Abstract
OBJECTIVE:
To evaluate whether early folic acid or multivitamin supplementation during pregnancy prevents diagnosis of hyperkinetic disorders (HKD), treatment for attention deficit hyperactivity disorder (ADHD) and ADHD-like behaviors reported by parents participating in the DNBC for children at age 7.
METHODS:
HKD diagnosis and ADHD medication use data was obtained from the Danish National Hospital, Central Psychiatric and Pharmaceutical registers. We estimated hazard ratios (HRs) for HKD diagnosis and ADHD medication use and risk ratios (RRs) for parent reported ADHD behavior collected with the Strength and Difficulties Questionnaire (SDQ), comparing children whose mothers took folic acid or multivitamin supplements early in pregnancy defined as starting peri-conceptionally (4-weeks prior to their last menstrual period (LMP)) through 8-weeks after their LMP (−4 to 8 weeks), to children whose mothers indicated no supplement use for the same entire period.
RESULTS:
We identified 384 children (1.1%) with a hospital diagnosis for HKD and 642 children (1.8%) treated with ADHD medication. We found no association between risk of HKD diagnosis or intake of ADHD medication and early maternal folic acid use. However, early multivitamin use was associated with an approximately 30% reduction in risk for HKD diagnosis (aHR: 0.70, 95% CI: 0.52–0.96) and 21% reduction in treatment with ADHD medication (aHR: 0.79, 95% CI: 0.62–0.98). We also observed a reduced risk in parent reported ADHD behaviors but these results were attenuated after adjustment.
CONCLUSION:
Our data suggest that multivitamin use in early pregnancy may reduce risk for HKD diagnosis and treatment for ADHD in the offspring.
Keywords: ADHD, protective factors, perinatal, nutrition
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD), characterized by inattention, hyperactivity, increased impulsivity and motivational/emotional dysregulation (1), is defined as one of the most common neurobehavioral disorders in children and has an estimated worldwide prevalence of 4–8% (2,3). Hyperkinetic disorder is often characterized as a particularly severe form of ADHD (4–6); however, these diagnoses are due to systems of disease classification that use somewhat different criteria. ADHD diagnostic criteria are derived from the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM-IV, 1994) (7), whereas HKD criteria are derived from the International Classification of Diseases (ICD-10) (8). While there is a substantial difference in the prevalence of ADHD and HKD in clinical samples(5), studies examining the predictive validity of the two main diagnostic schemata find large overlap in terms of important clinical characteristics (2,5,6). The etiology of ADHD/HKD is not well understood but both environmental and genetic factors are thought to contribute to disease onset (9,10). It has been argued that the rapid increase in ADHD/HKD observed over the past few decades (11,12) cannot solely be attributed to changes in diagnostic criteria (13) or parental awareness (4), necessitating a search for preventable causes.
The neuropathology of ADHD/HKD may already be present at birth or even before, thus exposures during pregnancy and/or infancy are of particular interest (14). Prenatal exposures that have been associated with ADHD/HKD include maternal diabetes (15), maternal smoking (3,16); maternal consumption of alcohol (17–19) or caffeine (20); and a modest contribution has been attributed to maternal psychological stress in pregnancy (21,22). Previous reports on folic acid supplementation starting a month before until two-months after conception suggested potential benefits in terms of behavioral outcomes associated with ADHD (23–25). Furthermore, there is growing theoretical and empirical evidence suggesting effective treatment of ADHD with broad spectrum micronutrients (26). Given the recent interest in research on early folic acid supplementation and neurodevelopmental deficits, we aim to assess whether dietary folic acid and multivitamin supplementation during a 12-week period, beginning 4-weeks prior from to the last menstrual period (LMP) through to 8-weeks after the LMP affects the diagnosis of HKD in the off-spring in the Danish National Birth Cohort (DNBC), treatment with ADHD medication, and/or parental reports of a child’s ADHD-like behaviors as documented in the Strength and Difficulties Questionnaire (SDQ) at age 7.
METHODS
The DNBC is a longitudinal population based cohort of pregnant women and their offspring with the overarching aim to study diseases in offspring from exposures operating in early life(27). Approximately 50% of all general practitioners in Denmark participated in the recruitment and 60% of invited women agreed to participate. Women were eligible if they spoke sufficient Danish and intended to carry their pregnancy to term. Women were recruited in early pregnancy during 1996–2002, interviewed twice during pregnancy and have been followed since (English versions of follow-up questionnaires can be found at:http://www.bsmb.dk) (27). This study was approved by the Institutional Review Board at the University of California, Los Angeles and the Danish Data Protection Agency.
Study Population
The DNBC participant recruitment form was revised and distributed on April 1, 2000 to newly allow for the ascertainment of supplement use in the 4-week preconception period. However, some participating general practitioners continued to use the original version of the recruitment form. Of the n=101,033 women recruited into the DNBC, n=48,184 (48%) women were recruited after April 2000. We excluded n=3,074 (6.4%) women with unsuccessful pregnancies, n=1,047 (2.2%) non-singleton births, n=22 (0.05%) pregnancies where mother’s emigrated, n=2 (0.004%) mothers who died and n=14 (0.03%) unknown birth outcomes. We also excluded n=8,966 (20.4%) women with missing values for weekly supplement use (for n=8,388 (93.6%) of these women, the original version of the recruitment form had been used for data collection while n=578 (6.4%) women completing the revised form had missing values for weekly use) and n=11,935 (34.0%) women who did not complete the 7-year follow-up. Derivation of the study sample is shown in Figure-1.
Figure 1.
