Abstract
Excessive sedentary time is related to poor mental health. However, much of the current literature uses cross-sectional data and/or self-reported sedentary time, and does not assess factors such as sedentary bout length. To address these limitations, the influence of objectively measured sedentary time including sedentary bout length (i.e. <30 min, ≥30 min) on mood, stress, and sleep, was assessed in 271 healthy adults (49% women; age 27.8 ± 3.7) across a 1-year period between 2011 and 2013 in Columbia, SC. Participants completed the Profile of Mood States and the Perceived Stress Scale, and wore a Sensewear Armband to assess sedentary time, physical activity, and sleep for ten days at baseline and one year. A series of fixed-effects regressions was used to determine the influence of both baseline levels and changes in daily sedentary time (total and in bouts) and physical activity on changes in mood, stress, and sleep over one year. Results showed that across the year, decreases in total sedentary time, and time in both short and long bouts, were associated with improvements in mood, stress and sleep (p < 0.05). Increases in physical activity were only significantly predictive of increases in sleep duration (p < 0.05). Thus, reductions in sedentary time, regardless of bout length, positively influenced mental wellbeing. Specifically, these results suggest that decreasing daily sedentary time by 60 min may significantly attenuate the negative effects of high levels of pre-existing sedentary time on mental wellbeing. Interventions manipulating sedentary behavior are needed to determine a causal link with wellbeing and further inform recommendations.
Keywords: Mental health, Longitudinal, Cohort, Mood, Stress, Sleep
Highlights
-
•
High amounts of sedentary time are associated with poor mood, stress, and sleep.
-
•
Decreased daily sedentary time predicted improved mental wellbeing over a year.
-
•
Changes in either longer or shorter bouts of sedentary time may influence wellbeing.
-
•
Reducing sedentary time by 1 h/day may benefit mood, stress, and sleep.
-
•
Recommendations for sedentary time should consider its effects on mental wellbeing.
1. Introduction
Much of the chronic disease burden in the United States is attributed to modifiable behavioral risk factors (e.g. diet, exercise) (Bauer et al., 2014). One such factor, excessive sedentary behavior, has recently received significant attention with evidence demonstrating deleterious effects for cardiometabolic health and all-cause mortality that may be independent of physical inactivity (Koster et al., 2012; Matthews et al., 2012). Sedentary behavior, defined as waking time spent sitting or reclining without being otherwise active (Sedentary Behaviour Research Network, 2012), is highly prevalent in the United States. Recent data from a sample of younger adults showed that this population sits for >9 h/day (Unick et al., 2017). Moreover, young adults have shown the largest increase in sedentary time coupled with the largest decrease in moderate to vigorous physical activity over the preceding decades, in comparison to other age groups, placing them at greater risk for development of chronic disease (Nelson et al., 2008).
Sedentary time also negatively influences mental health including increased risk for anxiety (Teychenne et al., 2015), depression (Teychenne et al., 2010), and lower levels of emotional wellbeing (Atkin et al., 2012a; Endrighi et al., 2015) in diverse populations including younger adults. Accumulating large amounts of sedentary time has also been linked with sleep disorders (Kline et al., 2016), which are frequently comorbid with mental health issues (Krystal, 2006). However, this research has largely relied on cross-sectional data. While longitudinal studies examining sedentary behavior and outcomes related to mental wellbeing exist (Hamer and Stamatakis, 2013; Lucas et al., 2011; Sanchez-Villegas et al., 2008; Teychenne et al., 2014), these have been conducted primarily in older populations and have relied on self-report and surrogate measures for sedentary time (e.g. hours of TV viewing), which typically have poor validity (Atkin et al., 2012b). Additionally, these measures do not usually consider the potential health consequences of different accumulation patterns of sedentary time. For example, previous research suggests that sedentary behavior accumulated in prolonged bouts (e.g. >30 min duration) may be a better predictor of cardiometabolic health outcomes than total sedentary time (Diaz et al., 2016; Dunstan et al., 2012; Honda et al., 2016; Júdice et al., 2015). However, given the limitations of the sedentary assessments used to date, it is unknown whether the total amount of sedentary time or the amount accumulated in longer bouts is most problematic for mental wellbeing. Lastly, research conducted in this area has largely focused on diagnosed mental health conditions (e.g. major depressive disorder) and less is known about the influence of sedentary time on sub-clinical mental health symptoms that affect a much larger segment of the population.
The purpose of this study was to address these limitations by examining the longitudinal association of changes in objectively measured sedentary time with changes in mood, stress, and sleep in a cohort of healthy young adults. Based on previous research demonstrating the detrimental health consequences of accumulating sedentary time in prolonged bouts, the influence of sedentary time accumulated in longer (≥30 min) and short (<30 min) bouts was also examined. It was hypothesized that as sedentary time increased, mood disturbance and stress would increase, while sleep duration would decrease, whereas these markers of wellbeing would improve with decreased sedentary time. Additionally, it was hypothesized that sedentary time accumulated in prolonged bouts (≥30 min) would have a greater influence on mental wellbeing than sedentary time accumulated in shorter bouts.
2. Methods
2.1. Participants
These data were drawn from a larger project designed to examine factors influencing energy balance in young adults (Hand et al., 2013). Participants were included in the present study if they had complete data at baseline and the 1-year follow up. Briefly, participants were healthy adults, ages 21 to 35 years, with a body mass index (BMI) between 20 and 35 kg/m2. Exclusionary criteria for the larger study included use of weight-loss medications, recent change of smoking status, planned weight-loss surgery, hypertension, high blood glucose, or a current chronic disease diagnosis requiring daily medication. Individuals were also excluded for a history of depression, anxiety, or panic disorder. All women were eumenorrheic, and those who gave birth in the previous year or were planning to become pregnant were excluded. All procedures were approved by the Institutional Review Board of the University of South Carolina and informed consent was obtained from each participant before data collection.
