.9 | 47.9 +/− 2.3 | 46.6 +/− 2.5 | 0.72 |
|
|
||||
| Sex, no. female (%) | 8 (67%) | 16 (52) | 15 (42) | 0.47 |
|
|
||||
| Asthma, no. (%) | 0 (0) | 9 (29) | 23 (64) | 0.007 |
|
|
||||
| Allergic rhinitis, no. (%) | 1 (8) | 17 (55) | 28 (78) | 0.07 |
|
|
||||
| SNOT-22 score | - | 46.3 +/− 16.4 | 46.8 +/− 19.2 | 0.95 |
|
|
||||
| CT score | 2.6 +/− 2.2 | 12.6 +/− 4.4 | 18.4 +/− 4.5 | <0.0001 |
|
|
||||
| SIT score | −3.9 +/− 3.6 | −6.5 +/− 9.3 | −16.6 +/− 10.2 | <0.0001 |
|
|
||||
| Prior surgery, no. (%) | 0 (0) | 8 (26) | 19 (53) | 0.05 |
|
|
||||
| Tissue eos/HPF | 0.4 +/− 0.9 | 21.6 +/− 35.0 | 75.5 +/− 71.5 | <0.0001 |
Mucus Cytokine Levels in the Olfactory Cleft
Mucus levels of 14 different cytokines and inflammatory mediators were measured from the olfactory clefts of CRS and control patients. Mean cytokine levels and ranges are presented in Table 2. As shown, CRSsNP was associated with reduced mucus levels of IL-7 (p = 0.02) when compared to healthy controls (Figure 1). Levels of IL-1β, IL-2, IL-5, IL-6, and IL-10 were moderately elevated, but these differences did not reach statistical significance. CRSwNP mucus contained significantly elevated levels of IL-5 (p < 0.0001) and IL-13 (p = 0.014) (Figure 1).
Table 2.
Mean olfactory mucus levels of 14 different cytokines and inflammatory mediators for CRSwNP, CRSsNP, and healthy control subjects.
| Control | CRSsNP | CRSwNP | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (range) | Mean (range) | p-value | Mean (range) | p-value | |
| IL-1β | 59.67 (0.335–295.7) | 143.2 (0.67–2351) | 0.97 | 94.38 (1.98–709) | 0.90 |
|
|
|||||
| IL-2 | 6.51 (0.07–46.0) | 95.54 (0.07–2797) | 0.59 | 19.55 (0.07–460) | 0.69 |
|
|
|||||
| IL-4 | 1.11 (0.005–6.22) | 0.42 (0.005–2.8) | 0.13 | 1.32 (0.005–14.5) | 0.85 |
|
|
|||||
| IL-5 | 0.52 (0.005–3.24) | 5.43 (0.005–83.3) | 0.23 | 42.46 (0.005–342.4) | <0.0001 |
|
|
|||||
| IL-6 | 94.73 (10.31–715.1) | 165.1 (1–2424) | 0.60 | 131.7 (2.94–1773) | 0.19 |
|
|
|||||
| IL-7 | 5.18 (0.63–14.4) | 2.74 (0.04–10.5) | 0.02 | 10.07 (0.3–55.1) | 0.57 |
|
|
|||||
| IL-8 | 3643 (118.9–22214) | 2673 (118.9–16532) | 0.40 | 4094 (282.7–32579) | 0.74 |
|
|
|||||
| IL-10 | 4.49 (0.07–9.3) | 10.27 (0.07–197.7) | 0.36 | 7.66 (0.07–69.2) | 0.99 |
|
|
|||||
| IL-12 | 128.3 (2.67–427) | 53.18 (0.04–257.1) | 0.23 | 94.52 (0.04–766.1) | 0.23 |
|
|
|||||
| IL-13 | 3.14 (0.005–17.2) | 2.51 (0.005–27.7) | 0.80 | 38.52 (0.005–334.9) | 0.01 |
|
|
|||||
| IL-17A | 1.66 (0.1–15.2) | 0.56 (0.1–6.07) | 0.50 | 7.94 (0.1–240.6) | 0.27 |
|
|
|||||
| Eotaxin | 18.16 (1.4–49.3) | 12.55 (0.065–44.1) | 0.11 | 18.45 (0.065–149.5) | 0.18 |
|
|
|||||
| TNF-α | 10.6 (0.025–63.1) | 8.99 (0.025–134.2) | 0.42 | 7.12 (0.025–7.12) | 0.85 |
|
|
|||||
| RANTES | 2500 (9.57–6600) | 1535 (18.97–6463) | 0.15 | 1675 (0.71–7471) | 0.26 |
Figure 1.
