Dear Editor,
The tumor-microenvironment interactions play important roles in tumor progression, metastasis, and therapeutic resistance,1 and increasing evidence indicates that tumor cell-derived exosomes can systematically modulate or reprogram the tumor microenvironment by transferring molecules, such as microRNAs, mRNAs, and proteins from donor cells to recipient cells.2 PD-L1 is a classical immune surface protein, which inhibits anti-tumor function of T cells by binding to its receptor programmed cell death-1 (PD-1) and effectively protects tumor from immune surveillance.3 Exosomes have been reported to contain certain types of proteins, including membrane proteins, e.g., EGFR and MET, that promote cancer metastasis.4,5 As a membrane-bound protein, whether PD-L1 exists in cancer cell-derived exosomes and whether it plays a role in tumor progress are largely unknown.
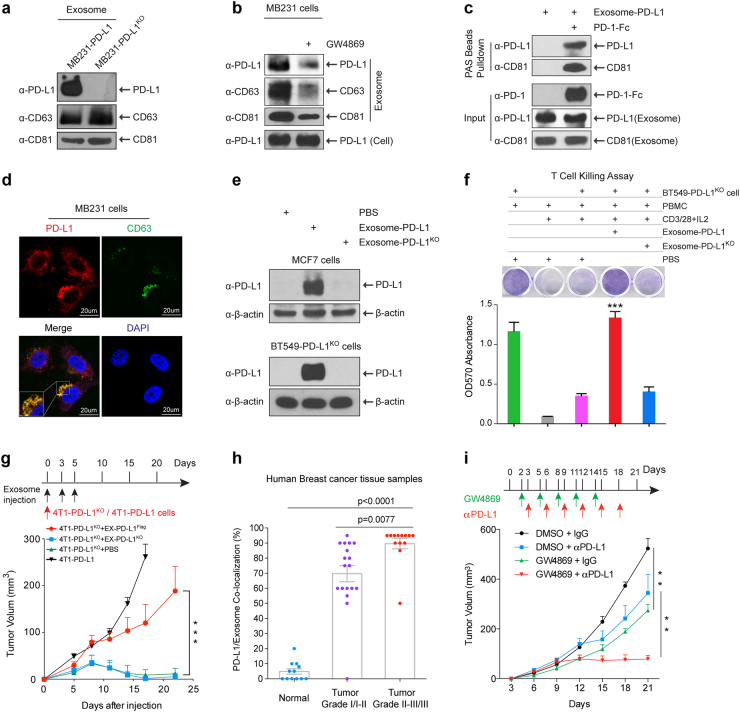
We isolated exosomes from the supernatant of cell cultures of MDA-MB-231 (231) human breast cancer cells and 4T1 mouse mammary tumor cells with PD-L1 expression or PD-L1 knockout (PD-L1KO) by sequential centrifugation. Transmission electron microscopy images showed that these exosomes are typically spherical and membrane encapsulated with a size of 30–100 nm (Supplementary information, Figure S1a). PD-L1 was detected in exosomes isolated from the culture media of PD-L1-expressing human breast cancer cells (231-PD-L1) and mouse mammary tumor cells (4T1-PD-L1), but not 231-PD-L1KO or 4T1-PD-L1KO cells with similar levels of exosome makers, CD63 and CD81, whose expression indicates exosome production6 (Fig. 1a; Supplementary information, Figure S1b and c). Notably, treatment with exosome secretion inhibitor, GW4869,7 reduced exosome production (as indicated by the reduction of exosome markers CD63 and CD81 or the total amount of exosomal protein) in 231 cells as well as the levels of PD-L1 in exosomes, but had no effect on PD-L1 expression in the cell lysates (Fig. 1b; Supplementary information, Figure S1d). In addition, in vitro binding assay showed that PD-1-Fc protein simultaneously pulled down PD-L1 and CD81 in 231-PD-L1-derived exosomes (term as exosome-PD-L1) (Fig. 1c). Immunofluorescence (IF) staining of 231 cells (Fig. 1d) and immunohistochemistry (IHC) double staining of human breast cancer tissues (Supplementary information, Figure S1e) demonstrated co-localization of PD-L1 and CD63 in the multivesicular bodies (MVBs), which are the precursor form of exosomes inside cells before released. These data further supported the presence of PD-L1 in exosomes in vitro and in vivo.
Fig. 1.
