Abstract
The interaction of amyloid β-peptide (Aβ) with the iron-storage protein ferritin was studied in vitro. We have shown that Aβ during fibril formation process is able to reduce Fe(III) from the ferritin core (ferrihydrite) to Fe(II). The Aβ-mediated Fe(III) reduction yielded a two-times-higher concentration of free Fe(II) than the spontaneous formation of Fe(II) by the ferritin itself. We suggest that Aβ can also act as a ferritin-specific metallochaperone-like molecule capturing Fe(III) from the ferritin ferrihydrite core. Our observation may partially explain the formation of Fe(II)-containing minerals in human brains suffering by neurodegenerative diseases.
Keywords: Ferritin, Aβ, Iron reduction, Alzheimer’s disease, Magnetite, Metallochaperone
Introduction
Alzheimer’s disease (AD) is an age-related, neurodegenerative disorder characterized by progressive cognitive decline, memory loss, extensive neuronal loss, decrease in cholinergic transmission, and psychosis. A pathological hallmark of Alzheimer’s disease is a deposition of amyloid β (Aβ) in the brain. Aβ is a proteolytic product of amyloid precursor protein by β- and γ-secretases. Hence, numerous different Aβ species exist; two major isoforms of Aβ, the 42-residue Aβ1–42 and the 40-residue Aβ1–40 peptides are ubiquitous in biological fluids at an approximate ratio of 1:9. The last two hydrophobic residues at the C-terminal of Aβ1–42 are proposed to be critical for its enhanced rate of nucleation, and are the principal species deposited in the brain [1–3]. However, the central sequence KLVFFAE is known to form amyloid on its own, and probably forms the core of the fibril [4–6]. Moreover, most studies have been performed on Aβ1–40 because of their greater stability and more defined structure over a long period of experimental time in solution compared to that of Aβ1–42.
AD is histologically characterized by the presence of extracellular amyloid plaques, intracellular neurofibrillary tangles, and increased concentration of Fe(II)-containing iron oxide (especially magnetite) nanoparticles in diseased brains [7, 8]. The total concentration of biogenic magnetite is up to 15 times higher in AD brains in comparison to healthy controls; there are also gender-based differences, with AD female subjects having significantly higher magnetite nanoparticles concentrations [9].
Under normal circumstances, brain iron is stored in a redox-inactive Fe(III) ferrihydrite mineral, which represents the inorganic part of iron-storage protein ferritin. However, detailed studies confirmed increased amounts of iron(II)-containing biominerals (magnetite) in AD brains when compared to disease-free brain tissue. Magnetite, a redox-active mineral, participates in the Fenton reaction [7, 10]. Dysfunctional homeostasis of iron and alterations in the levels and distribution of iron ions play an important role in the pathogenesis of AD [11, 12].
The formation of Fe(II)-containing biominerals in AD brains has to be accompanied by the formation of Fe(II) from Fe(III) precursors. It was shown that amyloid β-peptide (Aβ) interacts with synthetic Fe(III) ions; accumulation of ferric ions within amyloid aggregates resulted in Aβ-mediated reduction of Fe(III) to a redox-active Fe(II) [13]. Moreover, synthetic ferrihydrite can also interact with Aβ, resulting in the reduction of ferrihydrite to a Fe(II)-containing mineral (magnetite). Everett et al. demonstrated the capability of Aβ to induce redox-active biomineral formation in AD brain tissue from natural Fe(III)-containing precursors [7].
The main source of Fe(III) in brain tissue is the iron-storage protein ferritin. Ferrihydrite core is surrounded by a protein shell (apoferritin) that protects the iron mineral from direct contact with other protein molecules. As a continuation of previous studies, our research was designed to investigate the possible interaction of Aβ and ferritin and the potential production of Fe(II). We have shown that native ferritin is capable of generating Fe(II) ions during interaction with Aβ.
