Abstract
Purpose
Our aim was to explore whether antibiotic exposure in children and adolescents is associated with the later development of allergic diseases, using nationwide population-based claims data.
Methods
We collected information from the National Health Insurance Service (2006–2015) database. A total of 5,626,328 children and adolescents were eligible for the study. We explored whether exposure to antibiotics over the prior 7 years affects the later development of allergic diseases. We ran 3 analytical models after adjusting for confounding factors including age, sex, the number of visits to healthcare providers, income, and the place of residence (urban/rural).
Results
Allergic diseases were most common in male children and those aged < 10 years (atopic dermatitis, asthma and allergic rhinitis; all P < 0.01). Also, urban residents with higher incomes were more likely to develop allergic diseases (all P < 0.01). The annual number of days on which antibiotics were prescribed differed significantly between subjects with each allergic disease studied and a comparison group (all P < 0.01). Multiple logistic regression showed that as the duration of antibiotic exposure increased, the incidences of atopic dermatitis, asthma, and allergic rhinitis trended upward, even after adjusting for confounding factors (P for trend < 0.01).
Conclusions
Antibiotic use early in life is associated with an increased risk of allergic disease, especially in young children; the risk increases as the duration of antibiotic therapy rises. Moreover, urban residence was more strongly associated with a longer duration of antibiotic use than was rural residence.
Keywords: Asthma, atopic dermatitis, allergic rhinitis, antibiotics, epidemiologic
INTRODUCTION
Atopic dermatitis (AD), asthma, and allergic rhinitis (AR) are closely related diseases that principally affect children and adolescents.1 Especially, AD affects 17%–24% of the pediatric population.2,3 AD develops during the first 6 months of life in 45% of children and by 5 years of age in 85% of those affected.4 Several longitudinal studies have revealed the atopic march from AD to the development of asthma and AR.5,6 Moreover, the prevalences of AD, asthma, and AR have increased worldwide over the past 30 years, particularly in children and adolescents.7,8 The prevalence is relatively low in undeveloped countries, but increased greatly in children and adolescents in developing countries as hygiene practices improved.9,10
In 1980, Strachan proposed that repeated respiratory or other infections decrease the incidence of allergic diseases.11 Since that time, the hygiene theory has been developed to propose that an imbalance toward type 2 T-helper (Th2) responses attributable to a reduced infectious burden and subsequent reduction of the type 1 T-helper (Th1) response triggers allergic diseases.10 Furthermore, recent investigations have incorporated the microbiome hypothesis into the hygiene effect.12,13 Allergic diseases develop in mucosal tissues harboring commensal microbiota and are closely associated with changes in the nature of these microbiota in various tissues including the intestine, suggesting that normal microbiotic changes play important roles in the pathogenesis of allergic diseases.14 Disease-limiting effects may be mediated via changes in the resident microbiome associated with the production of protective metabolites such as short-chain fatty acids, tryptophan or polysaccharides.15 Several reports have found that exposure to antibiotics increased the incidence of allergic diseases.16,17,18 However, to the best of our knowledge, no study has yet explored the relationship between the duration of antibiotic exposure and the later development of allergic diseases (including AD, asthma, and AR) using nationwide population-based data. Therefore, our aim was to determine if the duration of exposure to antibiotics in children and adolescents is associated with the later development of allergic diseases. We examined nationwide population-based claims data spanning 10 years from 2006 to 2015.
MATERIALS AND METHODS
Data sources
We collected information from the National Health Insurance Service (NHIS) claims database (NHIS-2016-4-026) covering the interval 2006 to 2015. The study was approved by the Ethics Committee of the National Evidence-Based Healthcare Collaborating Agency and the need for informed subject consent was waived. The study adhered to all relevant tenets of the Declaration of Helsinki. In Korea, 97% of the population is covered by compulsory health insurance offered by the NHIS.19 Both outpatient and inpatient claims are reviewed by the NHIS, which records diagnoses, procedures, prescriptions, and demographic information.20 The NHIS also reviews claims from the Medical Assistance Program and the Medical Care for Patriots and Veterans Affairs Scheme, which cover medical expenses not reimbursed by the NHIS.21 Therefore, the NHIS database covers the entire Korean population.