2.2. Procedures
At baseline participants completed a demographic and health history form and questionnaires to assess mood and stress. Approximately one week later, height and weight were measured and participants were issued an activity monitor to objectively measure physical activity, sedentary behaviors, and sleep. Identical procedures were completed at a one-year follow-up.
2.3. Measures
2.3.1. Physical activity and sedentary behaviors
Physical activity and sedentary time were objectively measured using the SenseWear Mini Armband (SWA; BodyMedia Inc. Pittsburgh, PA), worn on the upper arm. Using a tri-axial accelerometer, and sensors for heat flux, galvanic skin response, and skin temperature with a proprietary algorithm, the SWA estimates time spent in different activity intensities and steps/day. This device has been validated for assessing energy expenditure against doubly-labeled water in a similar sample of young adults (intraclass correlation 95% confidence interval of 0.68–0.89) (St-Onge et al., 2007). Further, the SWA also has acceptable levels of validity for estimating energy expenditure during typical sedentary and light intensity activities when compared to indirect calorimetry under laboratory conditions with an ICC of 0.90 (Reece et al., 2015).
Participants were asked to wear the armband 24 h a day for 10 consecutive days, except during water-based activities (e.g., swimming or bathing) and were deemed compliant if they completed 7 days of wear (including two weekend days) with ≥21 h of wear time on each of the days (Hand et al., 2013). Average daily steps were used as a measure of physical activity. Sedentary time was calculated as total time spent ≤1.5 METs while awake. Lastly, sedentary time was divided into time accumulated in bouts of ≥30 (prolonged bouts) and < 30 min (short bouts). Thirty minutes was chosen as the cut-point for dividing prolonged from short bouts of sedentary time as this is a common operational definition used in previous literature (e.g. Diaz et al., 2016; Sloan et al., 2018) and all participants in the present study accumulated sedentary time in bouts of this length.
2.4. Sleep
Sleep duration was operationally defined as total nighttime sleep. This metric was derived from minute-by-minute sleep epoch data from the SWA. The SWA is a valid instrument for assessing sleep duration as well as several metrics of sleep quality (e.g. sleep onset latency, wake-after-sleep-onset) in comparison to polysomnography (Shin et al., 2015).
2.5. Mood
The Profile of Mood States (POMS) was used to assess mood over the past week (McNair et al., 1971). The POMS has six subscales (tension, depression, anger, vigor, fatigue, and confusion) and provides a summary score for total mood disturbance (TMD; tension + depression + anger + fatigue + confusion – vigor + 100). The POMS has acceptable levels of reliability and validity for use with the general adult population (McNair et al., 1971; Nyenhuis et al., 1999). Internal consistency reliability coefficients range from α = 0.63–0.96 with test–retest reliability estimates of 0.65 to 0.74 (McNair et al., 1971).
2.6. Stress
Stress was assessed using the Perceived Stress Scale (PSS) which measures the stressful perception of various life situations over the past month (Cohen et al., 1983). Validity and high internal consistency were demonstrated in the initial publication (r = 0.84–0.86) and the test-retest reliability was 0.55–0.85 (Cohen et al., 1983).
2.7. Statistical analyses
Demographic characteristics, baseline levels and changes in physical activity, sedentary time, and mental wellbeing-related outcomes were calculated using means and standard deviations for continuous variables and percentages for categorical variables. For descriptive purposes and to provide data to help inform future recommendations surrounding sedentary time and mental wellbeing, participants were divided based on objectively-monitored time spent per day in sedentary behaviors at baseline into low (<10.5 h), medium (10.5–12 h), and high (>12 h) subgroups. Demographics differences among subgroups were assessed with ANOVAs for continuous variables and chi-square analyses for categorical variables; significant results were followed by linear trend analyses.
To address the primary aims of this study, a series of fixed-effects longitudinal linear regression analyses estimated associations between changes in sedentary time and changes in mood, stress, and sleep over the duration of the study. Unlike conventional and random-effects regressions, fixed-effects models make comparisons within individuals rather than between groups. As such, each individual acts as her/his own control, thereby controlling for characteristics of the individual which do not vary with time (e.g. sex, race, education, etc.) (Allison, 2009). Consequently, fixed-effects models have the advantage of removing bias from time-invariant confounding factors from both measured and unobserved variables (Allison, 2009). Predictors included in the models were baseline sedentary time, changes in sedentary time, baseline steps, and changes in steps. Multicollinearity was assessed via variance inflation; variance inflation >2 was identified as collinearity. As none of the included predictors were collinear in any of the models, all proposed predictors were included. Dependent variables were changes in 1) POMS TMD, 2) perceived stress, and 3) sleep duration, each analyzed separately (three fixed-effects regressions). As a secondary evaluation of the predictors on mood, separate regression models were run with each of the six POMS subscales as the dependent variable.
Prediction equations were then created using the coefficients and intercepts from the regression analyses to examine the relative contributions of baseline sedentary subgroup membership (low, medium, high) and hypothetical changes in sedentary time on mental wellbeing. In these equations, the mean sedentary time at baseline was used to represent each of the three subgroups, with grand means for each of the other predictor variables in each equation held constant. The influence of increases and decreases of 30, 60 or 90 min as well as no change in sedentary time across the year on primary outcomes was predicted and illustrated graphically.