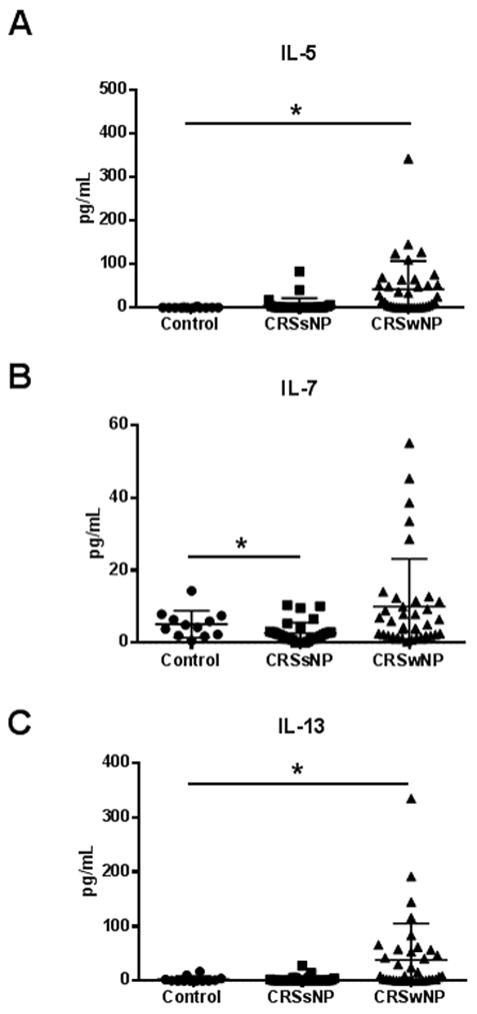
Olfactory cleft mucus from subjects with CRSwNP contained elevated levels of IL-5 (A) and IL-13 (C) while CRSsNP mucus contained reduced levels of IL-7 (B). Data is presented as a scatter plot with the mean and standard error for each group. *, p < 0.05 compared to control.
Comparison of Cytokine Levels Between the Olfactory Cleft and Middle Meatus
In order to determine whether the olfactory cleft represents a unique inflammatory microenvironment, cytokine levels in olfactory cleft mucus were compared to those of the middle meatus. Mean levels were lower in the olfactory cleft for 11 of 14 measured inflammatory mediators (IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, eotaxin, TNF-α, RANTES), however, none of these differences reached statistical significance (Table 3). With the exception of IL-17A (r = 0.17, p = 0.14), levels of all measured inflammatory mediators were moderately (IL-2, IL-10, IL-12) or strongly (IL-1β, IL-4, IL-5, IL-6, IL-7, IL-8, IL-13, eotaxin, TNF-α, RANTES) correlated between the olfactory cleft and middle meatus (Table 3).
Table 3.
Correlation between mucus cytokine levels of the olfactory cleft and middle meatus of all study patients.