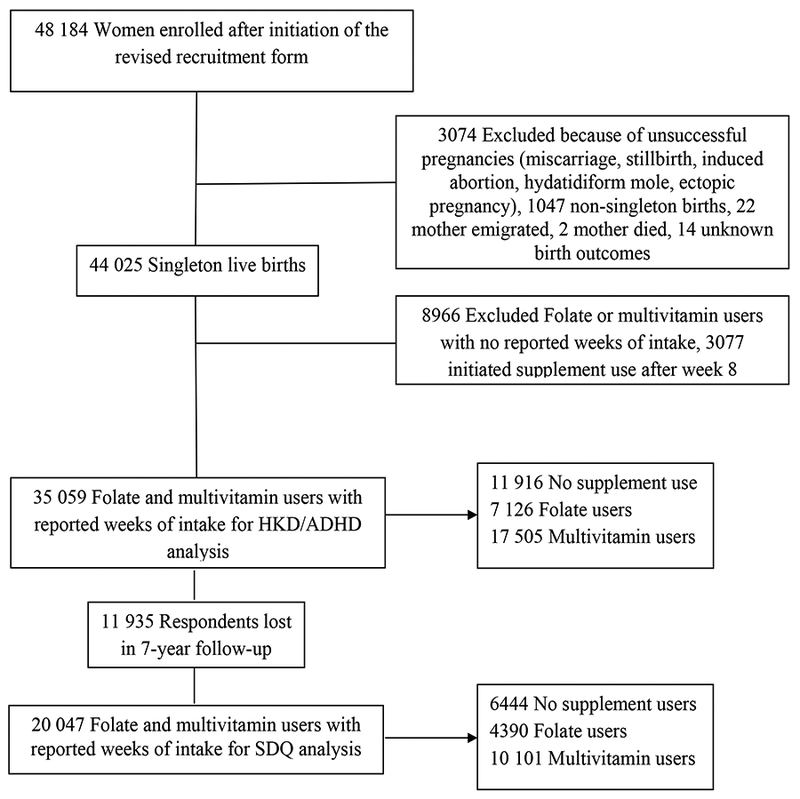
Flow chart of study population selection.
Supplement Use in Early Pregnancy
The Danish National Birth Cohort recruitment form was completed on average around 11.5 ± 3.9 weeks of gestation. Most folic acid supplements consumed by women in the cohort contained at least 400μg of folic acid. Contents of the most commonly used multivitamin are listed in E-Table 1. We considered women exposed if they indicated multivitamin or folic acid use beginning 4-weeks prior to their LMP through 8-weeks after their LMP (−4 to 8 weeks); specifically if they indicated supplement use in at least 10 of the 12 weeks. Women who indicated no supplement use for the same entire period were used as the unexposed group. There were n=3,077 (8.8%) women who initiated supplement use after the first 8 weeks of gestation who were included in the unexposed group.
TABLE 1:
Pregnancy characteristics of women enrolled in the DNBC who completed a revised recruitment form by prenatal intake of folate and multivitamins (−4 to 8 weeks), at baseline and 7-year Follow-up * ¥
| Non Supplement Users |
All Women | 7yr Follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Folic Acid | Multivitamin | Folic Acid | Multivitamin | |||||||
| 11916 | % | 7126 | % | 17 505 | % | 4390 | % | 10101 | % | |
| Maternal age (years) | ||||||||||
| ≤ 24 | 1606 | 13.5 | 407 | 5.7 | 1550 | 8.9 | 231 | 5.3 | 791 | 7.8 |
| 25–29 | 4304 | 36.2 | 2690 | 37.8 | 7112 | 40.7 | 1665 | 37.9 | 4124 | 40.8 |
| 30–34 | 4046 | 34.0 | 2807 | 39.5 | 6225 | 35.6 | 1732 | 39.5 | 3635 | 36.0 |
| ≥ 35 | 1940 | 16.3 | 1205 | 17.0 | 2605 | 14.9 | 762 | 17.4 | 1551 | 15.4 |
| Paternal age (years) | ||||||||||
| ≤ 24 | 701 | 6.1 | 161 | 2.3 | 650 | 3.8 | 86 | 2.0 | 356 | 3.6 |
| 25–29 | 3085 | 26.8 | 1801 | 26.0 | 4739 | 27.7 | 1124 | 26.2 | 2723 | 27.6 |
| 30–34 | 4215 | 36.7 | 2799 | 40.4 | 6661 | 39.0 | 1754 | 40.9 | 3844 | 38.9 |
| 35–39 | 2458 | 21.4 | 1596 | 23.0 | 3653 | 21.4 | 961 | 22.4 | 2116 | 21.4 |
| 40–44 | 790 | 6.9 | 452 | 6.5 | 1043 | 6.1 | 284 | 6.6 | 635 | 6.4 |
| ≥ 45 | 253 | 2.2 | 125 | 1.8 | 337 | 2.0 | 77 | 1.8 | 204 | 2.1 |
| Parity | ||||||||||
| 0 | 6571 | 56.4 | 3239 | 46.2 | 8299 | 48.3 | 1954 | 45.1 | 4717 | 47.5 |
| >1 | 5090 | 43.7 | 3766 | 53.8 | 8892 | 51.7 | 2380 | 54.9 | 5210 | 52.5 |
| Maternal pre-pregnancy RMI | ||||||||||
| <18.5 | 459 | 4.3 | 288 | 4.3 | 716 | 4.4 | 168 | 4.0 | 420 | 4.4 |
| 18.5–25 | 6827 | 63.5 | 4576 | 68.8 | 10932 | 67.4 | 2947 | 70.7 | 6635 | 69.8 |
| ≥26 | 3465 | 32.2 | 1788 | 26.9 | 4577 | 28.2 | 1051 | 25.2 | 2452 | 25.8 |
| Maternal smoking | ||||||||||
| Never | 7903 | 68.0 | 5684 | 80.7 | 13133 | 76.0 | 3595 | 82.0 | 7867 | 78.1 |
| ≤ 9 cigarettes/day | 1620 | 13.9 | 738 | 10.5 | 2176 | 12.6 | 434 | 9.9 | 1236 | 12.3 |
| > 9 cigarettes/day | 2102 | 18.1 | 622 | 8.8 | 1962 | 11.4 | 353 | 8.1 | 972 | 9.7 |
| Maternal alcohol consumption | ||||||||||
| Never | 3951 | 34.0 | 2248 | 31.9 | 5870 | 34.0 | 1343 | 30.7 | 3293 | 32.7 |
| 0–4 glasses per week | 3958 | 34.1 | 2907 | 41.3 | 6596 | 38.2 | 1842 | 42.0 | 3905 | 38.8 |
| more than 4 glasses per week | 3716 | 32.0 | 1889 | 26.8 | 4805 | 27.8 | 1197 | 27.3 | 2876 | 28.6 |
| History of Mental Health Illness§ | ||||||||||
| Yes | 287 | 2.4 | 137 | 1.9 | 389 | 2.2 | 66 | 1.5 | 171 | 1.7 |
| No | 11629 | 97.6 | 6989 | 98.1 | 17116 | 97.8 | 4324 | 98.5 | 9930 | 98.3 |
