Abstract
Purpose
Severe sepsis is associated with functional disability among patients surviving an acute phase of infection. Efforts to improve functional impairment are important. We assessed the effects of early exercise rehabilitation on functional outcomes in patients with severe sepsis.
Materials and Methods
A prospective, single-center, case-control study was conducted between January 2013 and May 2014 at a tertiary care center in Korea. Patients with severe sepsis and septic shock were enrolled and randomized to receive standard sepsis treatment or intervention. Intervention involved early targeted physical rehabilitation with sepsis treatment during hospitalization. Participants were assessed at enrollment, hospital discharge, and 6 months after enrollment. Functional recovery was measured using the Modified Barthel Index (MBI), Functional Independence Measure (FIM), and Instrumental Activities of Daily Living (IADL).
Results
Forty participants (21 intervention patients) were included in an intention-to-treat analysis. There were no significant differences in baseline MBI, FIM, and IADL between groups. Intervention yielded greater improvement of MBI, FIM, and IADL in the intervention group at hospital discharge, but not significantly. Subgroup analysis of patients with APACHE II scores ≥10 showed significantly greater improvement of physical function at hospital discharge (MBI and FIM) in the intervention group, compared to the control group (55.13 vs. 31.75, p=0.048; 52.40 vs. 31.25, p=0.045). Intervention was significantly associated with improvement of MBI in multiple linear regression analysis (standardized coefficient 0.358, p=0.048).
Conclusion
Early physical rehabilitation may improve functional recovery at hospital discharge, especially in patients with high initial severity scores.
Keywords: Sepsis, septic shock, exercise rehabilitation, functional outcome, functional recovery
INTRODUCTION
Severe sepsis is an acute-phase complication of an infection that causes organ dysfunction or death. Even after an acute episode, substantial and persistent functional disability and low health-related quality of life has been observed.1,2,3 This can be a burden for patients and their families and can also increase their healthcare expenses.4 Patients often need additional support or rehabilitation in long-term care facilities after discharge.4,5 Therefore, efforts to improve long-term functional impairment in severe sepsis patients are important.
Similar problems have been reported in critically ill patients who were treated at intensive care units (ICUs).6,7,8 Among several attempted interventions for improving functional disability in this population, exercise rehabilitation was shown to be an effective measure to improve long-term functional status of patients.9 Improved quality of life, physical function, muscle strength, ventilator-free days, and decreased duration of hospitalization were observed in critically ill patients in ICUs who received early physical intervention.10,11,12,13,14
The noted benefits of physical rehabilitation in critically ill patients cannot be directly applied to sepsis. Survivors of sepsis often have worse functional impairment than critically ill patients without sepsis.1,3 Decreased muscle mass can be aggravated due to inflammatory responses in sepsis and initial immobility.15 Therefore, early exercise rehabilitation may be more beneficial in patients with severe sepsis.
Systematic research on the effects of early exercise rehabilitation in patients with severe sepsis is required. Although several animal studies and retrospective analyses have evaluated the benefits of physical therapy in septic conditions,16,17,18,19 only one published prospective human trial has demonstrated functional improvement at 6 months after discharge via physical rehabilitation in sepsis syndrome.20 In the current study, we aimed to investigate the effectiveness of early exercise rehabilitation on functional recovery in patients with severe sepsis.
MATERIALS AND METHODS
Study design and population
A prospective assessor-blinded case-control study was conducted at a 2500-bed tertiary care medical center in Seoul, Korea. Patients who visited the hospital via the emergency department due to severe sepsis between January 2013 and May 2014 were screened for enrollment. Participants over the age of 20 years who met the criteria for severe sepsis (proven or suspected infection with two or more criteria of systemic inflammatory response syndrome plus organ dysfunction or hypotension) were eligible. Patients were excluded if they met any of the following conditions: pregnancy, central nervous system infection as the focus of sepsis, underlying cognitive or functional deficits causing dependent daily living at baseline, not expected to survive for an additional 24 hours, having any condition contraindicated to exercise rehabilitation, and at risk of worsening condition by exercise rehabilitation.
The enrolled patients were randomly assigned at a 1:1 ratio to receive standard treatment (control group) or standard treatment plus exercise rehabilitation (intervention group). Computer-generated randomization was performed using concealed allocation. All of the groups shared the allocation process of one doctor of rehabilitation medicine prescribing physical therapy and one physiotherapist performing intervention according to protocols; the outcome assessor and researchers providing standard care to patients were blinded. The study was conducted according to the Declaration of Helsinki of 1975 and in compliance with local institutional review board (IRB) guidelines (Severance Hospital IRB, approval number: 4-2012-0710), with participants' written informed consent.