Study population
We identified all children and adolescents (aged < 19 years) diagnosed with AD, asthma, and AR from 2006 to 2015 using the diagnostic codes of the 10th revision of the International Classification of Diseases (ICD-10). Between 2013 and 2015, data on 51,834,660 individuals were recorded; 10,328,983 were children and adolescents. We excluded patients diagnosed with allergic disease prior to 2013 (n = 4,702,655). Thus, the final number examined was 5,626,328. The comparison group was defined as other children and adolescents who were not diagnosed with AD, asthma or AR (n = 3,326,948). Because NHIS claims database was in service since 2006, we measured the durations of exposure to antibiotics from 7 years prior to the development of allergic disease. In the cases of children younger than 7-year-old, their life time data were used in this study. The antibiotics prescription days were counted before the diagnosed day in the disease group and before the last day of 2015 in the comparison group. Annual average antibiotics prescription days were measured from this data. The diagnostic codes used were L20 for AD; J301, J302, J303, and J304 for AR; and J45 and J46 for asthma, as previously reported.22,23 Principal and additional diagnoses were all analyzed as diagnostic codes. In the cases of 2 or more overlapping codes, the prior claimed code was decided as diagnosis. The day allergic diseases were first diagnosed was set as an index date. Metropolitan cities and cities were defined as urban, and the other provinces were defined as rural. The classification of the antibiotics was defined by Ministry of Food and Drug Safety classification code: 600 (antibiotics). The classification code was determined before the market release of the drug. The duration of the antibiotics were measured according to prescription days. Incomes were measured by insurance premium. Workplace health insurance, regional health insurance, and the people receiving social security payments were all included in the analysis. Inpatient and outpatient days were investigated by the day claimed to NHIS.
Statistical analysis
Statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous variables are presented as the mean ± standard error (SE). Categorical variables are presented as proportions (with SE). The clinical characteristics of subjects who did and did not develop allergic diseases were compared using Student's t-test or the Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical variables. The incidence of allergic diseases among decile groups was compared via one-way analysis of variance followed by Bonferroni's multiple comparisons test. A potential source of bias was the use of conventional medicine rather than alternative treatments. Therefore, we used 3 models to adjust for potential confounders including contact with medical professionals (inpatient and outpatient days). We employed multiple logistic regression to analyze the associations between antibiotic exposure duration and the incidence of allergic diseases, using all 3 models. Model 1 was the crude model. Model 2 was model 1 adjusted for age and sex. Model 3 was model 2 additionally adjusted for the number of days on which healthcare providers were visited (on an inpatient or outpatient basis), income, and the place of residence (urban or rural). All P values were 2-sided, and a P value of < 0.05 was considered to reflect statistical significance.
RESULTS
A total of 5,626,328 children and adolescents were included, and factors influencing the incidences of various diseases are shown in Table 1. Allergic diseases were more common in males and subjects aged < 10 years (AD, asthma and AR; all P < 0.01). Also, urban residence and a higher income were associated with higher incidences of allergic diseases (AD, asthma, and AR; all P < 0.01).
Table 1. Data on the total study population by factors known to affect the development of different allergic diseases.
| Variables | Comparison group | Atopic dermatitis | Asthma | Allergic rhinitis | Total | |
|---|---|---|---|---|---|---|
| No. (%) | 3,326,948 (59.13) | 134,050 (2.38) | 623,461 (11.08) | 1,541,869 (27.4) | 5,626,328 (100) | |
| Sex (male) | 1,665,301 (50.05) | 73,561 (54.88) | 336,265 (53.94) | 786,866 (51.03) | 2,861,993 (50.87) | |
| Age (yr) | ||||||
| < 10 | 938,901 (28.22) | 115,705 (86.31) | 603,273 (96.76) | 1,253,294 (81.28) | 2,911,173 (51.74) | |
| 10–19 | 2,388,047 (71.78) | 18,345 (13.69) | 20,188 (3.24) | 288,575 (18.72) | 2,715,155 (48.26) | |
| Residence (urban) | 1,429,720 (42.97) | 59,651 (44.5) | 272,764 (43.75) | 668,745 (43.37) | 2,424,651 (43.10) | |
| Income | ||||||
| Basic + lower 50% | 1,560,659 (46.90) | 58,558 (43.68) | 264,893 (42.49) | 661,509 (42.90) | 2,545,619 (45.24) | |
| Upper 50% | 1,766,289 (53.10) | 75,492 (56.32) | 358,568 (57.51) | 880,360 (57.10) | 3,080,709 (44.76) | |
All comparisons with the comparison group were significant (P < 0.01 by one-way analysis of variance). All differences between subjects of various ages, the two genders, the place of residence, and income category, were significant at P < 0.01 as revealed by the χ2 test. Basic: Receiving social security payments.