| Offspring sex¶§ | ||||||||||
| Female | 5843 | 49.1 | 3468 | 48.8 | 8498 | 48.6 | 2137 | 48.7 | 4901 | 48.5 |
| Male | 6053 | 50.9 | 3641 | 51.2 | 8994 | 51.4 | 2253 | 51.3 | 5200 | 51.5 |
| Birth weight (grams) | ||||||||||
| <2500 | 483 | 4.1 | 220 | 3.1 | 593 | 3.4 | 128 | 2.9 | 281 | 2.8 |
| 2500–4500 | 10917 | 91.8 | 6576 | 92.5 | 16132 | 92.2 | 4070 | 92.7 | 9385 | 92.9 |
| >4500 | 496 | 4.2 | 313 | 4.4 | 767 | 4.4 | 192 | 4.4 | 435 | 4.3 |
| Gestational age (weeks)¶ | ||||||||||
| <37 | 587 | 4.9 | 353 | 5.0 | 846 | 4.8 | 221 | 5.0 | 463 | 4.6 |
| 37–42 | 10186 | 85.8 | 6153 | 86.7 | 15200 | 87.0 | 3800 | 86.7 | 8815 | 87.3 |
| > 42 | 1102 | 9.3 | 593 | 8.4 | 1428 | 8.2 | 364 | 8.3 | 815 | 8.1 |
| Apgar Score¶§ | ||||||||||
| Less than 10 | 900 | 7.6 | 566 | 8.0 | 1364 | 7.9 | 338 | 7.7 | 747 | 7.5 |
| Score of 10 | 10924 | 92.3 | 6500 | 92.0 | 16032 | 92.1 | 4028 | 92.3 | 9310 | 92.5 |
| Season when Pregnancy Started | ||||||||||
| Fall | 2422 | 20.4 | 1670 | 23.5 | 4290 | 24.6 | 1041 | 23.7 | 2561 | 25.4 |
| Winter | 2969 | 25.0 | 1833 | 25.8 | 4337 | 24.8 | 1137 | 25.9 | 2542 | 25.2 |
| Spring | 3674 | 30.9 | 1878 | 26.4 | 4531 | 25.9 | 1166 | 26.6 | 2571 | 25.5 |
| Summer | 2810 | 23.7 | 1718 | 24.2 | 4316 | 24.7 | 1041 | 23.7 | 2419 | 24.0 |
| Household Socio-economic status | ||||||||||
| Higher-grade professionals | 2084 | 19.0 | 1977 | 29.3 | 3963 | 24.1 | 1257 | 29.9 | 2420 | 25.1 |
| Middle-grade professionals | 2953 | 26.9 | 2359 | 35.0 | 5485 | 33.3 | 1554 | 37.0 | 3367 | 34.9 |
| Skilled work | 3567 | 32.5 | 1543 | 22.9 | 4407 | 26.8 | 917 | 21.8 | 2506 | 26.0 |
| Unskilled work | 1958 | 17.8 | 652 | 9.7 | 2091 | 12.7 | 355 | 8.4 | 1079 | 11.2 |
| Student | 261 | 2.4 | 171 | 2.5 | 362 | 2.2 | 100 | 2.4 | 200 | 2.1 |
| Unemployed > 1year | 134 | 1.2 | 26 | 0.4 | 122 | 0.7 | 15 | 0.4 | 48 | 0.5 |
| Unclassified | 33 | 0.3 | 13 | 0.2 | 33 | 0.2 | 8 | 0.2 | 20 | 0.2 |
Missing values: 46 maternal age, 984 paternal age, 592 smoking, 592 alcohol consumption, 658 parity, 2820 pre-pregnancy BMI, 46 sex, 46 birth weight, 92 gestational age, 92 season when pregnancy started, 227 APGAR, 2273 household socio-economic status
P-values comparing folate and multivitamin supplement users to non-users are <0.05 unless otherwise specified
Chi-squared p-value comparing folate supplement users (−4 to 8 weeks) to non-users >0.05
Chi-squared p-value comparing multivitamin users (−4 to 8 weeks) to non-users >0.05
HKD Diagnosis and treatment with ADHD Medication
Information on HKD diagnosis was obtained from the National Hospital Register and Danish Psychiatric Central Register using ICD-10; (F90.0-F90.9) for either a primary or secondary diagnosis category (28); 97.5% of HKD cases received a primary diagnosis and mainly during outpatient visits (96%). The Danish Psychiatric Central Research Register contains data on psychiatric admissions to psychiatric hospitals and wards in Denmark, the Faroe Islands and Greenland. Since 1995, these data also include psychiatric outpatients (29). Children suspected of having a HKD are referred by general practitioners or school psychologists to a child psychiatric ward, where they are evaluated by a multidisciplinary team and assigned a final diagnosis by a child psychiatrist. All HKD cases are registered in the Psychiatric Register once a formal diagnosis has been established, without regard for treatment or educational provisions.