To examine the influence of sedentary bout length on mental wellbeing, total sedentary time was divided into time accumulated in bouts of greater than or equal to 30 min (prolonged) or <30 min (short). Changes in these two metrics across the year were included in place of total sedentary time in the models described above; all other predictors and outcomes were consistent with the primary analyses. The α-level was set to 0.05 for all analyses. Statistical Analyses were performed using SAS® v.9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Participant characteristics
A total of 271 participants (48.7% female) from the larger study (n = 430) had complete data at both baseline and 1 year and were included in the analyses. Baseline characteristics for the total sample and for each subgroup are presented in Table 1. Baseline characteristics of the participants with complete data at both time points did not differ significantly from the 159 with complete data only at baseline for any variable reported in Table 1 (all p > 0.05 for independent-samples t-tests). Participants in the present analysis were 27.8 ± 3.7 years old with an average BMI of 25.4 ± 3.9 kg/m2. At baseline, subgroups were similar in age, height, income, presence of children in the home, and education level (p > 0.05). Subgroups based on baseline levels of sedentary time (low, medium, high) were significantly different for BMI and body weight (p < 0.001), as well as the percent of the sample reporting ‘White’ as their racial or ethnic background (p = 0.006). Trend analyses showed that BMI and weight increased linearly across subgroups while percent reporting their race as White decreased linearly as sedentary time increased (all p < 0.05).
Table 1.
Demographic characteristics for the total sample and by baseline sedentary subgroup. Data were collected between 2011 and 2013 in Columbia, SC.
| Measure | Total sample | Subgroup (sedentary hours/day) |
Group differences (p) | ||
|---|---|---|---|---|---|
| <10.5 | 10.5–12 | >12 | |||
| n | 271 | 70 | 110 | 91 | |
| Gender (% female) |
48.7% | 54.3% | 46.4% | 47.3% | >0.05 |
| Age | 27.8 (3.7) | 27.22 (3.98) | 27.84 (3.66) | 28.31 (3.48) | >0.05 |
| BMI | 25.4 (3.9) | 23.78 (3.29) | 24.61 (3.19) | 27.59 (4.14) | <0.0001⁎ |
| Height | 172.2 (9.5) | 170.29 (7.86) | 173.58 (10.18) | 172.22 (9.47) | >0.05 |
| Weight | 75.5 (14.0) | 69.06 (10.93) | 74.38 (12.77) | 81.86 (15.00) | <0.0001⁎ |
| Income (% >$50,000) | 35% | 42% | 38.7% | 25.3% | >0.05 |
| Children in home (% without) |
87% | 85.7% | 89.3% | 85.6% | >0.05 |
| Race/ ethnicity (% White) |
66% | 77.1% | 68.8% | 53.8% | 0.006⁎ |
| Education (% w college degree) | 86% | 81.4% | 87.5% | 89.0% | >0.05 |
Values are ‘Mean (sd)’ unless otherwise noted. BMI = body mass index.
Indicates a significant linear trend (p < 0.05).
3.2. Physical activity and primary outcomes
3.2.1. Baseline data
Baseline levels of physical activity, sedentary behaviors, and primary outcomes are shown in Table 2. At baseline, participants averaged just under 11.5 h/day of sedentary time, accumulated approximately 7400 steps per day, and slept for about 6.5 h each night. Participants accumulated just under 5 h/day (41%) of their sedentary time in prolonged bouts (>30 min). Baseline values for most predictor and outcome variables differed by subgroup including total sedentary time (p < 0.001), short bouts (p = 0.018), prolonged bouts (p < 0.001), sleep duration (p < 0.0001) and total mood disturbance (p = 0.042) as well as steps (p < 0.001) with those with lower baseline sedentary time generally having the more favorable profile (e.g. longer sleep, lower levels of mood disturbance and more steps).
Table 2.
Measures of physical activity, sedentary time and outcomes variables at baseline. Data were collected between 2011 and 2013 in Columbia, SC.
| Measure | Total sample | Subgroup (sedentary hours/day) |
Group differences (p) | |||
|---|---|---|---|---|---|---|
| <10.5 | 10.5–12 | >12 | ||||
| n | 271 | 70 | 110 | 91 | ||
| Total sedentary time | 11.4 (1.61) | 9.42 (0.88) | 11.22 (0.44) | 13.15 (0.91) | <0.0001⁎ | |
| Sedentary time accumulated in bouts ≥30 min | 4.84 (1.95) | 3.06 (0.99) | 4.44 (0.94) | 6.63 (1.68) | <0.0001⁎ | |
| Sedentary time accumulated in bouts <30 min | 6.58 (1.01) | 6.34 (1.08) | 6.78 (0.82) | 6.52 (1.12) | 0.018 | |
| Steps/day | 7401.4 (2548.5) | 8931.8 (2798.2) | 7420.5 (2101.5) | 6200.7 (2213.5) | <0.0001⁎ | |
| Profile of mood states | TMD | 108.9 (22.6) | 106.7 (17.6) | 106.6 (21.4) | 113.6 (26.5) | 0.042⁎ |
| Ten. | 5.4 (4.9) | 4.9 (4.2) | 4.9 (4.5) | 6.3 (5.8) | 0.10 | |
| Dep. | 3.5 (5.8) | 2.7 (3.8) | 3.1 (5.2) | 4.7 (7.4) | 0.027⁎ | |
| Ang. | 2.7 (3.9) | 2.6 (4.1) | 2.5 (3.7) | 3.0 (4.0) | 0.61 | |
| Vig. | 13.7 (6.5) | 14.1 (6.4) | 14.7 (6.5) | 12.0 (6.2) | 0.011⁎ | |
| Fat. | 6.3 (5.2) | 6.1 (5.2) | 6.1 (4.9) | 6.7 (5.4) | 0.72 | |
| Con. | 4.7 (3.5) | 4.6 (3.0) | 4.7 (3.3) | 5.0 (4.1) | 0.78 | |
| Perceived stress scale | 12.5 (5.8) | 12.3 (5.5) | 11.9 (5.4) | 13.6 (6.5) | 0.10 | |
| Sleep duration | 6.59 (0.87) | 6.90 (0.81) | 6.75 (0.84) | 6.16 (0.81) | <0.0001⁎ | |
All time metrics are in hours/day. Values are ‘Mean (sd)’. TMD = Total Mood Disturbance; Ten = tension; Dep = depression; Ang = anger; Vig = vigor; Fat = fatigue; Con = confusion.