Interventions
All patients received standard treatment for severe sepsis or septic shock according to the Surviving Sepsis Campaign Guidelines.21 Patients in the intervention group underwent one to two times of daily targeted exercise rehabilitation for at least 1 hour by physiotherapist, from the day after randomization to the date of discharge. Exercise rehabilitation was performed according to the strategy used by Kayambu, et al.,22 which consisted of four stages of passive range of motion, active range of motion, electrical muscle stimulation, sitting, tilting, standing, ambulation, and other mobilization techniques depending on the patient's condition. Every day, the physiotherapist performed physical treatment and provided the doctor of rehabilitation medicine with feedback on the proper stage of subsequent intervention for each patient. Patients in the control group were able to receive general bedside exercise rehabilitation, depending on the doctor's decision. General bedside exercise rehabilitation took about 10 to 20 minutes a day and included passive range of motion, active range of motion, and sitting up in bed. Electrical muscle stimulation or exercise rehabilitation performed outside of the bed was usually not included. During the study period, one physical therapist and one doctor of rehabilitation medicine were consistently responsible for all enrolled patients' exercise rehabilitation regimens.
Assessment and measurements
Physical function was assessed three times throughout the study period: at the time of enrolment as “baseline,” just before discharge from the hospital, and 6 months after enrollment. We also conducted interviews to investigate the patients' usual physical function before admission. Physical function was evaluated according to the ability to perform Activities of Daily Livings (ADLs) and Instrumental Activities of Daily Livings (IADLs). In detail, ADLs were assessed by the Modified Barthel Index (MBI) and Functional Independence Measure (FIM), which have been validated and used extensively among patients with disabilities to assess functional measures and effects of rehabilitation.23,24,25 MBI measures patient performance on 10 items of ADL functions, is scored from 0 to 100 points, and is divided into six grades (total, severe, moderate, mild, minimal, independent) according to scores.26 FIM evaluated functional ability in 18 tasks in six areas of ADLs that were scored from 18 to 126 points.27 Performing IADLs were measured based on the total scores of eight items,28 and each item was scored from 1 to 3 points based on the degree of required assistance in this study. Assessments were conducted by blinded doctors of rehabilitation. The primary outcome of interest was the functional disability of participants 6 months after enrollment. Secondary outcome measures were 1) functional disabilities of participants at the time of discharge from hospital and 2) amount of recovery in physical function at every assessment point from baseline. Age, sex, body mass index, comorbidities, Charlson comorbidity score, septic focus, hemodynamic parameters, Sequential Organ Failure (SOFA) score, Acute Physiological and Chronic Health Evaluation (APACH II) score, and initial procalcitonin and C-reactive protein levels for the participants were collected at baseline.
Statistical analysis
Data were analyzed using the intention-to-treat principle, including data of all participants with at least one outcome assessment. Continuous variables are presented as means [standard deviation (SD)] or medians (inter-quartile range), and categorical variables are presented as numbers and percentages. For continuous variables, Student's t test or Mann-Whitney U test was used depending on the validity of normality assumption. Chi-square test or Fisher's exact test was used to assess categorical variables. Clinically significant changes could be determined using an effect size (calculated by dividing the mean absolute change score by SD of baseline scores), which standardizes raw score change.29 Outcomes were analyzed based on a linear mixed model using group (control or intervention) and time (at hospital discharge and 6 months after enrollment). We used multiple linear regression to determine interactions between outcomes and variables in subgroup analysis. P values ≤0.05 were considered statistically significant. All statistical analyses were performed using SPSS software, version 21 (IBM Corp., Armonk, NY, USA).