HKD diagnosis was ascertained for all children at or after their fifth birthday. If children received diagnoses solely prior to the age of 5 (n = 26) but not afterwards, they were not considered an HKD case owing to higher diagnostic uncertainty at younger ages. Prescription data is available from the Danish Prescription Registry (30), which receives data on dispensed prescriptions including drug Anatomical Therapeutic Chemical Classification (ATC) codes and dispensing date from all pharmacies in Denmark since January 1995. Children who had filled 2 or more prescriptions for either methylphenidate/Ritalin (ATC: N06BA04), atomoxetine (ATC: N06BA09), or modafinil (ATC: N06BA07) were classified as having been prescribed and taken ADHD medications.
Parent Reports of Social and Behavioral Development at Age −7
The Strength and Difficulties Questionnaire (SDQ) is a screening tool that was designed to assess five areas of social-behavioral development that consist of emotional symptoms, conduct problems, hyperactivity, peer relationship and pro-social behavior in children and adolescents ages 4 to 16 years (31). The SDQ has been shown to be a reliable screening instrument for emotional and behavioral problems in school-age children (32,33). At the 7-year follow-up all DNBC participants were asked 25 questions to assess their child’s ADHD-like behaviors using the standardized Strength and Difficulties Questionnaire (SDQ). The interview was conducted after the child’s 7th birthday with either the mother or the child’s primary caregiver and the questions ascertained behaviors observed in the previous 6 months. We followed the official recommendations for scoring the SDQ (Youth in mind 2009 http://www.sdqinfo.com), and created a “total difficulties score” (range 0–40) by summing over four subscales (emotional symptoms, conduct problems, hyperactivity, and peer problems) that range from 0–10 each, with higher scores indicating an increasing number of behavioral problems with the exception of the pro-social behavior subscale (range 0–10) for which higher scores indicate positive social behaviors.
Statistical Analysis
Covariate data on smoking and alcohol consumption during pregnancy, maternal pre-pregnancy BMI, maternal mental-health status (indicated by mother’s positive self-report of psychiatric illnesses, such as having been seen by a physician or psychologist due to depression, anxiety, childhood psychiatric disorders, family problems/life crisis, or other mental health problems, before or during pregnancy), and socioeconomic status was captured in the DNBC participant interviews (27). Information on gestational age at birth and birth weight were obtained from the Danish Medical Birth Registry (35). We used Cox regression to estimate crude and adjusted hazard ratios for (cHR/aHR) for HKD diagnosis and ADHD medication use. Person-time follow-up started at the child’s fifth birthday and ended at the time of HKD diagnosis from hospital records or the date ADHD prescription medication was dispensed (i.e., at the time of receiving the first medication if ≥ 2 prescriptions were filled in total), death, emigration, or end of follow-up, whichever came first. Follow-up ended on the last date of each respective record linkage (i.e., for HKD diagnoses on August 1, 2011, and for ADHD medications on December 31, 2011). For the SDQ analyses, we used generalized linear models with a log-link function and a Poisson distribution to estimate risk ratios and 95% confidence intervals for prenatal maternal folic acid supplementation, HKD and ADHD-like behaviors – as measured with the SDQ (31). Following the advice of Goodman we dichotomized SDQ scores using a cut-off point that results in high specificity for ADHD-like behaviors (“total difficulties scores” ≥ 17) (31,32,34).
We evaluated all demographic variables in Table-1 for possible confounding but included only those that yielded a greater than 5% difference in the final effect estimates or have been cited as confounders in prior research. Adjusted models controlled for maternal age (continuously); household socio-economic status (low {unskilled work, student, unemployed >1 year, unclassified}, medium {middle grade professionals and skilled work}, high {higher grade professional}); maternal smoking (never, ever); and alcohol consumption during pregnancy (never, 0–4 glasses per week, more than 4 glasses per week), maternal pre-pregnancy body mass index (0-<18.5, 18.5-<25, ≥26) birth year (2001, 2002, 2003) and offspring sex (male, female), reference categories have been italicized. Missing values for covariates were imputed using multiple imputation techniques (PROC MI and PROC MIANALYZE in SAS version 9.2), which consists of generating five simulated complete data sets and using standard analytical procedures proposed for combining the complete data sets and generating final estimates (36).