Indicates a significant linear trend, p < 0.05.
3.2.2. Changes over one year
Participants averaged small, non-meaningful changes in physical activity, sedentary time, mood, stress and sleep. However, the range of changes in sedentary time was large, from −5.55 h to +4.25 h. Approximately 10% of the total sample changed their total sedentary time by >2 h, ~30% by 1–2 h and ~60% by <1 h. The changes in steps/day averaged a decrease 15 steps with a range from −8886 to +7775. The changes in TMD averaged an increase of 2 points with a range from −95 to +125, while changes in stress averaged an increase of 0.5 points with a range from −20 to +19. Lastly, the change in sleep duration averaged an increase of 4 min with a range from −2.6 h to +4.5 h.
3.2.3. Fixed-effects regressions
As shown in Table 3, the overall models from the fixed effects regressions were significant for mood, stress, and sleep duration, with change in sedentary time as a significant predictor in each model. Baseline sedentary time only significantly predicted changes in total mood disturbance while changes in steps were only a significant predictor of changes in sleep duration. For POMS subscales, significant overall regression equations were found for anger, fatigue and confusion with changes in sedentary time a significant predictor in all three while baseline sedentary time only significantly predicted changes in anger and depression. Both baseline and changes in steps did not significantly predict changes in any of the subscales.
Table 3.
Results from the fixed-effects regressions for total sedentary time. Data were collected between 2011 and 2013 in Columbia, SC.
| Predictors | Dependent variables: change across 1 year |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆ POMS |
∆ PSS |
∆ Sleep duration |
||||||||||||||||
| ∆ TMD |
∆ Ten |
∆ Dep |
∆ Ang |
∆ Vig |
∆ Fat |
∆ Con |
||||||||||||
| Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | |
| Baseline sedentary time | 0.14 | 0.053 | 0.07 | 0.34 | 0.14⁎ | 0.049 | 0.24⁎ | 0.001 | 0.00 | 0.99 | 0.07 | 0.34 | 0.14 | 0.06 | 0.08 | 0.29 | −0.04 | 0.52 |
| ∆ sedentary time | 0.23⁎ | 0.001 | 0.13 | 0.07 | 0.19⁎ | 0.009 | 0.18⁎ | 0.01 | −0.13 | 0.08 | 0.19⁎ | 0.008 | 0.21⁎ | 0.003 | 0.20⁎ | 0.006 | −0.58⁎ | <0.0001 |
| Baseline steps | 0.04 | 0.55 | −0.00 | 0.99 | 0.08 | 0.29 | 0.11 | 0.12 | −0.04 | 0.59 | −0.06 | 0.44 | 0.01 | 0.86 | 0.03 | 0.71 | −0.03 | 0.70 |
| ∆ steps | 0.01 | 0.86 | −0.03 | 0.71 | 0.07 | 0.36 | 0.13 | 0.08 | 0.06 | 0.43 | −0.07 | 0.33 | 0.04 | 0.55 | −0.07 | 0.30 | −0.29 | <0.0001 |
| Overall Model (R2) | 0.04⁎ | 0.018 | 0.02 | 0.33 | 0.03 | 0.09 | 0.05⁎ | 0.008 | 0.03 | 0.08 | 0.04⁎ | 0.02 | 0.04⁎ | 0.04 | 0.04⁎ | 0.01 | 0.27⁎ | <0.0001 |
∆ = change; POMS=Profile of Mood States; Ten = tension; Dep = depression; Ang = anger; Vig = vigor; Fat = fatigue; Con = confusion; TMD = Total Mood Disturbance; PSS=Perceived Stress Scale.
Significant at p < 0.05.
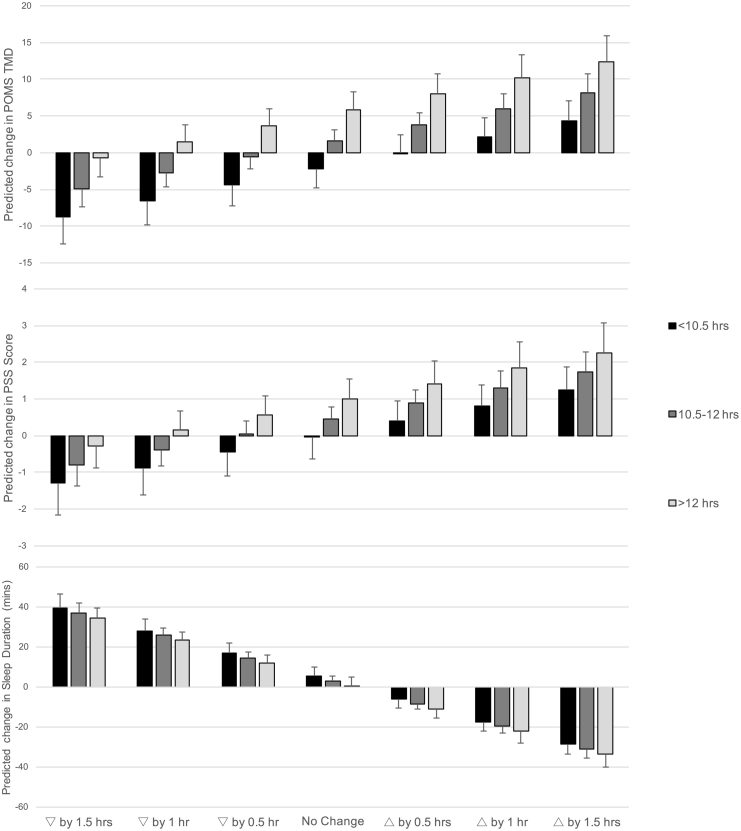
The results generated by the prediction equations are graphically represented in Fig. 1. This figure demonstrates the combined theoretical influence of baseline sedentary time (subgroup) and changes in sedentary time on changes in outcome variables (TMD, PSS, Sleep Duration) over the year. For example, for those with low sedentary time at baseline (<10.5 h/day), the cumulative effect of pre-existing sedentary time and decreasing sedentary time by 90 min is predicted to result in an improvement in TMD of 8.7 points or 0.49 standard deviation (SD) units. In contrast to this, for those with high sedentary time at baseline (12+ hours/day), the cumulative effect of pre-existing sedentary time and the same decrease of 90 min of sedentary behavior is predicted to result in a minimal improvement in TMD of 0.7 points or 0.03 SD units. Thus, Fig. 1 highlights that both baseline levels of sedentary time and potential changes in this set of behaviors play a role in future mental mood, stress and sleep.