PD-L1 existed in exosomes and played important roles in breast tumor growth. a Western blot of PD-L1 in exosomes isolated from the media of 231-PD-L1 and 231-PD-L1KO cells with the indicated antibodies. CD63 and CD81, exosome markers. b Western blot of PD-L1, CD63, CD81 in exosomes isolated from 231-PD-L1 cells with or without GW4869 (10 μM) treatment for 48 h with the indicated antibodies. c PD-L1-containing exosome were incubated with PD-1-Fc recombinant proteins followed with protein A sepharose pulldown. PD-L1 and CD81 were detected by Western blotting. One percent of PD-1-Fc proteins and exosome-PD-L1 served as input. d Confocal microscopy of PD-L1 (red) and CD63 (green) in 231 breast cancer cells. Yellow, co-localization of PD-L1 and CD63 in MVBs. e Exosome-PD-L1, exosome-PD-L1KO, or PBS was added to MCF7 (PD-L1 low) or BT549-PD-L1KO cells. After 24-h incubation, cells were washed with PBS 4 times, lysed in lysis buffer, and subjected to Western blotting using the indicated antibodies. f Top, T-cell-meditated tumor cell-killing assay in BT549-PD-L1KO cells incubated with exosome-PD-L1, exosome-PD-L1KO, or PBS. Bottom, quantification of cell viability. g Tumor growth of PD-L1 deficient 4T1 cells (4T1-PD-L1KO) in BALB/c mice injected with EX-PD-L1Flag or EX-PD-L1KO as indicated. Tumor volume was measured at the indicated time points (n = 10 mice per group). h Co-localization analysis of PD-L1 and exosomes in human breast cancer tissue microarray. Samples were double stained with PD-L1 and CD63 antibody. The percentage of PD-L1 and CD63 co-localization was determined by the green/brown color as described in Supplementary information, Figure S1e. i Tumor growth of 4T1 cells in BALB/c mice treated with GW4869 and/or PD-L1 antibody. Tumor volume was measured at the indicated time points (n = 8 mice per group). Data represent mean ± SEM, **P < 0.01, ***P < 0.001 or as indicated, Student’s t-test
To evaluate the biological functions of exosome-PD-L1, we first asked whether it could transfer PD-L1 to other cells with low (MCF-7) or no PD-L1 expression (BT549-PD-L1KO). We detected the transfer of PD-L1 from exosome-PD-L1 to MCF7 or BT549-PD-L1KO cells but not from exosomes derived from 231-PD-L1KO cells (exosome-PD-L1KO; Fig. 1e). The acquisition of PD-L1 protein was not a result of PD-L1 gene expression as indicated by the lack of PD-L1 mRNA in these cells (Supplementary information, Figure S1f). We also established 231-PD-L1EGFP cells and visually demonstrated the transfer of PD-L1EGFP from 231-PD-L1EGFP-derived exosomes to BT549 cells (Supplementary information, Figure S1g). To examine whether this also occurs in vivo, we co-injected mouse 4T1-PD-L1KO cells with exosomes derived from 4T1-PD-L1Flag (EX-PD-L1Flag), 4T1-PD-L1KO (EX-PD-L1KO), or PBS into the mammary fat pad of BALB/c mice. Tumors were harvested after 5 days. IF staining of tumor tissue sections showed that EX-PD-L1Flag but not EX-PD-L1KO rendered 4T1-PD-L1KO cells PD-L1Flag positive (Supplementary information, Figure S1h). Importantly, results from flow cytometric analysis further revealed that the PD-L1 transported by exosomes was located on the surface of target cells and able to bind to PD-1 (Supplementary information, Figure S1i). These results indicated that exosomes are capable of transferring functional PD-L1 to other cells.
Given that PD-L1 of exosomes can directly bind to PD-1 (Fig. 1c), we next examined whether exosomal PD-L1 affects T cell functions. As shown in Fig. 1f, exosome-PD-L1, but not exosome-PD-L1KO or PBS, significantly inhibited the T cell killing effect on BT549-PD-L1KO cells. Next, to explore whether exosomal PD-L1 inhibits CD3/CD28-triggered T cell activation signaling pathway, we generated T cell blasts by treating peripheral blood mononuclear cells (PBMCs) with phytohemagglutinin (PHA) to induce PD-1 expression.8 The results showed that exosome-PD-L1 but not exosome-PD-L1KO markedly inhibited CD3/CD28-induced ERK phosphorylation and NF-κB activation of T cells in a dose-dependent manner (Supplementary information, Figure S2a and b) as well as PHA-induced interleukin-2 (IL-2) secretion (Supplementary information, Figure S2c), all of which are indicators of T cell activation.9 Furthermore, exosomal PD-L1 from other cancer cell lines such as colon (RKO) and lung (HCC827) cancer cells has similar function in blocking T cell activation (IL-2 production; Supplementary information, Figure S2d and e). Together, these data supported that exosomal PD-L1 inhibits T-cell activation.