| Middle Meatus pg/mL, mean (range) | Olfactory Cleft pg/mL, mean (range) | r | p-value | |
|---|---|---|---|---|
|
| ||||
| IL-1β | 264.67 (0.18–7159) | 108.112 (0.34–2351) | 0.78 | <0.0001 |
|
|
||||
| IL-2 | 35.68 (0.19–1855) | 47.04 (0.07–2797) | 0.55 | <0.0001 |
|
|
||||
| IL-4 | 1.26 (0–21.4) | 0.94 (0.005–14.5) | 0.63 | <0.0001 |
|
|
||||
| IL-5 | 31.21 (0.005–431.6) | 21.82 (0.005–342.4) | 0.89 | <0.0001 |
|
|
||||
| IL-6 | 333.99 (1.35–9621) | 141.79 (1–2424) | 0.77 | <0.0001 |
|
|
||||
| IL-7 | 5.69 (0.17–30.6) | 6.50 (0.04–55.1) | 0.69 | <0.0001 |
|
|
||||
| IL-8 | 4912.41 (83.47–42119) | 3475.83 (118.9–32579) | 0.69 | <0.0001 |
|
|
||||
| IL-10 | 13.90 (0.04–307) | 8.20 (0.07–197.7) | 0.58 | <0.0001 |
|
|
||||
| IL-12 | 92.94 (0.27–2401) | 83.57 (0.04–766.1) | 0.56 | <0.0001 |
|
|
||||
| IL-13 | 32.20 (0.005–414.3) | 19.26 (0.005–334.9) | 0.75 | <0.0001 |
|
|
||||
| IL-17A | 2.34 (0.05–29.9) | 4.14 (0.01–240.6) | 0.17 | 0.14 |
|
|
||||
| Eotaxin | 23 (0.69–311.4) | 16.12 (0.065–149.5) | 0.73 | <0.0001 |
|
|
||||
| TNF-α | 8.63 (0.08–128.6) | 8.37 (0.025–134.2) | 0.69 | <0.0001 |
|
|
||||
| RANTES | 2030.6 (1.94–8253) | 1744.32 (0.71–7471) | 0.69 | <0.0001 |
Relationship Between Olfactory Cleft Cytokines and Olfactory Function
Levels of individual cytokines were next correlated with age- and sex-adjusted SIT scores. Olfactory function was inversely correlated with olfactory cleft levels of IL-2 (p=0.006), IL-5 (p<0.0001), IL-6 (p=0.0009), IL-10 (p<0.0001), and IL-13 (p<0.0001) (Table 4). Patients with and without polyps were then analyzed separately in order to eliminate this variable as a possible confounding factor. Consistent with results from the overall cohort, olfactory function in CRSwNP subjects was inversely correlated with levels of IL-5 (p=0.01), IL-6 (p=0.01), IL-10 (p<0.001), and IL-13 (p=0.001). For CRSsNP subjects, correlations did not reach statistical significance for any cytokines, with the exception of IL-7, which demonstrated a weak positive correlation with SIT scores (p=0.05).
Table 4.
Correlation between mucus cytokine levels of the olfactory cleft and age- and sex-adjusted SIT scores.
| All CRS | CRSsNP | CRSwNP | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Spearman r | p value | Spearman r | p value | Spearman r | p value | |
| IL-1β | −0.12 (−0.35–0.12) | 0.31 | −0.07 (−0.42–0.29) | 0.69 | −0.11 (−0.42–0.23) | 0.52 |
|
|
||||||
| IL-2 | −0.32 (−0.52– −0.09) | 0.006 | −0.24 (−0.55–0.13) | 0.18 | −0.19 (−0.49–0.14) | 0.25 |
|
|
||||||
| IL-4 | 0.18 (−0.11–0.45) | 0.21 | ND | - | 0.16 (−0.17–0.46) | 0.32 |
|
|
||||||
| IL-5 | −0.51 (−0.67– −0.31) | <0.0001 | −0.11 (−0.45–0.26) | 0.54 | −0.41 (−0.65– −0.09) | 0.01 |
|
|
||||||
| IL-6 | −0.38 (−0.57– −0.16) | 0.0009 | −0.23 (−0.54–0.14) | 0.20 | −0.39 (−0.64– −0.08) | 0.01 |
|
|
||||||
| IL-7 | −0.02 (−0.26–0.22) | 0.84 | 0.35 (−0.01–0.63) | 0.05 | −0.15 (−0.46–0.18) | 0.35 |
|
|
||||||
| IL-8 | −0.12 (−0.35–0.12) | 0.32 | −0.08 (−0.43–0.28) | 0.66 | −0.10 (−0.41–0.23) | 0.53 |
|
|
||||||
| IL-10 | −0.51 (−0.67– −0.31) | <0.0001 | −0.17 (−0.50–0.20) | 0.35 | −0.51 (−0.72– −0.23) | <0.001 |
|
|
||||||
| IL-12 | −0.12 (−0.35–0.12) | 0.32 | −0.06 (−0.41–0.31) | 0.76 | −0.03 (−0.35–0.29) | 0.84 |
|
|
||||||
| IL-13 | −0.46 (−0.63– −0.25) | <0.0001 | 0.15 (−0.22–0.48) | 0.41 | −0.51 (−0.71– −0.22) | 0.001 |
|
|
||||||
| IL-17A | −0.10 (−0.39–0.20) | 0.49 | ND | - | −0.17 (−0.47–0.1) | 0.31 |
|
|
||||||