RESULTS
In our study population, we observed lower paternal and maternal age among women who reported no supplement intake compared to women reporting folic acid and multivitamin supplementation. Zero parity, maternal pre-pregnancy BMI greater than 26, positive maternal self-report of smoking and alcohol consumption were also associated with no supplement use. Furthermore, women who resided in households with higher and middle-grade professionals were more likely to report taking supplements. Women in the 7-year cohort had similar demographic characteristics compared to all women at baseline. Among women reporting multivitamin use, women in the 7-year cohort tended to smoke less and come from households with higher-grade and middle-grade professionals. Among women reporting folic acid use, women in the 7-year cohort tended to have a pre-pregnancy BMI within a healthy range (18.5–25), and report less smoking and alcohol consumption during their pregnancy. There were also more women in the 7-yr cohort from households with middle-grade professionals reporting folic acid use, see Table-1.
In our study population, we found no evidence for an association between early folic acid supplementation and risk of HKD diagnosis or prescription of ADHD medication. This lack of association persisted even after including women into the exposed group who had indicated folic acid supplementation for only 6 (instead of 10) weeks out of the 12 week period. However, even after controlling for potential confounders early multivitamin use in pregnancy was associated with an approximately 30% reduction in risk for HKD diagnosis (aHR: 0.70, 95% CI: 0.52 – 0.96) and 21% reduced risk for ADHD medication prescriptions (aHR: 0.79, 95% CI: 0.62–0.98), potential confounders consisted of maternal age, household socio-economic status, maternal smoking and alcohol consumption during pregnancy, maternal pre-pregnancy body mass index, birth year and offspring sex, see Table-2 Upon stratification by season of conception, we observed a strong reduction in risk for HKD diagnosis in offspring of women who had conceived their children in the fall and reported early multivitamin intake (aHR: 0.47, 95% CI: 0.26–0.84), however this finding relies on a small sample size. We observed a reduced risk in some parent reported ADHD behaviors for children born to women who reported both early folic acid and multivitamin use, including conduct problems, hyperactivity, peer problems, and total SDQ difficulties. However, most risk estimates based on the SDQ became null once confounders were included in the model with the exception of maternal report of early folic acid intake and parent reported hyperactivity (aRR: 0.62, 95% CI: 0.47–0.84), see Table-3. We also found folic acid intake to be associated with emotional problems in children ascertained in the seven year follow up (aRR: 1.46, 95% CI: 1.21–1.75), but find it likely that this finding is spurious since it’s inconsistent with other results.
Table 2.
: Hazard Ratios (HR) for the Association Between Hospital diagnosed Hyperkinetic Disorders (HKD), Attention Deficit Hyperactivity Disorder (ADHD) Medication and Early Folic Acid and Multivitamin Supplementation (from −4 weeks to 8 weeks of gestation), in the DNBC ¥
| Exp. Cases/Total Exp. | Unexp. cases/Total Unexp. | cHR | 95% Confidence |
aHR* | 95% Confidence |
|||
|---|---|---|---|---|---|---|---|---|
| Folic acid users | ||||||||
| HKD Diagnosis | 20/2661 | 164/11916 | 0.59 | 0.37 | 0.95 | 0.87 | 0.54 | 1.41 |
| ADHD Medication | 39/2661 | 268/11916 | 0.70 | 0.50 | 0.97 | 0.96 | 0.68 | 1.37 |
| Multivitamin users | ||||||||
| HKD Diagnosis | 55/7901 | 164/11916 | 0.53 | 0.39 | 0.73 | 0.70 | 0.52 | 0.96 |
| ADHD Medication | 107/7901 | 268/11916 | 0.63 | 0.50 | 0.79 | 0.78 | 0.62 | 0.98 |
Consumed supplements in at least 10 of the 12 weeks, some folic acid users may have used multivitamins, multivitamin category excludes folic acid users
Adjusted for maternal age (continuously); household socio-economic status (low, medium, high); maternal smoking (never, ever); and alcohol consumption during pregnancy (never, 0–4 glasses per week, more than 4 glasses per week), maternal pre-pregnancy body mass index (0-<18.5, 18.5-<25, ≥26) birth year (2001, 2002, 2003), offspring sex (male, female) reference categories have been italicized.
Table 3:
Risk Ratios (RR) for the Association Between Attention Deficit Hyperactivity Disorder-Like Behavioral Problems at Age 7 and Maternal Intake of Folate and Multivitamin Supplements during Early Gestation and in the DNBC (N=20 247) ǂ *
| Exp. Cases/Total Exp. | Unexp. cases/Total Unexp. | cRR | 95% Confidence Interval |
aRR* | 95% Confidence Interval |
|||
|---|---|---|---|---|---|---|---|---|
| Folic acid supplement usersǂ | ||||||||
| Weeks −4 to 8 | ||||||||
| Emotional symptoms (score ≥ 5) | 159/1711 | 499/6444 | 1.20 | 1.00 | 1.43 | 1.46 | 1.21 | 1.75 |
| Conduct problems (score ≥ 4) | 72/1711 | 388/6444 | 0.70 | 0.54 | 0.90 | 0.87 | 0.68 | 1.13 |
| Hyperactivity (score ≥ 7) | 53/1711 | 399/6444 | 0.50 | 0.38 | 0.67 | 0.62 | 0.47 | 0.84 |
| Peer problems (score ≥ 4) | 58/1711 | 327/6444 | 0.67 | 0.51 | 0.88 | 0.84 | 0.63 | 1.12 |
| Prosocial behavior (score ≤ 6) | 27/1711 | 145/6444 | 0.70 | 0.47 | 1.06 | 0.75 | 0.49 | 1.15 |
| SDQ Total Difficulties (score ≥ 17) | 37/1711 | 255/6444 | 0.55 | 0.39 | 0.77 | 0.79 | 0.56 | 1.13 |
| Multivitamin users | ||||||||
| Weeks −4 to 8 | ||||||||
| Emotional symptoms (score ≥ 5) | 379/4935 | 499/6444 | 0.99 | 0.87 | 1.13 | 1.17 | 1.02 | 1.34 |
| Conduct problems (score ≥ 4) | 222/4935 | 388/6444 | 0.75 | 0.63 | 0.88 | 0.89 | 0.75 | 1.06 |
| Hyperactivity (score ≥ 7) | 231/4935 | 399/6444 | 0.76 | 0.64 | 0.89 | 0.90 | 0.76 | 1.06 |
| Peer problems (score ≥ 4) | 179/4935 | 327/6444 | 0.71 | 0.60 | 0.86 | 0.84 | 0.70 | 1.01 |
| Prosocial behavior (score ≤ 6) | 93/4935 | 145/6444 | 0.84 | 0.65 | 1.09 | 0.86 | 0.66 | 1.13 |
| SDQ Total Difficulties (score ≥ 17) | 125/4935 | 255/6444 | 0.64 | 0.52 | 0.79 | 0.84 | 0.67 | 1.05 |
Consumed supplements in at least 10 of the 12 weeks, some folic acid users may have used multivitamins, multivitamin category excludes folic acid users
Adjusted for maternal age (continuously); household socio-economic status (low, medium, high); maternal smoking (never, ever); and alcohol consumption during pregnancy (never, 0–4 glasses per week, more than 4 glasses per week), maternal pre-pregnancy body mass index (0-<18.5, 18.5-<25, ≥26) birth year (2001, 2002, 2003), offspring sex (male, female) reference categories have been italicized.