RESULTS
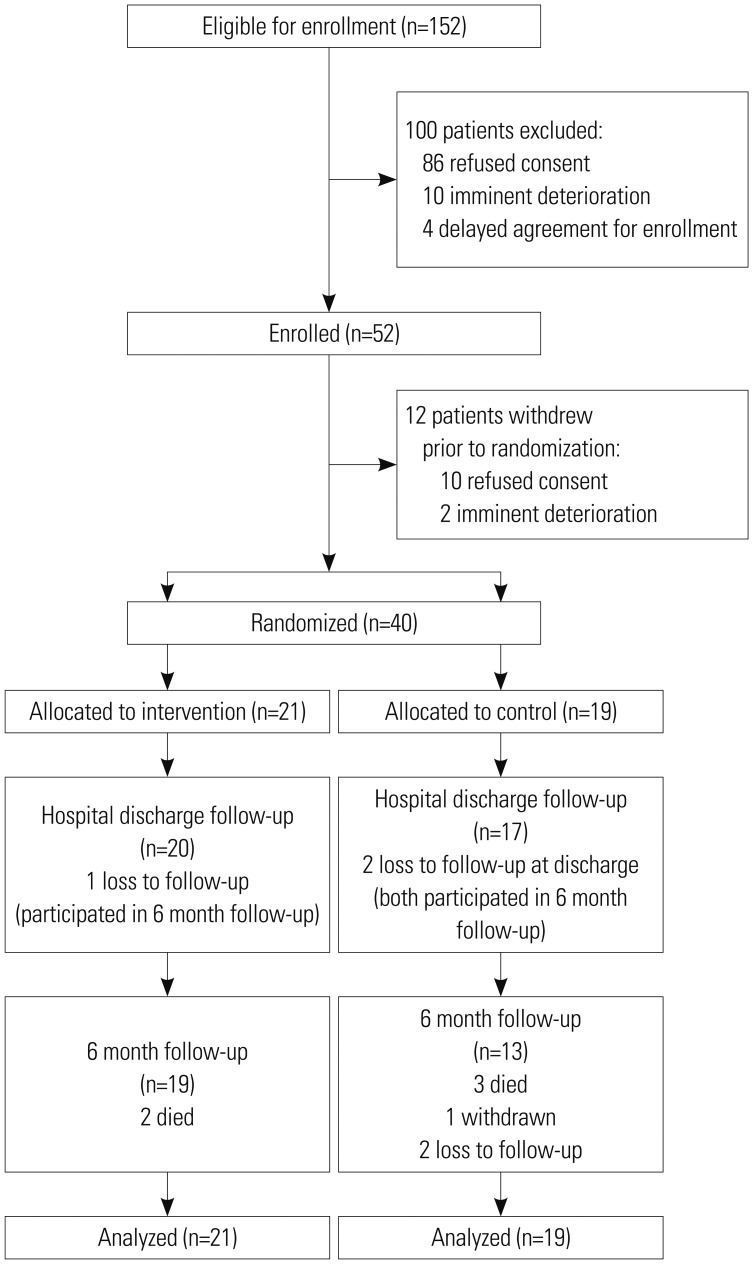
Participants were recruited from January 2013 to May 2014, and their follow-up was completed by November 2014. Forty patients were randomized (21 to the intervention group and 19 to the control group). Nineteen patients in the intervention group and 13 patients in the control group completed 6-month follow-up. All 40 patients were included in the intention-to-treat population (Fig. 1).
Fig. 1. Flow chart of the study participation.
The baseline characteristics of the participants are shown in Table 1. The median length of exercise rehabilitation in the intervention group was 8 days. There were no significant differences between the two groups with respect to demographic factors, comorbidities, septic focus, and severity of illness. Charlson comorbidity score was higher in the control group than the intervention group (6.0 vs. 4.0, p=0.007). No participant died during hospitalization. All-cause 6-month mortality rate was slightly higher in the control group than in the intervention group (21.1% vs. 14.3%, p=0.574), without statistical significance.
Table 1. Baseline Characteristics of Study Participants.
| Factors | Intervention group (n=21) | Control group (n=19) | p value |
|---|---|---|---|
| Demographic factors | |||
| Male | 8 (38.1) | 9 (47.4) | 0.342 |
| Age (yr) | 77.0 [69.5–78.5] | 70.0 [66.0–77.0) | 0.797 |
| BMI (kg/m2) | 22.1 [19.3–24.6] | 23.4 [20.2–26.6] | 0.303 |
| Length of hospital stay (day) | 12 [9.0–29.5] | 11 [8.0–16.0] | 0.221 |
| Total intervention day (day) | 8 [6.0–16.5] | - | |
| Comorbidities | |||
| HTN | 12 (57.1) | 13 (68.4) | 0.528 |
| DM | 7 (33.3) | 8 (42.1) | 0.319 |
| Cardiovascular disease | 3 (14.3) | 3 (15.8) | 1.000 |
| CHF | 2 (9.5) | 1 (5.3) | 1.000 |
| CVA | 3 (14.3) | 2 (10.5) | 1.000 |
| Chronic lung disease | 2 (9.5) | 0 (0) | 0.488 |
| Chronic renal disease | 3 (14.3) | 7 (36.8) | 0.104 |
| Chronic liver disease | 1 (4.8) | 5 (26.3) | 0.085 |
| Metastatic malignancy | 2 (9.5) | 6 (31.6) | 0.120 |
| Organ transplant recipient | 1 (4.8) | 0 (0) | 1.000 |
| Rheumatologic disease | 3 (14.3) | 3 (15.8) | 1.000 |
| Charlson comorbidity score | 4.0 [3.0–6.0] | 6.0 [5.0–8.0] | 0.007 |
| Clinical characteristics on admission | |||
| Shock | 21 (100) | 18 (94.7) | 0.475 |
| Use of inotropics | 21 (100) | 17 (89.5) | 0.219 |
| Acute kidney injury | 13 (61.9) | 12 (63.2) | 0.936 |
| RRT | 3 (14.3) | 1 (5.3) | 0.607 |
| Acute lung injury | 4 (19.0) | 0 (0) | 0.108 |
| Ventilator care | 4 (19.0) | 0 (0) | 0.108 |
| ICU care | 4 (19.0) | 2 (10.5) | 0.664 |
| CRP (mg/dL) | 177.0 [96.0–230.5] | 117.0 [56.0–234.0] | 0.255 |
| Procalcitonin | 23.6 [7.1–111.5] | 21.4 [2.8–44.4] | 0.642 |
| SOFA score | 6.0 [4.5–8.5] | 6.0 [5.0–10.0] | 0.990 |
| APACHE II score | 16.0 [9.0–20.5] | 17.0 [14.0–20.0] | 0.642 |
| Primary source of infection | 0.625 | ||
| Pneumonia | 4 (19.0) | 2 (10.5) | |
| Urinary tract infection | 9 (42.9) | 9 (47.4) | |
| Intra-abdominal infection | 5 (23.8) | 4 (21.1) | |
| Gastroenteritis | 3 (14.3) | 1 (5.3) | |
| Others | 0 (0) | 3 (15.8) | |
| Outcome | |||
| Overall 1 month mortality | 0 (0) | 0 (0) | - |
| Overall 6 month mortality | 3 (14.3) | 4 (21.1) | 0.574 |
BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; CHF, congestive heart failure; CVA, cerebrovascular accident; RRT, renal replacement therapy; ICU, intensive care unit; CRP, C-reactive protein; SOFA score, the Sequential Organ Failure Assessment score; APACHE II score, Acute Physiology and Chronic Health Evaluation II score.