| Eotaxin | −0.13 (−0.36–0.12) | 0.29 | 0.15 (−0.22–0.48) | 0.43 | −0.17 (−0.46–0.17) | 0.31 |
|
|
||||||
| TNF-α | −0.17 (−0.39–0.07) | 0.16 | −0.03 (−0.39–0.33) | 0.85 | 0.08 (−0.26–0.39) | 0.65 |
|
|
||||||
| RANTES | −0.01 (−0.25–0.23) | 0.93 | −0.13 (−0.47–0.24) | 0.47 | 0.15 (−0.19–0.45) | 0.38 |
DISCUSSION
The current study is among the first to specifically assess markers of inflammation in the human olfactory cleft. We chose to measure the levels of inflammatory mediators in sinonasal mucus, as it is easily collected and can be standardized between patients. A previous study also confirmed that mucus cytokine levels directly correlate with adjacent tissue cytokine levels 28. This study utilized mucus collected at the time of surgical intervention, however, collection of mucus is relatively non-invasive and has the potential for office-based sample collection in non-anesthetized patients.
The present data reveals that olfactory cleft mucus from CRSwNP patients contains elevated levels of IL-5 and IL-13, cytokines that have been closely associated with Th2-mediated inflammation. Interestingly, a previous report evaluating mucus cytokine levels in CRS patients reported results that conflict with the present study 29. No statistically significant difference in IL-5 levels between healthy control and CRSwNP subjects was identified, and IL-13 levels were actually lower in CRSwNP than in control patients. However, it should be noted that these results are in contrast to multiple previously published studies that have confirmed elevated levels of IL-5 and IL-13 in CRSwNP tissue 30–32, and the authors themselves questioned whether these conflicting results might be due to methodological errors. Our findings suggest that mucus IL-5 and IL-13 levels may have potential as non-invasive biomarkers of CRSwNP.
CRSsNP mucus was associated with reduced levels of IL-7. This cytokine is known to be important for B- and T-cell maturation, but a role for IL-7 in CRS has not previously been defined. In asthmatic patients, IL-7 is rapidly secreted following allergen challenge and has been associated with the number of eosinophils in bronchoalveolar lavage fluid 33. While multiple other cytokines were elevated in CRSsNP mucus, none of these reached statistical significance. Substantial variability in cytokine levels were identified in CRSsNP mucus, suggesting that this disease phenotype likely has multiple etiologies with no single immunologic basis. Future studies with a larger patient cohort may help to further delineate some of these potential disease endotypes.
The effect of pro-inflammatory cytokines on olfactory function remains largely unknown. In a study of 59 patients with olfactory loss due to multiple etiologies, Henkin et al. found that these patients had higher plasma, urine and mucus levels of IL-6 compared to patients with normal olfactory function 34. Our study found higher mucus levels of IL-6 in both CRSwNP and CRSsNP patients when compared to healthy controls, however, these differences did not reach statistical significance. We did, however, find that IL-6 levels were associated with significantly lower SIT scores. This result is in contrast to that by Schlosser et al. which conversely demonstrated a weak positive correlation between mucus IL-6 levels and olfactory identification scores 24. Of note, both IL-6 and IL-6 receptor expression have been reported in olfactory mucosa 35, and elevations in IL-6 have been associated with reduced neurogenesis 17.