Methods
Aβ1–40 (Cat # A-1001-2, rPeptide, USA) was dissolved in 10 mM NaOH to stock concentration of 665 μM, sonicated for 1 min in a bath sonicator, and centrifuged at 4 °C for 10 min (12,000 × g) to remove large aggregates. The Aβ1–40 stock solution was then diluted to a concentration of 10 μM in 150 mM 3-(N-morpholino)-propanesulfonic acid (MOPS), pH 7.0. After addition of ferritin (Sigma Aldrich, F4503/SLBC0504V; total concentration 106 μM), samples were incubated 11 days at 37 °C. At selected time intervals, the quantification of free Fe(II) in solution was performed using a ferrozine assay. To assess the Fe(II) content of Aβ/ferritin mixture, ferrozine was added to the mixture and absorbance was measured after 1 min of incubation at 562 nm with precision of about 1% (temperature 25 °C; ten measurements per sample). As a reference, Aβ-free ferritin controls were prepared. The whole experiment was performed in duplicate and average values are presented.
For kinetic measurements, the aliquots of Aβ1–40 peptide solution alone and with ferritin incubated at 37 °C and pH 7.0 were withdrawn at varying times, mixed with Thioflavin T (ThT) and incubated at 37 °C for 1 h. The final concentrations of ThT and Aβ1–40 peptide were always 20 and 10 μM, respectively. The fluorescence intensity was measured using a 96-well plate by a Synergy MX (BioTek) spectrofluorometer. The excitation was set at 440 nm and the emission was recorded at 485 nm. The excitation and emission slits were adjusted to 9.0/9.0 nm and the top probe vertical offset was 6 nm. Each experiment was performed in triplicate; the error bars represent the standard deviation for repeated measurements of three separate samples. The kinetic data were fitted into a sigmoidal function to obtain the growth curves.
The presence and morphology of amyloid fibrils was examined by atomic force microscopy (AFM; Bruker AXS) in a tapping mode after drop-casting of sample on freshly cleaved mica surface using an NCHV cantilever with specific resistance of 0.01–0.025 Ω cm, antimony (n) doped Si, radius of the tip curvature of 10 nm. The resolution of the image was 512 pixels per line (512 × 512 pixels/image) and the scan rate was 0.5 kHz. No smoothing or noise reduction was applied.
Results and discussion
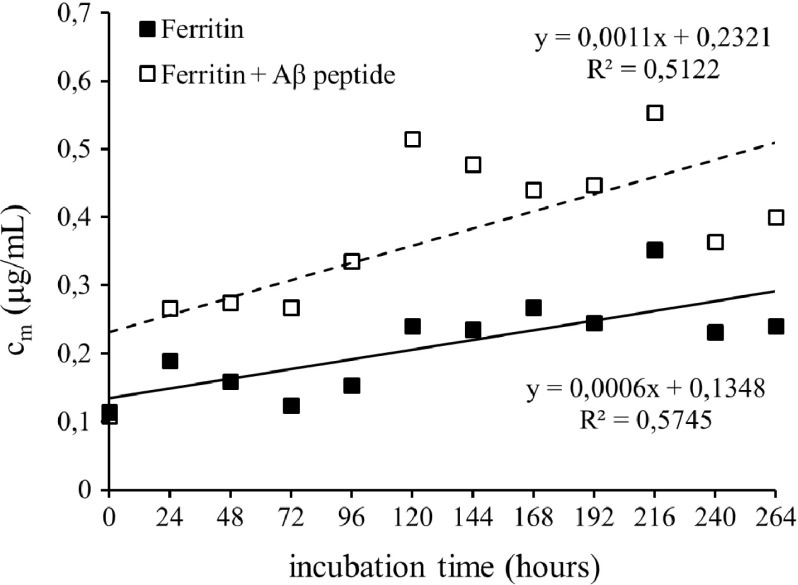
Considering the increased accumulation of Fe(II)-containing minerals in AD brain tissue, we assumed that free Fe(II) ions could be formed from ferritin upon interaction with Aβ peptide during fibrils formation. A ferrozine assay was used to quantify free Fe(II) ions formed both from pure native ferritin and a mixture of ferritin + Aβ. The Fe(II) concentration was plotted against the incubation time (Fig. 1). Comparison of both released amounts of Fe(II) from ferritin with and without Aβ and the slopes of the regression lines (0.0011 μg/ml.h and 0.0006 μg/ml.h, respectively) suggest that Aβ is capable of inducing faster conversion of Fe(III) to Fe(II), resulting in almost twice-higher concentration of free reduced iron ions in the reaction mixture. It has to be taken into account that the ferrozine assay can determine only free Fe(II) ions [14]. It can be expected that part of the reduced iron is bound to Aβ, which can specifically bound both Fe(II) and Fe(III) ions [15]; this iron is not determined using the ferrozine assay. The measured free Fe(II) ions represent the unbound part of the reduced iron.