Fig. 1.
Output of prediction equations demonstrating the influence of theoretical changes in sedentary time, from decreasing by 1.5 h to increasing by 1.5 h in 30 min increments, (represented by bars) on changes in mood (Panel A), stress (Panel B), and sleep duration (Panel C). To illustrate the cumulative influence of baseline sedentary time combined with changes in this behavior, predictions are broken down based on baseline levels of sedentary time in subgroups (<10.5, 10.5–12, >12 h/day). This shows that greater baseline sedentary time predicts somewhat worse mental wellbeing and shorter sleep one year later, but that changes in sedentary time over the year have the potential to overcome or exacerbate the predicted changes in mental wellbeing and sleep at the end of the year. Data were collected between 2011 and 2013 in Columbia, SC.
3.2.4. Bouts regressions
Results from the analyses examining sedentary time accumulated in bouts ≥30 min (prolonged) and < 30 min (short) are shown in Table 4. For changes in TMD, the overall model was significant and changes in prolonged and short bouts were both significant predictors. For changes in stress, the overall model was significant. However, only changes in prolonged bouts of sedentary time were a significant predictor. For changes in sleep duration, the overall model was significant with significant negative associations between changes in both prolonged and short bouts of sedentary time and change in steps.
Table 4.
Results from the fixed-effects regressions for sedentary time broken down into prolonged (30+ minutes) and short (<30 min) bouts. Data were collected between 2011 and 2013 in Columbia, SC.
| Predictors | Dependent variables: change across 1 year |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆POMS |
∆PSS |
∆Sleep Duration |
||||||||||||||||
| ∆ TMD |
∆ Ten |
∆ Dep |
∆ Ang |
∆ Vig |
∆ Fat |
∆ Con |
||||||||||||
| Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | Std. ß | p | |
| ∆ sed bouts of 30+ | 0.29⁎ | 0.003 | 0.16 | 0.09 | 0.03 | 0.19 | 0.25⁎ | 0.01 | −0.15 | 0.11 | 0.25⁎ | 0.01 | 0.27⁎ | 0.006 | 0.27⁎ | 0.005 | −0.70⁎ | <0.0001 |
| ∆ sed bouts of <30 | 0.27⁎ | 0.004 | 0.17 | 0.07 | 0.23⁎ | 0.02 | 0.17 | 0.07 | −0.18 | 0.06 | 0.20⁎ | 0.04 | 0.27⁎ | 0.003 | 0.17 | 0.08 | −0.84⁎ | <0.0001 |
| Baseline sed time in bouts of 30+ | 0.16 | 0.06 | 0.08 | 0.34 | 0.24⁎ | 0.01 | 0.28⁎ | 0.001 | 0.01 | 0.93 | 0.08 | 0.34 | 0.16 | 0.07 | 0.10 | 0.25 | −0.02 | 0.74 |
| Baseline sed time in bouts of <30 | 0.08 | 0.29 | −0.01 | 0.89 | 0.16 | 0.06 | 0.17⁎ | 0.04 | −0.05 | 0.54 | 0.01 | 0.89 | 0.05 | 0.52 | 0.00 | 0.97 | −0.00 | 0.97 |
| Baseline steps | 0.04 | 0.59 | −0.00 | 0.98 | 0.11 | 0.18 | 0.11 | 0.12 | −0.03 | 0.67 | −0.05 | 0.46 | 0.01 | 0.90 | 0.03 | 0.67 | −0.00 | 0.98 |
| ∆ steps | 0.01 | 0.90 | −0.03 | 0.69 | 0.17 | 0.34 | 0.13 | 0.07 | 0.06 | 0.38 | −0.07 | 0.34 | 0.04 | 0.59 | −0.07 | 0.34 | −0.27⁎ | <0.0001 |
| Overall Model (R2) | 0.05⁎ | 0.049 | 0.03 | 0.36 | 0.06 | 0.41 | 0.05⁎ | 0.031 | 0.03 | 0.17 | 0.04 | 0.06 | 0.05 | 0.06 | 0.05⁎ | 0.033 | 0.37⁎ | <0.0001 |
∆ = change; POMS=Profile of Mood States; TMD = Total Mood Disturbance; Ten = tension; Dep = depression; Ang = anger; Vig = vigor; Fat = fatigue; Con = confusion; PSS=Perceived Stress Scale.
Significant at p < 0.05.