Previous studies have shown that olfactory-specific expression of TNF-α results in olfactory dysfunction and loss of olfactory neurons in mice 20–22. However, our study comparing mucus from CRS and healthy control subjects showed that TNF-α levels are not altered in the human olfactory cleft, suggesting that this particular cytokine may not have an important function in CRS-associated olfactory loss. We did, however, find that the Th2-associated cytokines IL-5 and IL-13 are secreted in high levels within olfactory cleft mucus of patients with CRSwNP. Interestingly, Schlosser et al. recently reported that mucus IL-5 levels were inversely correlated with objective olfactory function in all patients with CRS 24. We identified this association as well, while noting similar findings for the Th2-associated cytokine IL-13, though in our case these associations seemed to be driven primarily by the CRSwNP phenotype. Reduced SIT scores were also associated with elevated mucus levels of IL-2 and IL-10. IL-2 is a T-cell effector that regulates immunity and tolerance, but its role in chronic rhinosinusitis is poorly defined. IL-10 is a Th2-associated anti-inflammatory cytokine that inhibits the activity of Th1 cells 36, and in this case may represent a marker of elevated Th2 inflammation, despite its anti-inflammatory properties. Of note, our group recently reported that elevated tissue eosinophilia is associated with worse objective sense of smell in CRSwNP patients, suggesting that eosinophils or eosinophil-associated Th2 cytokines may have direct roles in olfactory function 37.
It should be noted that relationships between inflammatory mediators and olfactory function highlighted in our study were somewhat dependent on polyp status. On the surface, this would suggest that identified correlations could be driven by factors specific to the CRSwNP phenotype, rather than the cytokines themselves. Theoretically, if the effect of cytokines on olfactory function is truly causative, then the phenotype should be irrelevant. However, it should be noted that while correlations identified in the overall cohort did not reach statistical significance in the CRSsNP subgroup, a consistent negative correlation did persist in these patients for IL-2, IL-5, IL-6, and IL-10. Our study may therefore simply be underpowered to identify statistically significant correlations in the CRSsNP phenotype, largely due to a more compact range of smell function and lower overall SIT scores in these patients. Further attempts to decipher causation vs. association will likely require larger translational and basic science studies.
We found that cytokine levels in the olfactory cleft directly correlated with levels in the middle meatus. These results suggest that the olfactory cleft does not represent a unique inflammatory microenvironment, and is instead reflective of inflammatory changes within the remainder of the sinonasal cavity. This finding should facilitate future studies that evaluate inflammatory mediators in sinonasal mucus, since the middle meatus is easily accessible even in awake patients. Several previous studies have specifically evaluated olfactory cleft inflammation 24,38,39, however, we would propose that subsequent studies could instead utilize mucus from the middle meatus or ethmoid cavity as an indirect reflection of olfactory cleft inflammation.
There are several limitations to our study that deserve further mention. First, while we have used olfactory cleft mucus as a gauge of local inflammatory burden, it is not clear that this is indicative of changes at the level of the olfactory epithelium itself. The olfactory epithelium is unique in its cellular makeup and function, and its role in immune system function and mucus secretion is poorly understood. It remains possible that levels of olfactory cleft cytokines may be indicative of levels found in adjacent respiratory epithelium proximal to the olfactory mucosa. While direct assessment of olfactory tissue itself would circumvent this issue, the difficulty in obtaining adequate and consistent olfactory biopsies and the small amount of available material for study, makes this approach less than ideal. Second, it is likely that mucus cytokine levels may vary considerable over time and in relation to CRS or comorbid disease exacerbations. We have attempted to negate the impact of temporal variations by assessing olfactory function and cytokine levels on the same day. However, subsequent studies that assess temporal variations in individual cytokines and any potential effects on olfactory function, may help to clarify this issue. Of note, we did not exclude patients with a history of prior endoscopic sinus surgery from our study, and it remains possible that scarring, fibrosis or other adverse effects could alter olfactory function in these patients, independent of inflammatory markers. Subsequent larger studies that specifically assess this variable on olfaction will likely be forthcoming. Finally, it should be clarified that the results of the current study do not suggest clear causation. While our data suggests an association between specific cytokines and olfactory loss in CRS patients, certain disease-specific factors could also plan an important role, and this question will certainly require further investigation.