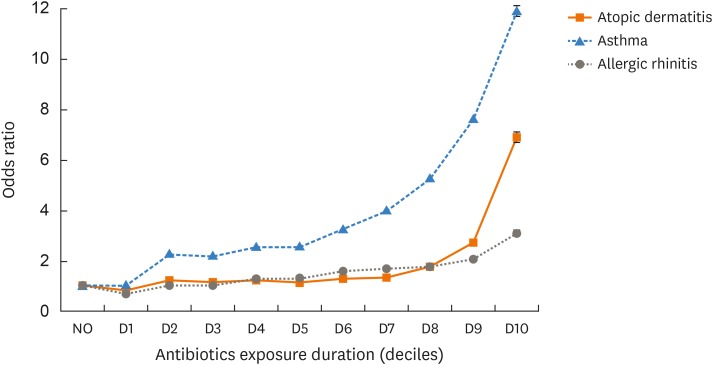
Table 2 shows the relationship between the annual average number of days (the mean for each year) on which antibiotics were prescribed and the incidences of allergic diseases. The mean annual prescription days of subjects with each allergic disease differed significantly from those of the comparison group, even after adjusting for potential confounding factors (all P < 0.01, Table 2). Subanalysis was performed by sex, age, and the place of residence (model 3). Male sex and rural residence of subjects aged < 10 years were associated with more antibiotic prescription days (all P < 0.01, Table 2). The average number of annual prescription days for both rural and urban residents was associated with the development of allergic diseases, but the relationship was stronger in urban residents (Table 3). As antibiotic exposure duration increased, the incidences of AD, AR, and asthma also trended higher (P for trend < 0.01) even after adjusting for confounding factors (all P < 0.01, Table 4 and Figure). The trend was strongest for asthma.
Table 2. Relationships between annual average antibiotic prescription days and atopic dermatitis, asthma or allergic rhinitis as analyzed using a generalized linear model.
| Variables | Comparison group | Atopic dermatitis | Asthma | Allergic rhinitis | |
|---|---|---|---|---|---|
| Sex | Male | 9.20 ± 0.01 | 24.64 ± 0.09 | 21.67 ± 0.04 | 13.04 ± 0.03 |
| Female | 8.92 ± 0.01 | 23.24 ± 0.08 | 20.53 ± 0.04 | 12.02 ± 0.03 | |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Age | < 10 | 14.89 ± 0.02 | 30.27 ± 0.10 | 26.39 ± 0.04 | 20.36 ± 0.03 |
| 10–19 | 6.04 ± 0.003 | 8.03 ± 0.02 | 11.61 ± 0.07 | 11.61 ± 0.07 | |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Place | Urban | 8.63 ± 0.01 | 22.92 ± 0.09 | 20.68 ± 0.04 | 12.06 ± 0.03 |
| Rural | 9.38 ± 0.01 | 24.88 ± 0.10 | 21.52 ± 0.04 | 12.92 ± 0.03 | |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Adjusted model | Model 1* | 7.40 ± 0.01 | 36.36 ± 0.05 | 31.78 ± 0.03 | 16.87 ± 0.02 |
| Model 2* | 8.33 ± 0.01 | 31.69 ± 0.05 | 26.96 ± 0.03 | 13.20 ± 0.02 | |
| Model 3* | 9.06 ± 0.01 | 25.04 ± 0.04 | 21.81 ± 0.03 | 12.06 ± 0.02 | |
Data are expressed as the mean ± standard error. Model 1: Crude. Model 2: Adjusted for age and sex. Model 3: Adjusted further for inpatient and outpatient days, income, and place of residence. Model 3 was used for subanalysis by sex, age, and place of residence.
*All comparisons with the comparison group were significant (P < 0.01 by one-way analysis of variance).