DISCUSSION
We observed a reduction in risk for receiving a HKD diagnosis and being prescribed ADHD medication among offspring whose mothers consumed multivitamins in the −4 to 8 week pregnancy period. We did not find support for an inverse association between early folic acid supplementation and HKD diagnosis or ADHD medications in children except for parent reported hyperactivity in the SDQ at age 7. We had, however, low power to examine this association, and the estimated protective effects size is small. Previous studies have reported increased childhood hyperactivity, peer (23), behavioral (24) and emotional problems (25) in offspring whose mothers had low maternal folate status in pregnancy. We do not know which single vitamin or combination of vitamins contributed most to our estimates but since our findings suggested seasonal variation we suspect vitamin D may play a role. Recently, higher levels of maternal plasma concentrations of 25-hydroxyvitamin D3 in pregnancy have been shown to be associated with lower risk of ADHD symptoms in childhood (37). Though it has also been suggested that inconsistent results from a single micronutrient could be due to an imbalance in other nutrients, and that a combination or broader spectrum of micronutrients is required for optimal brain function and development (26). To the best of our knowledge this is the first study to assess the association between early maternal multivitamin intake and report an inverse association with HKD diagnosis and prescription use of ADHD medication.
This study has a number of strengths, first the DNBC has collected detailed data in early pregnancy concerning maternal health status, health behaviors, nutritional and supplement intake as well as occupational exposures – all of which can be examined as potential confounders. Second, the DNBC is a large cohort, which is necessary since the outcome of interest is not very common. Third, members of the DNBC have aged beyond the age of typical HKD diagnosis allowing us to evaluate childhood onset of these outcomes at the present time. And fourth, the SDQ measures have previously been validated for the Danish population and the internal reliability of the original five factor structure and its usefulness as a screening tool to assess emotional and behavioral problems in children has been confirmed (38). A recent study published results for the Danish SDQ among 71,840 parent and teacher raters of 5, 7, 10 and 12 year old children, utilizing 4 large Danish cohorts - one of which was the DNBC - and confirming internal reliability of the five factor structure and its usefulness as a screening tool (38). Some important limitations of the DNBC should be acknowledged. First, the DNBC is a homogenous, predominantly Caucasian population and does not allow for ethnicity based comparisons. Second, women in the DNBC represent a relatively affluent and well educated group of women with full access to health care, conditions that may differ from other populations. Third, there is significant loss of follow up at seven years (34%) and this could affect the results we have presented on the parent reported SDQ behaviors. Fourth, there is a possibility of residual confounding for family history of HKD disease since we have only included maternal self reports and not sibling, paternal or grandparent reports of mental illness.
Previously, the SDQ has been employed to evaluate the effects of maternal prenatal smoking, drinking and acetaminophen use on children’s ADHD-like behaviors (39–42). To the best of our knowledge, this is the first report of maternal supplement use and reported behaviors in the SDQ. It is possible that maternal self-reports of supplement use is associated with reporting behavioral or emotional problems in children; this may be one explanation for the lack of association we observe between multivitamin use and problems reported in the SDQ, and also the observed association between emotional symptoms and supplement use. However, it is unlikely that the observed association between maternal multivitamin use, HKD diagnosis, and ADHD medication use is influenced by diagnostic bias since these outcomes depend on contact, evaluation and approval by a medical professional.
In conclusion, early maternal multivitamin supplementation during −4 to 8 weeks of gestation may reduce the risk of HKD diagnosis and prescription of ADHD medication in offspring. However, folic acid supplementation during this same early period does not appear to reduce risk of HKD diagnosis, ADHD medication or parental assessments of social and behavioral problems (with the exception of hyperactivity) in this population, thus we are unable to corroborate previous reports. There is some evidence to suggest that ADHD can effectively be treated with broad spectrum micronutrients, it is possible that early prevention of this disease can be prevented with prenatal use of the same treatment. Replication of these findings and additional research that assesses individual micronutrients may provide additional insights.
Supplementary Material
Footnotes
The authors have no funding, conflicts of interest or acknowledgements to declare.