Fig. 1.
The mass concentration of Fe(II) ions complexes with ferrozine after reduction of Fe(III) from ferritin (■) and ferritin in the presence of Aβ peptide (□)
Ferric ion accumulation within amyloid aggregates resulted in Aβ-mediated reduction of Fe(III) to Fe(II) [13]. The results obtained by our measurement suggest that ferric ions released from ferritin were bound to Aβ and subsequently reduced to ferrous ions; one part of Fe(II) remained bound to Aβ, while the excess was released from the peptide into the surrounding solution.
Native ferritin can release iron under physiological conditions through nanocage pores on the ferritin surface [16]; their size is about ~ 0.4 nm [17]. The in vivo mechanism of iron release from ferritin is still undetermined. However, it was shown that the ferroxidase centers of the H-subunits, responsible for the ferritin iron uptake, are not involved in iron release. The ferritin iron-uptake and the ferritin iron-release processes utilize distinct pathways [18].
Four models have been proposed to describe the ferritin iron release: (i) the existence of an equilibrium between the iron stored in ferritin and the iron in cytoplasm, (ii) the ferritin capsid degradation, (iii) the participation of a chaperone that would dock with ferritin and directly remove iron(III), and (iv) the existence on an electron-donor biomolecule that would dock with ferritin to reduce the iron(III) of the ferrihydrite mineral and facilitate iron(II) mobilization, which would be chelated by a chaperone molecule outside the ferritin molecule [18].
In our experiment, no low molecular weight electron donor was used, so the last model is not applicable. Due to the amyloid fibrils size, the transition of ~ 4 kDa Aβ into the ferritin mineral core is improbable. We suppose that Aβ can behave as a chaperone docking with ferritin and removing Fe(III). Currently, a series of metallochaperones has been described, including a poly C binding protein (PCBP) family of proteins. These chaperons can bind ferrous ions. Purified PCBPs exhibited no significant interaction with ferritin in the absence of iron. However, PCBP1 loaded anaerobically with ferrous iron exhibited affinities for apoferritin that were 30-fold higher than the affinity of free ferrous iron for ferritin. Approximately, nine Fe-PCBP1 molecules bind to a ferritin oligomer. As the number of putative iron-delivery channels is eight per ferritin polymer, this stoichiometry supports a model of PCBP1 facilitating iron incorporation into ferritin via direct binding at pores formed by the threefold axes of symmetry [19].
As stated above, one of the model mechanisms proposed to describe the ferritin iron release expects the participation of a chaperone that would dock with ferritin and directly remove iron(III) [18]. We suggest that Aβ (in addition to other functions) can also act as a ferritin-specific metallochaperone-like biomolecule responsible for Fe(III) release from ferritine. The mechanism of this process is unknown; it can be expected that fourfold hydrophobic, nonpolar channels of ferritin are involved in such interactions. The Fe(III) released from ferritin is captured by the soluble Aβ and during amyloid fibrillization reduced to Fe(II) [7, 13]. The excess of Fe(II) is probably released from the Aβ (see the experimental results) and free ferrous ions are subsequently captured by other ferritin molecules, not interacting with Aβ.