Data are presented as number (%) or median [interquartile range].
Physical function performance
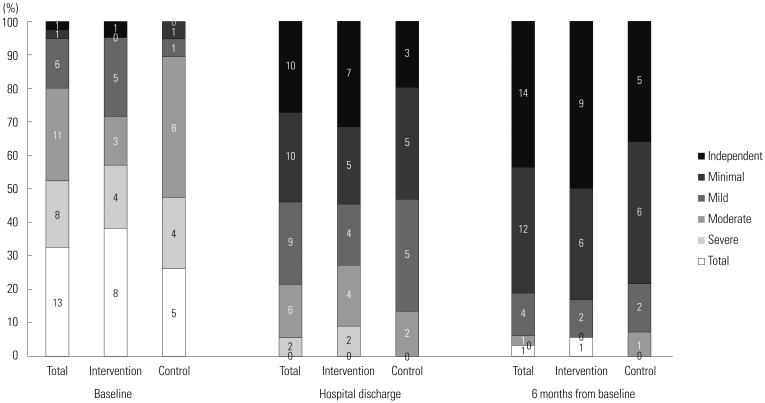
There were no significant differences in physical function represented by mean MBI, FIM, and IADL scores between the two groups at hospital discharge, during 6-month follow up, and at baseline. Patient interviews revealed no meaningful difference in usual physical function before admission between groups (Table 2). In the analysis of MBI grade distribution (Fig. 2), a definite improvement in physical function was shown over time from baseline to 6-month follow-up in both groups. More than half (21/40, 52.5%) of the participants belonged to the total or severe dependent grade, and two participants (2/40, 5%) had minimally dependent or independent status at baseline. However, at 6-month follow up, only one patient (1/32, 3.12%) showed total dependent performance, while 26 patients (26/32, 81.25%) improved to minimally dependent or independent status.
Table 2. Functional Outcomes at Time of Assessment according to Study Group.
| Outcome measure | Intervention group (n=21) | Control group (n=19) | p value |
|---|---|---|---|
| MBI score, mean (±SD) | |||
| Before admission | 95.43 (±8.54) | 97.05 (±5.28) | 0.479 |
| At study enrollment | 41.43 (±34.76) | 48.42 (±27.09) | 0.486 |
| At hospital discharge | 89.45 (±12.67) | 80.82 (±19.96) | 0.120 |
| At 6 month follow-up | 91.33 (±22.02) | 93.86 (±11.92) | 0.702 |
| FIM score, mean (±SD) | |||
| Before admission | 121.48 (±8.64) | 122.58 (±8.64) | 0.644 |
| At study enrollment | 64.38 (±33.30) | 72.89 (±21.31) | 0.347 |
| At hospital discharge | 114.30 (±12.94) | 104.29 (±23.35) | 0.129 |
| At 6 month follow-up | 116.88 (±24.93) | 119.21 (±14.22) | 0.759 |
| IADL score, mean (±SD) | |||
| Before admission | 21.43 (±3.65) | 22.68 (±2.38) | 0.203 |
| At study enrollment | 12.10 (±4.41) | 13.37 (±3.73) | 0.316 |
| At hospital discharge | 19.50 (±5.23) | 18.41 (±5.23) | 0.532 |
| At 6 month follow-up | 21.67 (±4.03) | 21.93 (±3.69) | 0.851 |
MBI, Modified Barthel Index; FIM, Functional Independence Measure; IADL, Instrumental Activity of Daily Living; SD, standard deviation.
Fig. 2. Distribution of MBI grades over time according to study group. Numbers within bars represent the number of participants. MBI, Modified Barthel Index.