4. Discussion
The present results show that changes in sedentary time over a year were significantly associated with changes in multiple aspects of mental wellbeing in a sample of young healthy adults. Specifically, increases in sedentary time were associated with less sleep and greater levels of mood disturbance and stress when controlling for baseline amounts and changes in physical activity (i.e., steps). When overall sedentary time was partitioned based on the amount of time spent in short and prolonged bouts, results indicated that changes in either of these metrics significantly affected outcomes. However, changes in prolonged bouts were more predictive (larger beta coefficients) of changes in mood and stress and changes in short bouts were more predictive for changes in sleep duration. Neither baseline levels of total sedentary time or baseline time spent in both short and prolonged bouts significantly predicted changes in general mental wellbeing (total mood disturbance, stress and sleep) one year later. Overall, these data indicate that decreasing sedentary behavior, regardless of bout length, may positively affect future mental health and sleep.
Mental health is a rising societal concern, with 18% of adults suffering from a diagnosable mental illness each year (Hedden et al., 2013), and an even greater number with subclinical symptoms, costing the United States more than $200 billion annually (Levit et al., 2008). As such, understanding modifiable factors that could improve mental health has great public health significance. A growing body of work demonstrates a link between sedentary behaviors and various aspects of mental health including diagnosable conditions such as depression, anxiety, and bipolar disorder (Teychenne et al., 2015, Teychenne et al., 2010), and subclinical issues including stress, poor sleep and lower levels of wellbeing (Atkin et al., 2012a; Kline et al., 2016). Adding to this cross-sectional work are several longitudinal investigations showing that self-reported sedentary time predicts future mental health and wellbeing (Lucas et al., 2011; Sanchez-Villegas et al., 2008). Additionally, a few brief, small-scale interventions show that changing sedentary behavior results in concomitant changes in mental health-related outcomes (Barwais et al., 2013; Ellingson et al., 2016; Endrighi et al., 2015; Pronk et al., 2012). However, this link has not been universally supported (van Uffelen et al., 2013) and there is some evidence of reverse causality showing that changes in mental health may result in changes in sedentary time (Teychenne et al., 2014). The present work adds to these findings by demonstrating significant associations between natural fluctuations in sedentary time and changes in several aspects of mental wellbeing over a year's time in healthy young adults. Specifically, we found that changes in sedentary time were positively related to changes in several aspects of mood disturbance, including depression, anger, fatigue, and confusion, and were positively associated with changes in stress and negatively associated with changes in sleep duration. Thus, increasing sedentary time may put individuals at greater risk for worsening mental wellbeing.
Previous research suggests that prolonged sedentary behavior may be a better predictor of health outcomes than total sedentary time. For example, only time spent in prolonged sedentary bouts and not total sedentary time or time spent in short bouts was shown to have a significant effect on development of metabolic syndrome (Honda et al., 2016). Further, changing the number of prolonged bouts had a greater effect on waist circumference than changing the number of short bouts (Júdice et al., 2015). Our study supports this potentially greater impact of prolonged bouts of sedentary time for aspects of mental wellbeing including mood and stress. However, changes in short bouts of sedentary time were more predictive of changes in sleep duration (larger beta coefficient) and were also significantly associated with changes in mood. In other words, decreasing sedentary time, regardless of bout length, may also have relevance for outcomes related to mental wellbeing and should be considered in future intervention design and public health messages.
4.1. Toward sedentary behavior recommendations
There is a growing interest in developing evidence-based recommendations for sedentary time (Hamilton et al., 2008). While a number of countries and agencies have noted the importance of limiting sedentary time in children, to our knowledge only Australia and the American College of Sports Medicine have officially recommended that adults limit the amount of time they spend sitting each day (ACSM, 2011; Australian Government Department of Health, 2014). However, these recommendations do not offer specific guidance regarding how to accumulate these often-requisite behaviors without negatively impacting health.
The prediction equations generated from our regression analyses are intended to help advance this cause by showing how changes in sedentary behaviors influence changes in mental wellbeing, accounting for an individual's pre-existing behavior. Decreasing sedentary time may be advisable and, depending on where an individual starts, greater changes in sedentary time may be needed in order to realize a meaningful improvement in wellbeing. For example, for participants accumulating the most sedentary time at baseline (>12 h/day), a decrease of 90 min/day may be needed to negate the detrimental influence of their high pre-existing sedentary time on mood disturbance, stress and sleep duration 1 year later. The potentially large effects of changing sedentary time contrast with the more minimal effects of pre-existing/baseline sedentary time where, even in those participants accumulating the least sedentary time at baseline, increasing time by 30 min/day was still predicted to worsen stress and decrease sleep duration. In this study, approximately 40% of participants changed their daily sedentary time by >60 min and another 25% changed their sedentary time between 30 and 60 min. This suggests that making subtle (i.e., >30 min/day) changes in sedentary time may be feasible with larger increases (60–90 min/day) also possible. Our data support that reducing sedentary time by even 60 min/day could prevent or significantly attenuate the negative effects of even high amounts of baseline sedentary behavior and, thus, could contribute to the development of sedentary behavior recommendations for mental wellbeing.
Decades of intervention research have demonstrated that promoting behavior change at the individual level can be challenging. This is certainly true for sedentary behavior, which has been strongly influenced by advances in technology and the built environment (Owen et al., 2010). As such, policy, system, and environmental changes will be important for promoting sustained decreases in this ubiquitous set of behaviors. For example, there is growing evidence that policy-level programs aimed at increasing availability of sit-to-stand desks in the workplace and other modifications to the internal built environment can effectively decrease sitting time (Marmot and Ucci, 2015). More recently, evidence has shown that low neighborhood walkability is predictive of high sedentary behavior (Sallis et al., 2018) suggesting that modifications like additions of sidewalks and greenspace may also be effective. Individual-level changes to reduce prolonged sedentary time could include more frequently breaking up sedentary activities with brief periods of standing/moving (e.g., standing during commercials), while reducing total time spent sedentary could be accomplished through standing or moving during more activities that are usually performed seated (e.g., standing while talking on the phone). The present results highlight the need for future sedentary intervention research informed by the important sociocultural and environmental factors that influence sedentary habits.