As already described, Aβ can efficiently capture Fe(III) and part of it reduces into Fe(II). The entrapment of a high concentration of both magnetite precursors in a reduced volume can lead to the formation of iron-oxide magnetite nanoparticles [20]. Similar reaction conditions are also employed during laboratory synthesis of magnetic iron-oxide nanoparticles [21].
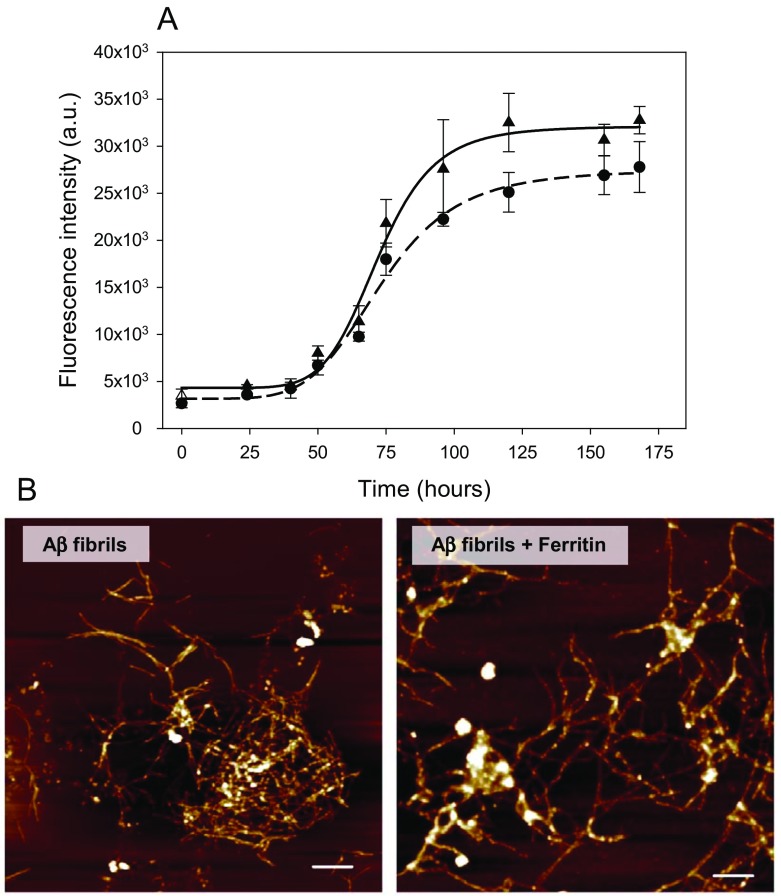
The kinetics of Aβ fibrils formation was estimated using a ThT assay [22]. The fluorescence intensity of ThT is not affected by the presence of the globular proteins in the native state, proteins in molten globule or unfolded state, or amorphous aggregates of protein [22–24]. Therefore, the ThT assay represents a good approach to study both the extent and kinetics of fibrillization. In absence of ferritin, the characteristic nucleation-dependent pattern, with three distinct phases, namely an initial lag-phase, elongation, and equilibration of amyloid formation, was observed (Fig. 2a). The equilibrium phase, suggesting that formation of the mature fibrils is completed, was observed after 5 days of incubation at 37 °C. In the presence of ferritin, the effect on the lag-phase was apparently insignificant and moderately shallow S-curve was observed in comparison to the Aβ fibrillization occurring in the absence of ferritin. The maximal fluorescence intensity in the plateau phase reached ~ 85% of that for control experiment; formation of Aβ1–40 fibrils was thus not substantially inhibited in the presence of ferritin. These observations indicate that the reduction of Fe(III) presented in Fig. 1 is taking place during the fibrillization process.