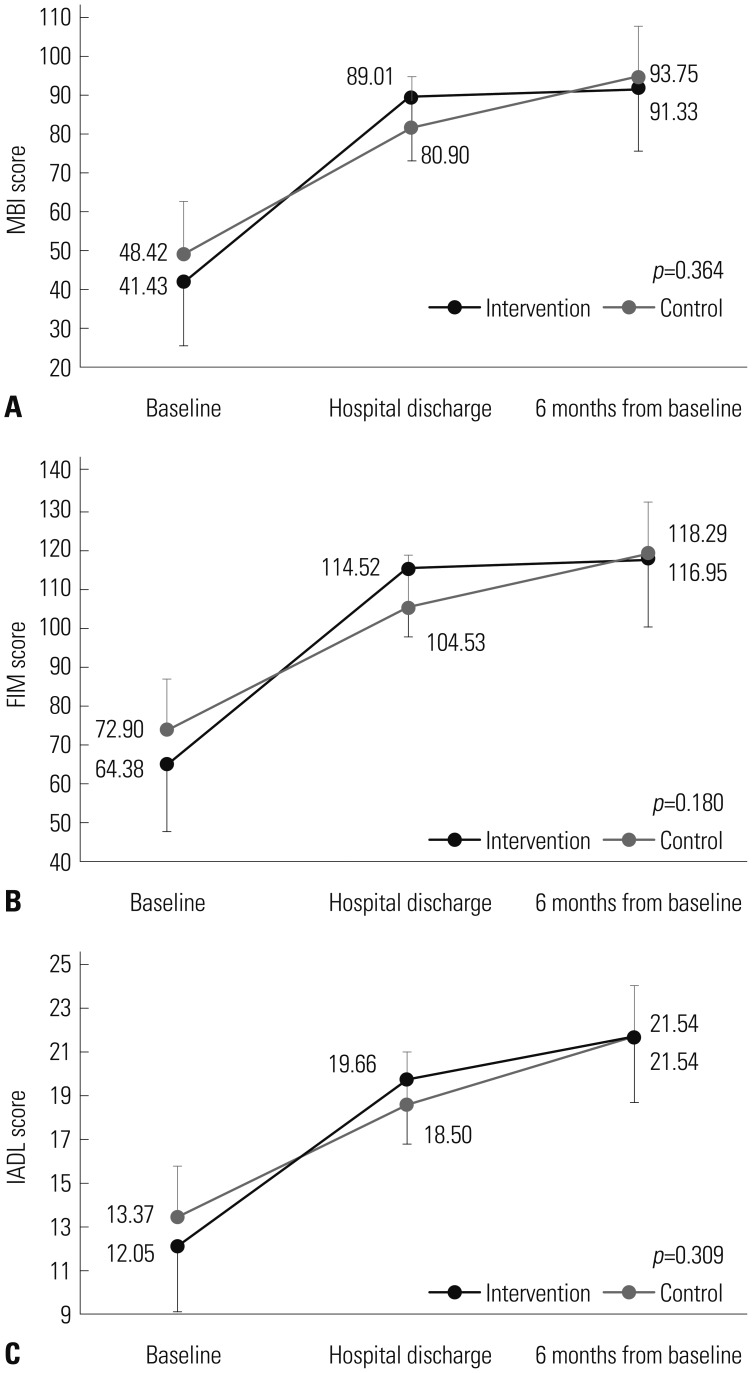
The extent of improvement in MBI, FIM, and IADL was not significantly different between control and intervention groups at any time point (group×time, p=0.364 for MBI, p= 0.180 for FIM, p=0.309 for IADL in a mixed linear model). However, when comparing changes in mean scores of MBI, FIM, and IADL over time between the two groups, a steeper upward gradient from baseline to hospital discharge was found in the intervention group, which might represent better functional recovery via exercise rehabilitation during hospitalization (Fig. 3).
Fig. 3. Change in functional outcome (MBI, FIM, and IADL) scores during the study period in the intervention and control groups. (A) Changes in mean MBI score. (B) Changes in mean FIM score. (C) Changes in mean IADL score. Means were calculated and compared between groups at each time point using a linear mixed model. Error bars represent standard error. MBI, Modified Barthel Index; FIM, Functional Independence Measure; IADL, Instrumental Activity of Daily Living.
Correlates of functional recovery
We performed subgroup analysis to identify the effects of exercise rehabilitation during hospitalization. We compared the mean change in physical functions between baseline and hospital discharge in subgroups. Analysis of patients with initial APACHE II scores ≥10 was performed (Table 3). Sixteen patients in the intervention group and 18 patients in the control group were thus included. There were no significant differences between the intervention and control groups, except for Charlson comorbidity score. Charlson comorbidity score was higher in the control group, compared to the intervention group (6.5 vs. 4.5, p=0.009). Mean changes in MBI and FIM from baseline to hospital discharge were significantly greater in the intervention group, although improvement in IADL was not statistically meaningful (55.13 vs. 31.75, p=0.048 for MBI; 52.40 vs. 31.25, p=0.045 for FIM; 7.60 vs. 5.06, p=0.112 for IADL). The effect size of MBI and FIM was also higher in the intervention group than in the control group (1.60 vs. 1.20 for MBI, 1.56 vs. 1.45 for FIM).