Interestingly, baseline physical activity did not significantly predict mood, stress or sleep duration, with changes in steps only significantly predicting changes in sleep duration at 1 year. Thus, in this sample of healthy adults, changes in sedentary time were more strongly and consistently associated with changes in mental wellbeing than changes in steps. Although previous research clearly indicates a link between physical activity and mental health (Zschucke et al., 2013), the present data indicate an important association between changes in sedentary behavior and mental wellbeing. Future research directly comparing changes in sedentary time with changes in physical activity will be instrumental in clarifying the relative importance of the effects of increasing activity and/or decreasing sedentary time for mental wellbeing.
4.1.1. Strengths and limitations
Limitations of this study include: lack of postural or contextual information for sedentary time, and the observational nature of the study. Additionally, our sample included primarily young, highly educated, adults with no children in the home, and low levels of psychopathology. However, it is plausible that the relationships between sedentary behavior and mental wellbeing may actually be stronger in older, less healthy populations with lower levels of wellbeing. Further, understanding the influence of sedentary behavior in a population that is establishing behavioral habits that may impact their future health is important. Nonetheless, future studies would benefit from sampling more diverse populations. Strengths include: objective 24-h sedentary monitoring, which allowed for examination of bout length, the longitudinal study design, and multiple assessments of mental wellbeing in a generally healthy population.
5. Conclusions
The present data show that, regardless of bout length, changes in sedentary behavior predicted changes in mental wellbeing in a sample of younger healthy adults. In pursuit of developing evidence-based recommendations, our data suggest that decreasing daily sedentary time by 60 min may prevent or significantly attenuate the negative effects of sedentary time on mental wellbeing. The powerful fixed-effects regression model and the coherence of these findings with the current literature demonstrating that sedentary behavior is detrimental for multiple aspects of health suggest that additional research is warranted. Interventions designed to alter sedentary time appear promising and are needed to assess a causal link with mental wellbeing.
Conflicts of interest and source of funding
The data reported here were collected as part of a larger study designed to assess factors associated with energy balance, which was supported by an unrestricted research grant from the Coca-Cola Company. The present work was conducted independently from Coca-Cola but the authors acknowledge that funding from Coca-Cola supported the data collection. The authors declare that they have no other potential conflicts of interest associated with this work.
References
- ACSM . 2011. ACSM Information of Reducing Sedentary Behaviors: Sitting Less and Moving More. [Google Scholar]
- Allison P.D. Sage Publications; London: 2009. Fixed Effects Regression Models (Quantitative Applications in the Social Sciences) [Google Scholar]
- Atkin A.J., Adams E., Bull F.C., Biddle S.J.H. Non-occupational sitting and mental well-being in employed adults. Ann. Behav. Med. 2012;43:181–188. doi: 10.1007/s12160-011-9320-y. [DOI] [PubMed] [Google Scholar]
- Atkin A.J., Gorely T., Clemes S.A. Methods of Measurement in epidemiology: Sedentary Behaviour. Int. J. Epidemiol. 2012;41:1460–1471. doi: 10.1093/ije/dys118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government Department of Health . 2014. Australia's Physical Activity and Sedentary Behaviour Guidelines. [Google Scholar]
- Barwais F.A., Cuddihy T.F., Tomson L.M. Physical activity, sedentary behavior and total wellness changes among sedentary adults: a 4-week randomized controlled trial. Health Qual. Life Outcomes. 2013;11:183. doi: 10.1186/1477-7525-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U.E., Briss P.A., Goodman R.A., Bowman B.A. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384:45–52. doi: 10.1016/S0140-6736(14)60648-6. Lond. Engl. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Diaz K.M., Howard V.J., Hutto B. Patterns of sedentary behavior in US middle-age and older adults: the REGARDS study. Med. Sci. Sports Exerc. 2016;48:430–438. doi: 10.1249/MSS.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan D.W., Kingwell B.A., Larsen R. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson L.D., Meyer J.D., Cook D.B. Wearable technology reduces prolonged bouts of sedentary behavior. Transl. J. Am. Coll. Sports Med. 2016;1:10–17. [Google Scholar]
- Endrighi R., Steptoe A., Hamer M. The effect of experimentally induced sedentariness on mood and psychobiological responses to mental stress. Br. J. Psychiatry J. Ment. Sci. 2015 doi: 10.1192/bjp.bp.114.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Stamatakis E. Prospective study of sedentary behavior, risk of depression, and cognitive impairment. Med. Sci. Sports Exerc. 2013 doi: 10.1249/MSS.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.T., Healy G.N., Dunstan D.W., Zderic T.W., Owen N. Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Curr. Cardiovasc. Risk Rep. 2008;2:292–298. doi: 10.1007/s12170-008-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand G.A., Shook R.P., Paluch A.E. The energy balance study: the design and baseline results for a longitudinal study of energy balance. Res. Q. Exerc. Sport. 2013;84:275–286. doi: 10.1080/02701367.2013.816224. [DOI] [PubMed] [Google Scholar]
- Hedden S.L., Bose J., Gfroerer J.C., Lipari R.N. The CBHSQ Report. Substance Abuse and Mental Health Services Administration (US); Rockville (MD): 2013. Revised estimates of mental illness from the national survey on drug use and health. [PubMed] [Google Scholar]