Next, to evaluate the role of exosomal PD-L1 in tumor microenvironment and tumor progression in vivo, we measured tumor growth of 4T1-PD-L1KO cells co-injected with EX-PD-L1Flag, EX-PD-L1KO, or PBS. Consistent with the previous report,10 PD-L1 deficiency in 4T1-PD-L1KO cells led to substantial tumor regression; however, EX-PD-L1Flag but not EX-PD-L1KO remarkably restored tumor growth of 4T1-PD-L1KO cells (Fig. 1g). We then exposed 4T1-PD-L1KO cells to increasing amounts of EX-PD-L1Flag and showed that EX-PD-L1Flag promoted tumor growth in a dose-dependent manner (Supplementary information, Figure S2f). Moreover, 4T1-PD-L1KO tumors with EX-PD-L1Flag co-injection exhibited much less granzyme B expression, indicating reduced cytotoxic T cell activity, in tumor area compared with those with EX-PD-L1KO or PBS co-injection (Supplementary information, Figure S2g). These results suggested that EX-PD-L1 protects and promotes tumor growth in vivo by inhibiting T-cell granzyme B secretion. These findings are consistent with the above in vitro studies in which exosomal PD-L1 can bind to PD-1 and inhibit T-cell killing activities (Fig. 1c, f).
On the basis of the above IHC staining showing co-localization of PD-L1 and exosome marker CD63 in MVBs in human tumor tissue samples (Supplementary information, Figure S1e), we sought to determine whether their co-localization is linked to the stages of breast cancer in patients. Analysis of a set of human breast cancer tissue microarray indicated higher levels of PD-L1-CD63 co-localization associated with higher stage of the disease (Fig. 1h), which is consistent with the recent report that PD-L1 levels in exosomes correlate with disease progression in head and neck squamous cell carcinomas patients.11 Based on the above results, we hypothesized that growing tumors continue to secret exosome-PD-L1 to inactive T cell functions in the tumor microenvironment, which may induce immunotherapy resistance. To explore the possibility that targeting exosome secretion enhances the immunotherapy efficacy, we treated tumors with exosome secretion inhibitor GW4869 combined with PD-L1 antibody in a mouse model. First, we showed that GW4869 inhibited exosome secretion of 4T1 mouse breast cancer cells (Supplementary information, Figure S2h). As shown in Fig. 1i, inhibition of exosome secretion by GW4869 inhibited 4T1 tumor growth in BALB/c mice (black vs. green line) but not 4T1 cell growth in vitro and in immunodeficient nude mice (Supplementary information, Figure S3a and b), indicating that GW4869 treatment itself did not inhibit 4T1 tumor growth but likely through modulation of anti-tumor immunity involving inhibition of exosome-PD-L1 secretion. Thus, as expected, GW4869 treatment significantly augmented anti-PD-L1 therapeutic efficacy in 4T1 tumor growth suppression (blue vs. red line; Fig. 1i). Furthermore, in order to specifically inhibit exosome secretion of 4T1 cells, we constructed Tet-on inducible Rab27a knockdown 4T1 cell line. Consistent with previous report,6,12 knockdown of Rab27a by Dox treatment significantly inhibited exosome secretion as measured by CD63 and CD81 (Supplementary Figure S3c, top) and the results further showed that knockdown of Rab27a significantly inhibited 4T1 tumor growth (black/purple vs. green line; Supplementary Figure S3c, bottom). Similar to GW4869 treatment, specific inhibition of exosome secretion by Rab27a knockdown in tumor cells strikingly augmented anti-PD-L1 therapeutic efficacy (blue vs. red line; Supplementary Figure S3c, bottom). Thus, inhibition of exosome secretion by both pharmacological inhibitor and genetic approach in tumor cells, namely Rab27a knockdown, significantly inhibited 4T1 tumor growth and augmented anti-PD-L1 therapeutic efficacy. It is worthwhile to mention that the tumor suppressive effect is relatively profound by knocking down Rab27a (Supplementary Figure S3c) or inhibitor GW4869 treatment (Fig. 1i) compared with anti-PD-L1 antibody treatment (green vs. blue line), suggesting that blockage of secreted exosome-PD-L1 in tumor cells contributes significantly to anti-tumor immunity. Furthermore, combination of both led to much stronger tumor suppression (red vs. green/blue line).