Table 3. Baseline Characteristics and Change in Functional Outcome Scores during Hospital Stay in a Subgroup with APACH II Scores ≥10.
| Factors | Intervention group (n=16) | Control group (n=18) | p value |
|---|---|---|---|
| Patient characteristics | |||
| Male | 7 (43.8) | 9 (50) | 0.716 |
| Age (yr) | 77 [68–78] | 70.5 [65.75–77.75] | 0.097 |
| BMI (kg/m2) | 22.4 [20.3–25.1] | 23.6 [20.3–26.7] | 0.523 |
| Length of hospital stay (day) | 14 [9.0–30.3] | 11 [8–18.3] | 0.200 |
| Total intervention day (day) | 9 [6.3–18.3] | - | |
| Charlson comorbidity score | 4.5 [3.0–6.5] | 6.5 [5.0–8.0] | 0.009 |
| Shock | 16 (100) | 17 (94.4) | 0.346 |
| ICU care | 3 (18.8) | 2 (11.1) | 0.536 |
| SOFA score | 7.5 [14.3–21.8] | 6.0 [5.0–9.3] | 0.522 |
| APACHE II score | 19.0 [14.3–21.8] | 17.0 [14.8–20.3] | 0.663 |
| Baseline MBI | 36.44 (±34.37) | 50.5 (±26.26) | 0.187 |
| Baseline FIM | 62.88 (±33.65) | 73.94 (±21.42) | 0.256 |
| Baseline IADL | 12.13 (±4.66) | 13.50 (±3.79) | 0.350 |
| Mean change of outcome (from baseline to hospital discharge) | |||
| MBI | 55.13 (±36.45) | 31.75 (±25.98) | 0.048 |
| FIM | 52.40 (±33.29) | 31.25 (±20.33) | 0.045 |
| IADL | 7.60 (±4.79) | 5.06 (±3.79) | 0.112 |
BMI, body mass index; ICU, intensive care unit; SOFA score, the Sequential Organ Failure Assessment score; APACHE II score, Acute Physiology and Chronic Health Evaluation II score; MBI, Modified Barthel Index; FIM, Functional Independence Measure; IADL, Instrumental Activity of Daily Living; SD, standard deviation. Data are presented as number (%), median [interquartile range], or mean (±standard deviation).
We further investigated the determinants of functional improvement among a subgroup of study participants with APACHE II scores ≥10 using stepwise multiple linear regression. When receiving intervention, length of hospital stay, age, sex, and APACHE II score were included in the model, and receiving intervention was selected as a single variable significantly associated with improvement in MBI (standardized coefficient 0.358, p=0.048). However, receiving intervention was not selected as a contributing variable in the regression model for improvement of FIM and IADL.
DISCUSSION
In this study, exercise rehabilitation did not have a statistically significant effect on functional status at assessment time points nor on the amount of functional improvement from baseline to hospital discharge or 6-month follow-up. However, in subgroup analysis of participants with high initial severity scores, the intervention group experienced significantly greater improvement in MBI and FIM during hospitalization.
To date, only one prospective randomized human clinical trial on physical rehabilitation of patients with sepsis has been published in 2015.20 The effect of early physical rehabilitation on functional recovery in severe sepsis has not been investigated enough to change current clinical practices. The present study provides more evidence on the potentially positive effects of exercise rehabilitation. In our study, physical function was evaluated by doctors of rehabilitation medicine at hospital discharge and at 6 months from baseline. Most (92% and 80%) participants were assessed at hospital discharge and at 6-month follow-up. These factors allowed us to make objective comparisons of functional status between groups over time.
APACHE II score has been validated for predicting hospital mortality in many studies. In-hospital mortality was reported as 12% to 18% in patients with APACHE II scores 10–19,30,31,32 and mortality rate was significantly higher in patients with APACHE II scores 10–19, compared to patients with APACHE II scores <10.31 Considering the actual mortality rates of study site and predicted hospital mortality of patients with particular APACHE II scores, participants with APACHE II score ≥10 were included in subgroup analysis.
In subgroup analysis, improvement of MBI and FIM during hospitalization was significantly greater in the intervention group. Greater mean improvement in MBI and FIM suggests that rehabilitation interventions can enhance the quality of ADL performance in patients with severe sepsis or septic shock. Functional improving quality of ADL performance during hospitalization can be one of the most important factors affecting long-term functional status and health care cost in severe sepsis. If patients do not acquire enough functional recovery before being discharged, they tend to enter long-term care facilities and experience deterioration, which may lead to further complications and re-admission.4,5
Receiving intervention was associated with a change in MBI, but was not a significant determinant of change in FIM. The reason for this remains unclear; however, there are numerous possible explanations. Although both MBI and FIM measure the performance of ADL with similar responsiveness to rehabilitation, FIM score consist of not only ADL performance but also social cognition (social interaction, problem solving, memory) and communication (comprehension, expression) abilities.27 In one study of rehabilitation after brain injury, MBI and physical FIM showed similar responses to rehabilitation, whereas cognitive FIM was less responsive.33 Therefore, improvement in MBI score could be more clearly achieved by physical exercise in this study. The small sample size might explain this discordance in our results. In this study, only six out of 40 participants received ICU care. Due to shortage of ICU rooms at the study site, patients were mostly moved from the emergency department to general wards despite receiving inotropes, unless they required mechanical ventilation or continuous renal replacement therapy. The lower rate of receiving ICU care could also be due to the initial exclusion of patients with refractory shock or ongoing multi-organ failure, who were not expected to survive for an additional 24 hours. This may also be a major reason for the lower 28-day mortality rate, compared to previous data from the sepsis registry of this study site,34 or the lower mean APACHE II score on admission, compared to results published in other papers concerning physical rehabilitation among patients with severe sepsis20 or critically ill patients.9,12,13 In this study, participants were not enrolled according to APACH II scores, but by the definition of severe sepsis and septic shock. All enrolled patients fulfilled the definition of severe sepsis or septic shock. Except for one patient, all of the participants had initial shock presentation. In general wards, a patient's physical activity is not completely recorded or controlled. Patients in general wards tend to perform voluntary physical exercise, irrespective of inclusion in a specific study group. This could represent a confounding factor in our analysis.