- Honda T., Chen S., Yonemoto K. Sedentary bout durations and metabolic syndrome among working adults: a prospective cohort study. BMC Public Health. 2016;16:888. doi: 10.1186/s12889-016-3570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Júdice P.B., Hamilton M.T., Sardinha L.B., Silva A.M. Randomized controlled pilot of an intervention to reduce and break-up overweight/obese adults' overall sitting-time. Trials. 2015;16:490. doi: 10.1186/s13063-015-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline C.E., Krafty R.T., Mulukutla S., Hall M.H. Associations of sedentary time and moderate-vigorous physical activity with sleep-disordered breathing and polysomnographic sleep in community-dwelling adults. Sleep Breath. 2016;1–8 doi: 10.1007/s11325-016-1434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A., Caserotti P., Patel K.V. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal A.D. Sleep and psychiatric disorders: future directions. Psychiatr. Clin. N. Am. 2006;29:1115–1130. doi: 10.1016/j.psc.2006.09.001. abstract xi. [DOI] [PubMed] [Google Scholar]
- Levit K.R., Kassed C.A., Coffey R.M. SAMHSA Publication; Rockville, MD: 2008. Projections of National Expenditures for Mental Health Services and Substance Abuse Treatment, 2004–2014. (No. SMA 08-4326) [Google Scholar]
- Lucas M., Mekary R., Pan A. Relation between clinical depression risk and physical activity and time spent watching television in older women: a 10-year prospective follow-up study. Am. J. Epidemiol. 2011;174:1017–1027. doi: 10.1093/aje/kwr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot A., Ucci M. Sitting less, moving more: the indoor built environment as a tool for change. Build. Res. Inf. 2015;43:561–565. [Google Scholar]
- Matthews C.E., George S.M., Moore S.C. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am. J. Clin. Nutr. 2012;95:437–445. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D.M., Lorr M., Droppleman L.F. Educational and Industrial Testing Service; San Diego, CA: 1971. Manual for the Profile of Mood States. [Google Scholar]
- Nelson M.C., Story M., Larson N.I., Neumark-Sztainer D., Lytle L.A. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity. 2008;16:2205–2211. doi: 10.1038/oby.2008.365. Silver Spring Md. [DOI] [PubMed] [Google Scholar]
- Nyenhuis D.L., Yamamoto C., Luchetta T., Terrien A., Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J. Clin. Psychol. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Owen N., Sparling P.B., Healy G.N., Dunstan D.W., Matthews C.E. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin. Proc. 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk N.P., Katz A.S., Lowry M., Payfer J.R. Reducing occupational sitting time and improving worker health: the Take-a-Stand Project, 2011. Prev. Chronic Dis. 2012;9 doi: 10.5888/pcd9.110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece J.D., Barry V., Fuller D.K., Caputo J. Validation of the SenseWear Armband as a measure of sedentary behavior and light activity. J. Phys. Act. Health. 2015;12:1229–1237. doi: 10.1123/jpah.2014-0136. [DOI] [PubMed] [Google Scholar]
- Sallis J.F., Conway T.L., Cain K.L., Carlson J.A., Frank L.D., Kerr J., Glanz K., Chapman J.E., Saelens B.E. Neighborhood built environment and socioeconomic status in relation to physical activity, sedentary behavior, and weight status of adolescents. Prev. Med. 2018;110:47–54. doi: 10.1016/j.ypmed.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A., Ara I., Guillén-Grima F., Bes-Rastrollo M., Varo-Cenarruzabeitia J.J., Martínez-González M.A. Physical activity, sedentary index, and mental disorders in the SUN cohort study. Med. Sci. Sports Exerc. 2008;40:827–834. doi: 10.1249/MSS.0b013e31816348b9. [DOI] [PubMed] [Google Scholar]
- Sedentary Behaviour Research Network Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- Shin M., Swan P., Chow C.M. The validity of Actiwatch2 and SenseWear armband compared against polysomnography at different ambient temperature conditions. Sleep Sci. 2015;8:9–15. doi: 10.1016/j.slsci.2015.02.003. São Paulo Brazil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan R.A., Kim Y., Sahasranaman A., Müller-Riemenschneider F., Biddle S.J.H., Finkelstein E.A. The influence of a consumer-wearable activity tracker on sedentary time and prolonged sedentary bouts: secondary analysis of a randomized controlled trial. BMC. Res. Notes. 2018;11:189. doi: 10.1186/s13104-018-3306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge M., Mignault D., Allison D.B., Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am. J. Clin. Nutr. 2007;85:742–749. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- Teychenne M., Ball K., Salmon J. Sedentary behavior and depression among adults: a review. Int. J. Behav. Med. 2010;17:246–254. doi: 10.1007/s12529-010-9075-z. [DOI] [PubMed] [Google Scholar]
- Teychenne M., Abbott G., Ball K., Salmon J. Prospective associations between sedentary behaviour and risk of depression in socio-economically disadvantaged women. Prev. Med. 2014;65:82–86. doi: 10.1016/j.ypmed.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Teychenne M., Costigan S.A., Parker K. The association between sedentary behaviour and risk of anxiety: a systematic review. BMC Public Health. 2015;15:513. doi: 10.1186/s12889-015-1843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen J.G.Z., van Gellecum Y.R., Burton N.W., Peeters G., Heesch K.C., Brown W.J. Sitting-time, physical activity, and depressive symptoms in mid-aged women. Am. J. Prev. Med. 2013;45:276–281. doi: 10.1016/j.amepre.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Unick J.L., Lang W., Tate D.F., Bond D.S., Espeland M.A., Wing R.R. Objective estimates of physical activity and sedentary time among young adults. J. Obes. 2017;2017 doi: 10.1155/2017/9257564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschucke E., Gaudlitz K., Ströhle A. Exercise and physical activity in mental disorders: clinical and experimental evidence. J. Prev. Med. Public Health. 2013;46:S12–S21. doi: 10.3961/jpmph.2013.46.S.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]