Table 3. Relationships between the annual average numbers of antibiotic prescription days and the place of residence as analyzed by multiple logistic regression.
| Days of prescription | OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1–15 | 16–30 | 31–60 | 61–90 | > 90 | ||
| Total | |||||||
| Rural | 1.000 | 1.283 (1.270–1.295) | 1.968 (1.946–1.990) | 2.535 (2.504–2.567) | 3.420 (3.356–3.485) | 4.772 (4.661–4.886) | |
| Urban | 0.916 (0.905–0.928) | 1.341 (1.328–1.355) | 2.1 (2.075–2.125) | 2.839 (2.800–2.878) | 4.115 (4.020–4.213) | 5.785 (5.609–5.966) | |
| Atopic dermatitis | |||||||
| Rural | 1.000 | 1.146 (1.109–1.185) | 1.535 (1.481–1.590) | 2.536 (2.449–2.626) | 4.719 (4.531–4.914) | 9.513 (9.123–9.919) | |
| Urban | 1.018 (0.978–1.060) | 1.248 (1.207–1.291) | 1.766 (1.703–1.832) | 3.047 (2.938–3.160) | 5.971 (5.707–6.246) | 12.031 (11.460–12.630) | |
| Asthma | |||||||
| Rural | 1.000 | 2.491 (2.443–2.540) | 4.582 (4.491–4.675) | 6.816 (6.678–6.957) | 9.518 (9.276–9.766) | 12.157 (11.800–12.525) | |
| Urban | 0.938 (0.915–0.962) | 2.429 (2.382–2.478) | 4.826 (4.727–4.927) | 7.754 (7.588–7.924) | 11.659 (11.315–12.013) | 14.635 (14.105–15.185) | |
| Allergic rhinitis | |||||||
| Rural | 1.000 | 1.162 (1.150–1.174) | 1.631 (1.612–1.651) | 1.826 (1.802–1.851) | 2.255 (2.210–2.301) | 3.213 (3.135–3.294) | |
| Urban | 0.907 (0.895–0.919) | 1.217 (1.204–1.230) | 1.731 (1.710–1.753) | 2.016 (1.986–2.046) | 2.659 (2.593–2.726) | 3.894 (3.771–4.021) | |
The values are multivariate-adjusted ORs (with 95% CIs).
OR, odds ratio; CI, confidence interval.
Table 4. Relationships between the annual average numbers of days on which antibiotics were prescribed and the odds ratios for atopic dermatitis, asthma, and allergic rhinitis, as analyzed using multiple logistic regression.
| Days of prescription | OR (95% CI) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Atopic dermatitis | ||||
| 0 | 1.000 | 1.000 | 1.000 | |
| 1–15 | 0.386 (0.377–0.395) | 1.130 (1.102–1.158) | 1.185 (1.157–1.215) | |
| 16–30 | 1.929 (1.882–1.977) | 2.744 (2.676–2.814) | 1.628 (1.586–1.672) | |
| 31–60 | 6.242 (6.094–6.393) | 6.700 (6.540–6.865) | 2.740 (2.669–2.813) | |
| 61–90 | 17.623 (17.141–18.119) | 17.208 (16.733–17.697) | 5.196 (5.037–5.360) | |
| > 90 | 55.294 (53.753–56.880) | 53.202 (51.706–54.742) | 10.446 (10.111–10.792) | |
| P for trend | < 0.01 | < 0.01 | < 0.01 | |
| Asthma | ||||
| 0 | 1.000 | 1.000 | 1.000 | |
| 1–15 | 0.441 (0.438–0.444) | 1.384 (1.374–1.395) | 1.242 (1.232–1.252) | |
| 16–30 | 1.861 (1.847–1.876) | 2.928 (2.903–2.953) | 1.753 (1.737–1.769) | |
| 31–60 | 3.876 (3.841–3.912) | 4.494 (4.451–4.537) | 1.993 (1.972–2.013) | |
| 61–90 | 7.385 (7.278–7.494) | 7.709 (7.595–7.824) | 2.518 (2.478–2.559) | |
| > 90 | 17.002 (16.696–17.313) | 17.090 (16.779–17.407) | 3.618 (3.546–3.691) | |
| P for trend | < 0.01 | < 0.01 | < 0.01 | |
| Allergic rhinitis | ||||
| 0 | 1.000 | 1.000 | 1.000 | |
| 1–15 | 0.511 (0.504–0.518) | 2.607 (2.570–2.644) | 2.539 (2.502–2.576) | |
| 16–30 | 4.410 (4.350–4.471) | 8.277 (8.158–8.398) | 4.833 (4.760–4.906) | |
| 31–60 | 13.796 (13.602–13.993) | 18.654 (18.381–18.931) | 7.416 (7.301–7.533) | |
| 61–90 | 30.240 (29.692–30.798) | 37.354 (36.657–38.064) | 10.629 (10.418–10.844) | |
| > 90 | 60.957 (59.673–62.269) | 73.965 (72.367–75.598) | 13.447 (13.133–13.768) | |
| P for trend | < 0.01 | < 0.01 | < 0.01 | |
The values are multivariate-adjusted ORs (with 95% CIs). Model 1: Basic. Model 2: Model 1 with age and sex. Model 3: Model 2 with inpatient and outpatient days, income, and place of residence.