The median length of hospital stay was 11–14 days in subgroup analysis. This was a relatively shorter period than previously reported on severe sepsis or septic shock.2,20,35 Over the study period (January 2013–May 2014), a total of 210 patients visited this study site via the emergency department due to severe sepsis or septic shock. The mean length of stay among the 210 patients was 12.3 days. The mean APACHE II score was 15.5, which was similar to that of our study participants. Similarly, at this study site, the mean length of hospital stay for a total of 436 patients with severe sepsis or septic shock who were registered between November 2007 and November 2011 was 14 days, and the mean APACHE II score was 18.29.34 Therefore, the length of hospital stay among our study participants was similar to that of the overall data from this study site. In other words, our study participants were not less severe or discharged earlier than the average patients with septic shock in this site. Similar data can also be found for other Korean sites. According to analysis of data from the sepsis registry of 591 patients with severe sepsis or septic shock in an emergency department, the median length of hospital stay was 12 days with a median APACHE II score of 15.36 Therefore, with early management of septic shock before massive progression of organ damage, patients could achieve enough recovery to be discharged in a relatively shorter period of inpatient treatment.
With a relatively shorter period of median hospitalization, the median duration of exercise rehabilitation was only 9 days in the subgroup analysis. Since the type of rehabilitation, duration of daily exercise, and patient conditions differ from study to study, it is difficult to define the minimum period of exercise rehabilitation that can accurately determine the effectiveness thereof. However, some studies have shown significant results of physical rehabilitation on critically ill patients over a time period similar to ours. In a randomized controlled trial of critically ill patients who were treated with mechanical ventilator care in the ICU,13 participants in the intervention group received early physical rehabilitation from the day of study enrollment to hospital discharge. Since the median length of hospital stay was 13.5 days in the intervention group, the actual duration of rehabilitation during intervention would be shorter. Early physical rehabilitation resulted in better functional outcomes at hospital discharge, shorter duration of ICU stay (5.9 days vs. 7.9 days, p=0.08), and shorter duration of delirium in ICU (2 days vs. 4 days, p=0.03) in that study. It is evident that even a few days of physical rehabilitation affect outcomes. In another study,12 critically ill patients in the ICU engaged in daily cycling exercise to improve functional outcomes. The intervention group showed significantly better functional status at the time of hospital discharge, and the median duration of cycling exercise was 7 days. Through these studies, we can deduce that just a short period of rehabilitation would be effective for those of a critically ill condition.
This study had a few other limitations. The sample size was small. Small sample size can lead to either lack of statistical power to prove the benefits of an intervention or inconsistency in study results. In the same context, two groups were not completely controlled due to the small sample size. In terms of baseline characteristics, Charlson comorbidity score was slightly higher in the control group with statistical significance. In subgroup analysis, when Charlson comorbidity index was included in the multiple linear regression model to investigate the determinants of functional improvement during hospitalization, Charlson comorbidity index was selected as a single associated variable. Therefore, it is a limitation that the effect of difference in Charlson comorbidity index between the groups on functional recovery was not completely controlled in this study. However, as shown in Table 1, rates of having other individual comorbidities were not statistically different between the two groups. Even considering the Charlson comorbidity score difference between groups, there was no difference in usual functional status before admission between the two groups (Table 2). We also failed to assess cognitive or emotional status to evaluate the effects of intervention on quality of life after severe sepsis.
In conclusion, we were unable to demonstrate the apparent benefits of exercise rehabilitation on functional recovery in patients with sepsis. However, some physical functions tend to be improved by exercise rehabilitation in patients with initial high severity scores. Further research, with larger study populations and more study outcomes, is warranted to define the role of early exercise rehabilitation in severe sepsis.