Fig. 2.
a Time dependence of Aβ peptide fibrillization alone (▲) and in the presence of ferritin (●) monitored using a ThT assay. b Atomic force microscopy visualization of Aβ fibrils formed alone and in the presence of ferritin. The xy scale is 10 × 10 μm. Scale bars are 1 μm
The ThT assay allows quantification of the amount of the protein in the form of amyloid fibrillar aggregates as the extent of aggregation is proportional to the dye fluorescence intensity; lowering ThT fluorescence intensity indicates a decrease of amyloid fibrils in the samples. We observed only slightly different results for Aβ1–40 fibrils formed in the presence of ferritin. Therefore, we did not expect any significant changes in morphology of fibrils. Indeed, representative AFM visualization confirmed that the morphology of Aβ1–40 fibrils formed alone and in the presence of ferritin possesses similar morphology. Aβ tended to form characteristic long unbranched twisting fibrils (Fig. 2b). It should be noted that some amorphous-like aggregates (white dots) observed in right panel of Fig. 2b correspond to the residues of ferritin molecules (protein shells) that were not removed during washing of samples.
To conclude, we have shown that protein-protected ferrihydrite (ferritin) can serve as a Fe(II) precursor after interaction with Aβ. We suggest that Aβ can fulfill the role of ferritin-specific metallochaperone-like biomolecule, enabling capture of Fe(III) from the ferrihydrite core. Aβ can subsequently reduce Fe(III) to Fe(II) and stimulate formation of magnetite nanoparticles. In addition, Aβ loaded with Fe(II) was reported to induce oxidative stress by trapping molecular oxygen and its subsequent reduction to hydrogen peroxide [25]. Ferritin can thus be an important source of Fe(II) necessary for Fe(II)-containing minerals formed in the brains of patient suffering from neurodegenerative diseases and also for Fe(II)-loaded Aβ formation exhibiting neurotoxicity in diseased brain.
Acknowledgements
This work was supported by VEGA Grant Agency (project No. 2/0062/16, 2/0016/17, 0045, 2/0062/14, 2/0009/17), the Slovak Research and Development Agency (contract No. APVV-015-0453) and the Ministry of Education Agency for European Structural Funds (projects No. 26220120021, 2622012033, 26220220061, and 26220220186).
References
- 1.Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 2.El-Agnaf OMA, Mahil DS, Patel BP, Austen BM. Oligomerization and toxicity of β-amyloid-42 implicated in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2000;273:1003–1007. doi: 10.1006/bbrc.2000.3051. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Hilbich C, Kisterswoike B, Reed J, Masters CL, Beyreuther K. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer’s disease βA4 peptides. J. Mol. Biol. 1992;228:460–473. doi: 10.1016/0022-2836(92)90835-8. [DOI] [PubMed] [Google Scholar]
- 5.Soto C, Castaño EM, Kumar RA, Beavis RC, Frangione B. Fibrillogenesis of synthetic amyloid-β peptides is dependent on their initial secondary structure. Neurosci. Lett. 1995;200:105–108. doi: 10.1016/0304-3940(95)12089-M. [DOI] [PubMed] [Google Scholar]
- 6.Tjernberg LO, Naslund J, Lindqvist F, Johansson J, Karlstrom AR, Thyberg J, Terenius L, Nordstedt C. Arrest of β-amyloid fibril formation by a pentapeptide ligand. J. Biol. Chem. 1996;271:8545–8548. doi: 10.1074/jbc.271.15.8545. [DOI] [PubMed] [Google Scholar]
- 7.Everett, J., Cespedes, E., Shelford, L.R., Exley, C., Collingwood, J.F., Dobson, J., van der Laan, G., Jenkins, C.A., Arenholz, E., Telling, N.D.: Evidence of redox-active iron formation following aggregation of ferrihydrite and the Alzheimer’s disease peptide β-amyloid. Inorg. Chem. 53, 2803–2809 (2014) [DOI] [PubMed]