Contributor Information
Zeyan Liew, Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA.
Jørn Olsen, Department of Public Health, Institute of Epidemiology and Social Medicine, Aarhus University, Aarhus, Denmark.
Ellen A Nohr, Department of Obstetrics and Gynecology, Odense University Hospital, Odense, Denmark.
Beate Ritz, Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA.
REFERENCES
- 1.Swanson J, Sergeant J, Taylor E, Sonuga-Barke E, Jensen P, Cantwell D. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet [Internet]. 1998. February [cited 2013 Sep 10];351(9100):429–33. Available from: 10.1016/S0140-6736(97)11450-7 [DOI] [PubMed] [Google Scholar]
- 2.Polanczyk G, Rohde L. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry [Internet]. 2007. [cited 2013 Sep 10]; Available from: http://journals.lww.com/co-psychiatry/Abstract/2007/07000/Epidemiology_of_attention_deficit_hyperactivity.13.aspx [DOI] [PubMed] [Google Scholar]
- 3.Spencer T, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. Ambul Pediatr [Internet]. 2007. [cited 2013 Sep 10]; Available from: http://www.sciencedirect.com/science/article/pii/S153015670600181X [DOI] [PubMed] [Google Scholar]
- 4.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World psychiatry Off J World Psychiatr Assoc WPA [Internet]. Masson; 2003;2(2):104–13. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1525089&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SI, Schachar RJ, Chen SX, Ornstein TJ, Charach A, Barr C, et al. Predictive validity of DSM-IV and ICD-10 criteria for ADHD and hyperkinetic disorder. J Child Psychol Psychiatry Allied Discip [Internet]. 2008;49(1):70–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17979965 [DOI] [PubMed] [Google Scholar]
- 6.Lahey BB, Pelham WE, Chronis A, Massetti G, Kipp H, Ehrhardt A, et al. Predictive validity of ICD-10 hyperkinetic disorder relative to DSM-IV attention-deficit/hyperactivity disorder among younger children. J Child Psychol Psychiatry Allied Discip. 2006;47(5):472–9. [DOI] [PubMed] [Google Scholar]
- 7.Diagnostic and statistical manual of mental disorders (4th ed.)American Psychiatric Association, Washington: (1994). 1994. [Google Scholar]
- 8.The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines 1992; diagnostic criteria for research 1993.
- 9.Halmøy A, Klungsøyr K, Skjærven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. Elsevier Inc; 2012;71(5):474–81. [DOI] [PubMed] [Google Scholar]
- 10.Millichap JG. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics [Internet]. Am Acad Pediatrics; 2008;121(2):e358–65. Available from: http://pediatrics.aappublications.org/cgi/content/abstract/121/2/e358 [DOI] [PubMed] [Google Scholar]
- 11.Pastor PN, Reuben CA. Diagnosed Attention Deficit Hyperactivity Disorder and Learning Disability: United States, 2004–2006. Data from the National Health Interview Survey Vital and Health Statistics. Series 10, Number 237 Centers Dis Control Prev [Internet]. Centers for Disease Control and Prevention. 1600 Clifton Road, Atlanta, GA: 30333. Tel: 800–311-3435; Tel: 404–639-3311; Web site: http://www.cdc.gov; 2008. June 30 [cited 2013 Sep 12]; Available from: http://eric.ed.gov/?id=ED502147 [PubMed] [Google Scholar]
- 12.Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH, Henriksen TB. Gestational age, birth weight, and the risk of hyperkinetic disorder. Arch Dis Child [Internet]. 2006. August 1 [cited 2013 Sep 25];91(8):655–60. Available from: http://adc.bmj.com/content/91/8/655.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Møller LR, Sørensen MJ, Thomsen PH. ICD-10 classification in Danish child and adolescent psychiatry--have diagnoses changed after the introduction of ICD-10? Nord J Psychiatry [Internet]. 2007. January [cited 2013 Sep 12];61(1):71–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17365792 [DOI] [PubMed] [Google Scholar]
- 14.Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry [Internet]. 2008. November [cited 2013 Sep 12];47(11):1233–51. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2759682&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab [Internet]. 2001. January [cited 2013 Sep 4];14 Suppl 1:681–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11393563 [DOI] [PubMed] [Google Scholar]
- 16.Eskenazi B, Trupin L. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. II. Effects on neurodevelopment at age 5. Am J Epidemiol [Internet]. 1995. [cited 2013 Sep 16]; Available from: http://aje.oxfordjournals.org/content/142/Supplement_9/S19.short [DOI] [PubMed] [Google Scholar]
- 17.Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr [Internet]. 2001. August [cited 2013 Sep 16];22(4):217–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11530894 [DOI] [PubMed] [Google Scholar]
- 18.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry [Internet]. RCP; 2002;180(JUNE):502–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12042228 [DOI] [PubMed] [Google Scholar]
- 19.Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol [Internet]. 2000. September [cited 2013 Sep 16];61(5):661–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11022804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnet KM. Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorder and Associated Behaviors: Review of the Current Evidence. Am J Psychiatry [Internet]. American Psychiatric Association; 2003. June 1 [cited 2013 Sep 16];160(6):1028–40. Available from: http://journals.psychiatryonline.org/article.aspx?volume=160&page=1028 [DOI] [PubMed] [Google Scholar]
- 21.McIntosh DE, Mulkins RS, Dean RS. Utilization of maternal perinatal risk indicators in the differential diagnosis of ADHD and UADD children. Int J Neurosci [Internet]. 1995. March [cited 2013 Sep 16];81(1–2):35–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7775071 [DOI] [PubMed] [Google Scholar]