OR, odds ratio; CI, confidence interval.
Figure. The odds ratios of developing allergic disease by the duration of exposure to antibiotics. The odds ratios reflect the risks in deciles compared with the average risk in the comparison group. The error bars reflect the standard error.
DISCUSSION
Microbial imbalances in the gut and airway mucosa have been implicated in the development of many diseases including inflammatory bowel disease, allergic conditions, rheumatoid arthritis, depression, and obesity.24 Most infections in children are viral, but antibiotics given during such infections may prevent secondary bacterial infection.18 Antibiotics change the mucosal microbiota and trigger dysbiosis of bacterial communities,17 which is associated with the development of allergic diseases upon maturation of the immune system during childhood. Thus, early changes in microbiome compositions and microbial dysfunction may explain the development of allergic diseases of childhood and adolescence.25,26 A possible immunological explanation is based on the hygiene hypothesis: exposure to microbial products such as endotoxins in early childhood, possibly associated with induction of Th1 lymphocytes, reduces the Th2-biased responses characteristic of allergies, including the persistent production of immunoglobulin E (IgE).27,28
We found that children and adolescents exposed to greater levels of antibiotics early in life were at increased risk of developing later allergic diseases compared with those receiving fewer antibiotics. The association remained significant even after adjustment for potential confounders. Our findings are in agreement with other studies, and may support the hygiene/microbiome hypothesis: childhood infections protect against later allergies.16,17,18 However, we gathered more historical data than previous studies and evaluated subjects aged 0 to 19 years, thus including those most affected by allergic diseases. We also interrogated a large nationwide database. In agreement with the hygiene/microbiome hypothesis, we found a lower incidence of allergic diseases in children living in rural areas, associated with higher-level exposure to bacteria. Reduced bacterial exposure was associated with atopy and asthma.29,30 Although children and adolescents living in urban areas were prescribed fewer antibiotics than rural subjects, they developed more allergic diseases. It is generally accepted that urban residents have a higher incidence of allergic diseases than rural residents. Notably, among subjects who did not take antibiotics, asthma and AR were more common in rural residents. However, the trend changed significantly (toward urban residents) as the number of antibiotic use days increased. Thus, urban residence may correlate not only with susceptibility to allergic diseases but also with the extent of antibiotic exposure.
Of the allergic diseases, asthma was most strongly associated with the duration of antibiotic use, consistent with the finding of a previous study which compared the associations between five levels of antibiotic use and the development of allergic diseases.31 However, the associations found were not clearly explained. The interaction between gut and lung microbiota (the gut-lung axis) may explain the strong association between asthma and antibiotic use. Microbial communication via microaspiration and inhalation, mucosal dispersion eliminating microbes via coughing and mucociliary clearance may create cross-links between the gut and lung microbiota.32 As most antimicrobial agents are oral and are thus absorbed through the gastrointestinal tract, mucosal dysbiosis and cross-talk with the lung may be closely related. Further studies are needed to clarify the effects of different antibiotics on allergic disease development.
Our study has both strengths and limitations. This is the largest population-based study to date; the NHIS database covers the entire Korean population. Over 5 million children and adolescents were followed up for up to 7 years. In contrast, many previous studies had small sample sizes and durations, and were limited in terms of geographic region and subject background. In addition, we assessed antibiotic exposure using claims data. The recall bias and other biases associated with self-reporting were thus not in play. Moreover, we adjusted for many potential confounding factors including age, sex, the number of days on which healthcare professionals were visited, income, and the place of residence (urban/rural). However, our study has several limitations. First, claims data do not capture some important risk factors for allergic diseases, including the mode of delivery during childbirth, family history, or family size. We sought to adjust for all other major confounding factors; however, the number of healthcare visits may in fact be a significant confounding factor when evaluating the association between antibiotic use and allergic disease. Secondly, any study that relies on administrative data is associated with a risk of misclassification. Therefore, there may be discrepancies of the real prevalence of allergic disease and claim data. Potential confounding factors and the risk of diagnostic misclassification could affect the interpretation of data. However, these limitations could be compensated with large study population data and high odds ratios. Thirdly, the cross-sectional study design has limitation on interpreting cumulative exposure to environment.