- 8.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/S1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 9.Pankhurst Q, Hautot D, Khan N, Dobson J. Increased levels of magnetic iron compounds in Alzheimer's disease. J. Alzheimers Dis. 2008;13:49–52. doi: 10.3233/JAD-2008-13105. [DOI] [PubMed] [Google Scholar]
- 10.Brillas E, Sirés I, Oturan MA. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009;109:6570–6631. doi: 10.1021/cr900136g. [DOI] [PubMed] [Google Scholar]
- 11.Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J. Neural Transm. 2011;118:301–314. doi: 10.1007/s00702-010-0470-z. [DOI] [PubMed] [Google Scholar]
- 12.Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 13.Everett, J., Cespedes, E., Shelford, L.R., Exley, C., Collingwood, J.F., Dobson, J., van der Laan, G., Jenkins, C.A., Arenholz, E., Telling, N.D.: Ferrous iron formation following the co-aggregation of ferric iron and the Alzheimer’s disease peptide β-amyloid (1-42). J. Royal Soc. Interface 11, 20140165 (2014) [DOI] [PMC free article] [PubMed]
- 14.Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004;331:370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Khan A, Dobson JP, Exley C. Redox cycling of iron by Aβ42. Free Radic. Biol. Med. 2006;40:557–569. doi: 10.1016/j.freeradbiomed.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Jutz G, van Rijn P, Santos Miranda B, Böker A. Ferritin: a versatile building block for bionanotechnology. Chem. Rev. 2015;115:1653–1701. doi: 10.1021/cr400011b. [DOI] [PubMed] [Google Scholar]
- 17.Watt RK, Hilton RJ, Graff DM. Oxido-reduction is not the only mechanism allowing ions to traverse the ferritin protein shell. Biochim. Biophys. Acta Gen. Subj. 2010;1800:745–759. doi: 10.1016/j.bbagen.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Carmona F, Palacios O, Galvez N, Cuesta R, Atrian S, Capdevila M, Dominguez-Vera JM. Ferritin iron uptake and release in the presence of metals and metalloproteins: chemical implications in the brain. Coor. Chem. Rev. 2013;257:2752–2764. doi: 10.1016/j.ccr.2013.03.034. [DOI] [Google Scholar]
- 19.Philpott CC, Ryu M-S. Special delivery: distributing iron in the cytosol of mammalian cells. Front. Pharmacol. 2014;5:173. doi: 10.3389/fphar.2014.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahirbegi, I.B., Pardo, W.A., Alvira, M., Mir, M., Samitier, J.: Amyloid Aβ 42, a promoter of magnetite nanoparticle formation in Alzheimer’s disease. Nanotechnology 27, 465102 (2016) [DOI] [PubMed]
- 21.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 22.Khurana R, Coleman C, Ionescu-Zanetti C, Carter SA, Krishna V, Grover RK, Roy R, Singh S. Mechanism of thioflavin T binding to amyloid fibrils. J. Struct. Biol. 2005;151:229–238. doi: 10.1016/j.jsb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Vassar PS, Culling CF. Fluorescent stains, with special reference to amyloid and connective tissues. Arch. Pathol. 1959;68:487–498. [PubMed] [Google Scholar]
- 24.LeVine III, H.: Thioflavine T interaction with synthetic Alzheimer's disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 2, 404–410 (1993) [DOI] [PMC free article] [PubMed]
- 25.Huang, X., Atwood, C.S., Hartshorn, M.A., Multhaup, G., Goldstein, L.E., Scarpa, R.C., Cuajungco, M.P., Gray, D.N., Lim, J., Moir, R.D., Tanzi, R.E., Bush, A.I.: The Aβ peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38, 7609–7616 (1999) [DOI] [PubMed]