Additionally, although we have shown that exosomes transported PD-L1 from PD-L1-positive to PD-L1-negative breast cancer cells and that exosomal PD-L1 bound to PD-1 on T cells to inhibit T cell activation and killing activities (Supplementary information, Figure S5), it has been reported that human myeloid APCs also express PD-L1 and critically contribute to immunotherapy.13,14 Thus, we also tested whether PD-L1-containing exosomes could transport PD-L1 to myeloid APCs. As shown in Supplementary Figure S4a, we detected the transfer of PD-L1 from exosome-PD-L1 to human monocyte cell line THP1 (precursor of macrophages and DCs) but not from exosome-PD-L1KO. Results from flow cytometric analysis further revealed that exosomes could transfer PD-L1EGFP to human macrophages and DCs in PBMCs in vitro (Supplementary information, Figure S4b). In vivo, IF staining results showed that EX-PD-L1Flag but not EX-PD-L1KO rendered tumor-infiltrated macrophages PD-L1Flag positive (Supplementary information, Figure S4c); however, we did not observe the positive staining in DCs (Supplementary information, Figure S4d). It is not yet clear why the transferring efficiency of PD-L1 to DCs by exosomes in vivo is so low compared with that to macrophages. This could be due to less amount of DCs (purple, Supplementary Figure S4d vs. red, Supplementary Figure S4c) and/or some unknown mechanisms to reduce the efficiency in the tumor microenvironment. Nevertheless, the results indicated that PD-L1 can be transferred to multiple cell types including tumor cells, macrophages and DCs (at least in vitro) through PD-L1-containing exosomes in the tumor microenvironment. Thus, the exosome appears to serve as a trafficking vehicle to deliver PD-L1 into different cell types in the tumor microenvironment to modulate immune surveillance. Although it is not yet clear whether the PD-L1-expressing macrophages or DCs might also secrete exosome-PD-L1, the results shown in Supplementary Figure S3c indicated that blockage of exosome secretion by Rab27a knockdown in tumor cells inhibited 4T1 tumor growth similar as GW4869 treatment (Fig. 1i). Together, our study suggested that exosome-PD-L1 from tumor cells plays an important role in modulating immune surveillance in the tumor microenvironment.
Collectively, our current findings suggested that PD-L1 plays a role in active defense in addition to passive protection by its presence in exosomes, and PD-L1-containing exosomes attenuate anti-tumor immunity in tumor microenvironment. Furthermore, our results suggested that blockage of exosome-PD-L1 secretion contributes significantly to anti-tumor immunity, and that the combination of exosome secretion inhibition and anti-PD-L1 therapy has the potential to improve anti-tumor response in the clinic.
Electronic supplementary material
Acknowledgements
This work was funded in part by the following: National Institutes of Health (CCSG CA016672); RO1 CA211615; Cancer Prevention & Research Institutes of Texas (DP150052 and RP160710); National Breast Cancer Foundation, Inc.; Breast Cancer Research Foundation (BCRF-17-069); Patel Memorial Breast Cancer Endowment Fund; The University of Texas MD Anderson-China Medical University and Hospital Sister Institution Fund; Center for Biological Pathways; Ministry of Science and Technology, International Research-intensive Centers of Excellence in Taiwan (I-RiCE; MOST 105-2911-I-002-302); Ministry of Health and Welfare, China Medical University Hospital Cancer Research Center of Excellence (MOHW106-TDU-B-212-144003); The University of Texas MD Anderson Cancer Center Odyssey Fellowship Program (to Y.Y.); The National Research Foundation of Korea grant for the Global Core Research Center funded by the Korean government (MSIP 2011-0030001 to J.-H.C)
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Supplementary information accompanies for this paper at 10.1038/s41422-018-0060-4.
References
- 1.Quail DF, Joyce JA. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. J. Control. Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Zou W, Wolchok JD, Chen L. Sci. Transl. Med. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, et al. Nat. Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peinado H, et al. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrowski M, et al. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 7.Li J, et al. Nat. Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 8.Saunders PA, Hendrycks VR, Lidinsky WA, Woods ML. Eur. J. Immunol. 2005;35:3561–3569. doi: 10.1002/eji.200526347. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Garvin JE, Koretzky GA, Jordan MS. Annu Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau J, et al. Nat. Commun. 2017;8:14572. doi: 10.1038/ncomms14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodoraki, M. N., Yerneni, S., Hoffmann, T. K., Gooding, W. E. & Whiteside, T. L. Clinical cancer research 2018;24:896-905. [DOI] [PMC free article] [PubMed]
- 12.Bobrie A, et al. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 13.Curiel TJ, et al. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, et al. J. Clin. Invest. 2018;128:805–815. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.