To our knowledge, the current study is the largest to date to evaluate inflammatory changes to the human olfactory cleft in CRS. Strengths of the study include its prospective design and simultaneous evaluation of multiple cytokines and inflammatory mediators. Our inability to identify significant differences for most cytokines between CRS and control patients suggests that much larger studies will ultimately be needed going forward. It also highlights the limitations of phenotypic categorization of CRS based on the presence or absence of nasal polyps alone.
CONCLUSION
Olfactory cleft mucus from patients with CRSwNP contained elevated levels of IL-5 and IL-13, while mucus from CRSsNP patients contained reduced levels of IL-7. The levels of cytokines and inflammatory mediators in the olfactory cleft were closely correlated with levels in the middle meatus, suggesting that the inflammatory environment in the olfactory cleft may be similar to that within the remainder of the sinonasal cavity. Elevated levels of IL-2, IL-5, IL-6, IL-10 and IL-13 were associated with reduced objective olfactory function in CRS patients.
Acknowledgments
This project was supported by NIH RO3 DC014809 (J.H.T.), L30 AI113795 (J.H.T.), and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Financial disclosures: No relevant disclosures
Conflicts of interest: None
References
- 1.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital and health statistics Series 10, Data from the National Health Survey. 2009:1–157. [PubMed] [Google Scholar]
- 2.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. The Journal of allergy and clinical immunology. 2010;125:S103–115. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 3.Rudmik L, Soler ZM, Mace JC, Schlosser RJ, Smith TL. Economic evaluation of endoscopic sinus surgery versus continued medical therapy for refractory chronic rhinosinusitis. The Laryngoscope. 2014 doi: 10.1002/lary.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Current opinion in otolaryngology & head and neck surgery. 2012;20:29–32. doi: 10.1097/MOO.0b013e32834dfb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. American journal of rhinology. 2008;22:445–448. doi: 10.2500/ajr.2008.22.3195. [DOI] [PubMed] [Google Scholar]
- 6.Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM. The prevalence of olfactory dysfunction in chronic rhinosinusitis. The Laryngoscope. 2017;127:309–320. doi: 10.1002/lary.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katotomichelakis M, Simopoulos E, Zhang N, et al. Olfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseases. American journal of rhinology & allergy. 2013;27:293–298. doi: 10.2500/ajra.2013.27.3903. [DOI] [PubMed] [Google Scholar]
- 8.Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Livaditis M, Danielides V. Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. The Laryngoscope. 2012;122:1450–1454. doi: 10.1002/lary.23349. [DOI] [PubMed] [Google Scholar]
- 9.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. American journal of rhinology & allergy. 2009;23:139–144. doi: 10.2500/ajra.2009.23.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinology. 2017 [Google Scholar]
- 11.Hox V, Bobic S, Callebaux I, Jorissen M, Hellings PW. Nasal obstruction and smell impairment in nasal polyp disease: correlation between objective and subjective parameters. Rhinology. 2010;48:426–432. doi: 10.4193/Rhino10.049. [DOI] [PubMed] [Google Scholar]
- 12.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. The Laryngoscope. 2001;111:409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern RC, Conley DB, Haines GK, 3rd, Robinson AM. Pathology of the olfactory mucosa: implications for the treatment of olfactory dysfunction. The Laryngoscope. 2004;114:279–285. doi: 10.1097/00005537-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Yee KK, Pribitkin EA, Cowart BJ, et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. American journal of rhinology & allergy. 2010;24:110–120. doi: 10.2500/ajra.2010.24.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstantinidis I, Witt M, Kaidoglou K, Constantinidis J, Gudziol V. Olfactory mucosa in nasal polyposis: implications for FESS outcome. Rhinology. 2010;48:47–53. doi: 10.4193/Rhin09.102. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon gamma directly regulates neural precursors in the non-inflammatory brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9038–9050. doi: 10.1523/JNEUROSCI.5691-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanekar S, Gandham M, Lucero MT. PACAP protects against TNFalpha-induced cell death in olfactory epithelium and olfactory placodal cell lines. Molecular and cellular neurosciences. 2010;45:345–354. doi: 10.1016/j.mcn.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Li K, Zhu L, et al. Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC immunology. 2013;14:20. doi: 10.1186/1471-2172-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner JH, Liang KL, May L, Lane AP. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. American journal of rhinology & allergy. 2010;24:336–340. doi: 10.2500/ajra.2010.24.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. American journal of rhinology & allergy. 2010;24:192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozharskaya T, Lane AP. Interferon gamma causes olfactory dysfunction without concomitant neuroepithelial damage. International forum of allergy & rhinology. 2013;3:861–865. doi: 10.1002/alr.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlosser RJ, Mulligan JK, Hyer JM, Karnezis TT, Gudis DA, Soler ZM. Mucous Cytokine Levels in Chronic Rhinosinusitis-Associated Olfactory Loss. JAMA otolaryngology-- head & neck surgery. 2016;142:731–737. doi: 10.1001/jamaoto.2016.0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology Supplement. 2012;3:1–298. p preceding table of contents. [PubMed] [Google Scholar]
- 26.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. International forum of allergy & rhinology. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 27.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. The Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. The Laryngoscope. 2013;123:E72–78. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 29.Konig K, Klemens C, Haack M, et al. Cytokine patterns in nasal secretion of non-atopic patients distinguish between chronic rhinosinusitis with or without nasal polys. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2016;12:19. doi: 10.1186/s13223-016-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Bruaene N, Perez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. The Journal of allergy and clinical immunology. 2008;121:1435–1441. 1441 e1431–1433. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 32.Kramer MF, Ostertag P, Pfrogner E, Rasp G. Nasal interleukin-5, immunoglobulin E, eosinophilic cationic protein, and soluble intercellular adhesion molecule-1 in chronic sinusitis, allergic rhinitis, and nasal polyposis. The Laryngoscope. 2000;110:1056–1062. doi: 10.1097/00005537-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 33.Kelly EA, Koziol-White CJ, Clay KJ, et al. Potential contribution of IL-7 to allergen-induced eosinophilic airway inflammation in asthma. J Immunol. 2009;182:1404–1410. doi: 10.4049/jimmunol.182.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henkin RI, Schmidt L, Velicu I. Interleukin 6 in hyposmia. JAMA otolaryngology-- head & neck surgery. 2013;139:728–734. doi: 10.1001/jamaoto.2013.3392. [DOI] [PubMed] [Google Scholar]
- 35.Nan B, Getchell ML, Partin JV, Getchell TV. Leukemia inhibitory factor, interleukin-6, and their receptors are expressed transiently in the olfactory mucosa after target ablation. The Journal of comparative neurology. 2001;435:60–77. doi: 10.1002/cne.1193. [DOI] [PubMed] [Google Scholar]
- 36.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 37.Hauser LJ, Chandra RK, Li P, Turner JH. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. International forum of allergy & rhinology. 2017;7:957–962. doi: 10.1002/alr.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sivam A, Jeswani S, Reder L, et al. Olfactory cleft inflammation is present in seasonal allergic rhinitis and is reduced with intranasal steroids. American journal of rhinology & allergy. 2010;24:286–290. doi: 10.2500/ajra.2010.24.3478. [DOI] [PubMed] [Google Scholar]
- 39.Debat H, Eloit C, Blon F, et al. Identification of human olfactory cleft mucus proteins using proteomic analysis. Journal of proteome research. 2007;6:1985–1996. doi: 10.1021/pr0606575. [DOI] [PubMed] [Google Scholar]