ACKNOWLEDGEMENTS
Dr. Jun Yong Choi was supported by the Ministry of Science, ICT, and Future Planning of Korea (H-GUARD_2013M3A6B2078953) and the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28:3599–3605. doi: 10.1097/00003246-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Hofhuis JG, Spronk PE, van Stel HF, Schrijvers AJ, Rommes JH, Bakker J. The impact of severe sepsis on health-related quality of life: a long-term follow-up study. Anesth Analg. 2008;107:1957–1964. doi: 10.1213/ane.0b013e318187bbd8. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43:738–746. doi: 10.1097/CCM.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofhuis JG, Spronk PE, van Stel HF, Schrijvers GJ, Rommes JH, Bakker J. The impact of critical illness on perceived health-related quality of life during ICU treatment, hospital stay, and after hospital discharge: a long-term follow-up study. Chest. 2008;133:377–385. doi: 10.1378/chest.07-1217. [DOI] [PubMed] [Google Scholar]
- 7.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 9.Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest. 2013;144:1469–1480. doi: 10.1378/chest.13-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–859. doi: 10.1007/s10350-004-6672-4. [DOI] [PubMed] [Google Scholar]
- 11.Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281. doi: 10.2522/ptj.20050036. [DOI] [PubMed] [Google Scholar]
- 12.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 13.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 15.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37(10 Suppl):S354–S367. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sossdorf M, Fischer J, Meyer S, Dahlke K, Wissuwa B, Seidel C, et al. Physical exercise induces specific adaptations resulting in reduced organ injury and mortality during severe polymicrobial sepsis. Crit Care Med. 2013;41:e246–e255. doi: 10.1097/CCM.0b013e31828a2ae3. [DOI] [PubMed] [Google Scholar]
- 17.Sossdorf M, Otto GP, Menge K, Claus RA, Lösche W, Kabisch B, et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958. doi: 10.1016/j.jcrc.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 18.de Araújo CC, Silva JD, Samary CS, Guimarães IH, Marques PS, Oliveira GP, et al. Regular and moderate exercise before experimental sepsis reduces the risk of lung and distal organ injury. J Appl Physiol (1985) 2012;112:1206–1214. doi: 10.1152/japplphysiol.01061.2011. [DOI] [PubMed] [Google Scholar]
- 19.Chen HI, Hsieh SY, Yang FL, Hsu YH, Lin CC. Exercise training attenuates septic responses in conscious rats. Med Sci Sports Exerc. 2007;39:435–442. doi: 10.1249/mss.0b013e31802d11c8. [DOI] [PubMed] [Google Scholar]
- 20.Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med. 2015;41:865–874. doi: 10.1007/s00134-015-3763-8. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 22.Kayambu G, Boots RJ, Paratz JD. Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i-PERFORM Trial (Protocol Article) BMC Anesthesiol. 2011;11:21. doi: 10.1186/1471-2253-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson K, Aito S, Atkins M, Biering-Sørensen F, Charlifue S, Curt A, et al. Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2008;31:133–144. doi: 10.1080/10790268.2008.11760704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granger CV, Cotter AC, Hamilton BB, Fiedler RC, Hens MM. Functional assessment scales: a study of persons with multiple sclerosis. Arch Phys Med Rehabil. 1990;71:870–875. [PubMed] [Google Scholar]
- 25.Granger CV, Cotter AC, Hamilton BB, Fiedler RC. Functional assessment scales: a study of persons after stroke. Arch Phys Med Rehabil. 1993;74:133–138. [PubMed] [Google Scholar]
- 26.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 27.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 28.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 29.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27(3 Suppl):S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 30.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 31.Chiavone PA, Sens YA. Evaluation of APACHE II system among intensive care patients at a teaching hospital. Sao Paulo Med J. 2003;121:53–57. doi: 10.1590/S1516-31802003000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong DT, Crofts SL, Gomez M, McGuire GP, Byrick RJ. Evaluation of predictive ability of APACHE II system and hospital outcome in Canadian intensive care unit patients. Crit Care Med. 1995;23:1177–1183. doi: 10.1097/00003246-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Houlden H, Edwards M, McNeil J, Greenwood R. Use of the Barthel Index and the Functional Independence Measure during early inpatient rehabilitation after single incident brain injury. Clin Rehabil. 2006;20:153–159. doi: 10.1191/0269215506cr917oa. [DOI] [PubMed] [Google Scholar]
- 34.Song JE, Kim MH, Jeong WY, Jung IY, Oh DH, Kim YC, et al. Mortality risk factors for patients with septic shock after implementation of the Surviving Sepsis Campaign Bundles. Infect Chemother. 2016;48:199–208. doi: 10.3947/ic.2016.48.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poulsen JB, Møller K, Jensen CV, Weisdorf S, Kehlet H, Perner A. Effect of transcutaneous electrical muscle stimulation on muscle volume in patients with septic shock. Crit Care Med. 2011;39:456–461. doi: 10.1097/CCM.0b013e318205c7bc. [DOI] [PubMed] [Google Scholar]
- 36.Joo YM, Chae MK, Hwang SY, Jin SC, Lee TR, Cha WC, et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency department. Clin Exp Emerg Med. 2014;1:35–40. doi: 10.15441/ceem.14.012. [DOI] [PMC free article] [PubMed] [Google Scholar]