In conclusion, we found that antibiotic use early in life was associated with increased risk of later allergic diseases, especially in young children, and the risk increased as the duration of antibiotic use rose. This was more marked in urban residents, supporting the hygiene/microbiome hypothesis. Although association does not prove causality, unnecessary use of antibiotics by children and adolescents should be avoided.
ACKNOWLEDGMENTS
This work was supported by grants from the Korean Society of Otorhinolaryngology-Head and Neck Surgery, 2017.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(Suppl):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 3.Yoon J, Choi YJ, Lee E, Cho HJ, Yang SI, Kim YH, et al. Allergic rhinitis in preschool children and the clinical utility of FeNO. Allergy Asthma Immunol Res. 2017;9:314–321. doi: 10.4168/aair.2017.9.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35–39. doi: 10.1016/s0190-9622(94)70004-4. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165:176–180. doi: 10.1164/ajrccm.165.2.2104032. [DOI] [PubMed] [Google Scholar]
- 7.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Han K, Kim SW. Relationship between allergic rhinitis and mental health in the general Korean adult population. Allergy Asthma Immunol Res. 2016;8:49–54. doi: 10.4168/aair.2016.8.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platts-Mills TA. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136:3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu AH. Revisiting the hygiene hypothesis for allergy and asthma. J Allergy Clin Immunol. 2015;136:860–865. doi: 10.1016/j.jaci.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The hygiene hypothesis: current perspectives and future therapies. ImmunoTargets Ther. 2015;4:143–157. doi: 10.2147/ITT.S61528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro-Nallar E, Shen Y, Freishtat RJ, Pérez-Losada M, Manimaran S, Liu G, et al. Integrating microbial and host transcriptomics to characterize asthma-associated microbial communities. BMC Med Genomics. 2015;8:50. doi: 10.1186/s12920-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 16.Farooqi IS, Hopkin JM. Early childhood infection and atopic disorder. Thorax. 1998;53:927–932. doi: 10.1136/thx.53.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickens K, Pearce N, Crane J, Beasley R. Antibiotic use in early childhood and the development of asthma. Clin Exp Allergy. 1999;29:766–771. doi: 10.1046/j.1365-2222.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- 19.Park SJ, Choi NK, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed retinal vein occlusion in Korea, 2008 through 2011: preponderance of women and the impact of aging. Ophthalmology. 2014;121:1274–1280. doi: 10.1016/j.ophtha.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Kim J, Park SY, Um HY, Kim K, Kim Y, et al. Effect of pregnancy in asthma on health care use and perinatal outcomes. J Allergy Clin Immunol. 2015;136:1215–1236.e1-6. doi: 10.1016/j.jaci.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Kwon KE, Choi NK, Park KH, Woo SJ. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015;122:2063–2070.e1. doi: 10.1016/j.ophtha.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Kim BK, Kim JY, Kang MK, Yang MS, Park HW, Min KU, et al. Allergies are still on the rise? A 6-year nationwide population-based study in Korea. Allergol Int. 2016;65:186–191. doi: 10.1016/j.alit.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Choi KH, Hwang SW, Lee YB, Park HJ, Bae JM. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40–48. doi: 10.1016/j.jaad.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 25.Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 26.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98:279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- 29.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134:593–601.e12. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valkonen M, Wouters IM, Täubel M, Rintala H, Lenters V, Vasara R, et al. Bacterial exposures and associations with atopy and asthma in children. PLoS One. 2015;10:e0131594. doi: 10.1371/journal.pone.0131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr Allergy Immunol. 2013;24:762–771. doi: 10.1111/pai.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung KF. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? J Allergy Clin Immunol. 2017;139:1071–1081. doi: 10.1016/j.jaci.2017.02.004. [DOI] [PubMed] [Google Scholar]