- 22.Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry [Internet]. 2010. October [cited 2013 Apr 3];19(10):747–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20495989 [DOI] [PubMed] [Google Scholar]
- 23.Schlotz W, Jones A, Phillips DIW, Gale CR, Robinson SM, Godfrey KM. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry [Internet]. 2010. May [cited 2013 Mar 15];51(5):594–602. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2862762&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roza SJ, van Batenburg-Eddes T, Steegers EAP, Jaddoe VWV, Mackenbach JP, Hofman A, et al. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br J Nutr [Internet]. 2010. February [cited 2013 Mar 15];103(3):445–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19772683 [DOI] [PubMed] [Google Scholar]
- 25.Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, et al. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr [Internet]. 2012. June 1 [cited 2014 May 13];95(6):1413–21. Available from: http://ajcn.nutrition.org/content/95/6/1413.short [DOI] [PubMed] [Google Scholar]
- 26.Rucklidge JJ, Kaplan BJ. Broad-Spectrum Micronutrient Treatment for Attention-Deficit/Hyperactivity Disorder: Rationale and Evidence to Date. CNS Drugs [Internet]. Springer International Publishing; 2014. September 24 [cited 2016 Oct 10];28(9):775–85. Available from: http://link.springer.com/10.1007/s40263-014-0190-2 [DOI] [PubMed] [Google Scholar]
- 27.Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health [Internet]. 2001. December [cited 2013 Mar 15];29(4):300–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11775787 [DOI] [PubMed] [Google Scholar]
- 28.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull [Internet]. 1999;46(3):263–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10421985 [PubMed] [Google Scholar]
- 29.Munk-Jørgensen P, Mortensen PB. The Danish Psychiatric Central Register. Dan Med Bull [Internet]. 1997. February [cited 2013 Sep 23];44(1):82–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9062767 [PubMed] [Google Scholar]
- 30.Gaist D, Sørensen HT, Hallas J. The Danish prescription registries. Dan Med Bull [Internet]. 1997. September [cited 2015 Jun 28];44(4):445–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9377907 [PubMed] [Google Scholar]
- 31.Goodman R The Strengths and Difficulties Questionnaire: A Research Note. J Child Psychol Psychiatry [Internet]. 1997. July [cited 2013 Aug 7];38(5):581–6. Available from: http://doi.wiley.com/10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- 32.Goodman A, Goodman R. Strengths and Difficulties Questionnaire scores and mental health in looked after children. Br J psychiatry [Internet]. 2012;200(5):426–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22550331 [DOI] [PubMed] [Google Scholar]
- 33.Koskelainen M, Sourander A, Kaljonen A. The Strengths and Difficulties Questionnaire among Finnish school-aged children and adolescents. Eur child Adolesc psychiatry [Internet]. 2000;9(4):277–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11202103 [DOI] [PubMed] [Google Scholar]
- 34.GOODMAN R Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry [Internet]. 2000. December 1 [cited 2013 Sep 16];177(6):534–9. Available from: http://bjp.rcpsych.org/content/177/6/534.full [DOI] [PubMed] [Google Scholar]
- 35.Knudsen LB. The Danish Fertility Database. Dan Med Bull. 1998;45(2):221–5. [PubMed] [Google Scholar]
- 36.Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development. Rockville, MD: SAS Institute Inc; 2001. [Google Scholar]
- 37.Morales E, Julvez J, Torrent M, Ballester F, Rodríguez-Bernal CL, Andiarena A, et al. Vitamin D in Pregnancy and Attention Deficit Hyperactivity Disorder-like Symptoms in Childhood. Epidemiology [Internet]. 2015. July [cited 2015 Oct 30];26(4):458–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25867115 [DOI] [PubMed] [Google Scholar]
- 38.Niclasen J, Teasdale TW, Andersen A-MN, Skovgaard AM, Elberling H, Obel C. Psychometric properties of the Danish Strength and Difficulties Questionnaire: the SDQ assessed for more than 70,000 raters in four different cohorts. Scott JG, editor. PLoS One [Internet]. Public Library of Science; 2012. January [cited 2014 May 29];7(2):e32025 Available from: http://dx.plos.org/10.1371/journal.pone.0032025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly YJ, Sacker A, Gray R, Kelly J, Wolke D, Head J, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Heal [Internet]. 2010;66(1):1–8. Available from: http://jech.bmj.com/cgi/doi/10.1136/jech.2009.103002 [DOI] [PubMed] [Google Scholar]
- 40.Obel C, Linnet KM, Henriksen TB, Rodriguez A, Järvelin MR, Kotimaa A, et al. Smoking during pregnancy and hyperactivity-inattention in the offspring--comparing results from three Nordic cohorts. Int J Epidemiol [Internet]. 2009. June 1 [cited 2013 Aug 11];38(3):698–705. Available from: http://ije.oxfordjournals.org/content/38/3/698.short [DOI] [PubMed] [Google Scholar]
- 41.Sayal K, Heron J, Golding J, Emond A. Prenatal alcohol exposure and gender differences in childhood mental health problems: a longitudinal population-based study. Pediatrics [Internet]. 2007. February 1 [cited 2013 Sep 16];119(2):e426–34. Available from: http://pediatrics.aappublications.org/content/119/2/e426.short [DOI] [PubMed] [Google Scholar]
- 42.Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr [Internet]. American Medical Association; 2014. April 1 [cited 2014 Jun 4];168(4):313–20. Available from: http://archpedi.jamanetwork.com/article.aspx?articleid=1833486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


