Abstract
Background
In infants with phenylketonuria (PKU), dietary management is based on lowering and titrating phenylalanine (Phe) intake from breast milk or standard infant formula in combination with a Phe-free infant formula in order to maintain blood Phe levels within target range. Professionals use different methods to feed infants with PKU and our survey aimed to document practices across Europe.
Methods
We sent a cross sectional, survey monkey® questionnaire to European health professionals working in IMD. It contained 31 open and multiple-choice questions. The results were analysed according to different geographical regions.
Results
Ninety-five centres from 21 countries responded. Over 60% of centres commenced diet in infants by age 10 days, with 58% of centres implementing newborn screening by day 3 post birth. At diagnosis, infant hospital admission occurred in 61% of metabolic centres, mainly in Eastern, Western and Southern Europe. Breastfeeding fell sharply following diagnosis with only 30% of women still breast feeding at 6 months.
53% of centres gave pre-measured Phe-free infant formula before each breast feed and 23% alternated breast feeds with Phe-free infant formula. With standard infant formula feeds, measured amounts were followed by Phe-free infant formula to satiety in 37% of centres (n = 35/95), whereas 44% (n = 42/95) advised mixing both formulas together. Weaning commenced between 17 and 26 weeks in 85% centres, ≥26 weeks in 12% and < 17 weeks in 3%.
Discussion
This is the largest European survey completed on PKU infant feeding practices. It is evident that practices varied widely across Europe, and the practicalities of infant feeding in PKU received little focus in the PKU European Guidelines (2017). There are few reports comparing different feeding techniques with blood Phe control, Phe fluctuations and growth. Controlled prospective studies are necessary to assess how different infant feeding practices may influence longer term feeding development.
Abbreviations: PKU, Phenylketonuria; Phe, Phenylalanine; IMD, Inherited Metabolic Disorders
Keywords: Breastfeeding, Infant practices, Phenylketonuria, Phenylalanine, Phe-free infant formula
1. Background
Infants with phenylketonuria (PKU) with blood Phe >360 μmol/L are treated with a low phenylalanine (Phe) diet following diagnosis after a positive newborn screening test. This involves restricting natural protein intake by lowering the amount of breast milk or standard infant formula given in combination with a protein substitute (Phe-free infant formula). The practical feeding process can be conducted in different ways and it is likely that practices may vary throughout Europe. Although it is well established that commencing treatment early is associated with better outcome in PKU, it is possible that the choice and application of infant feeding practices may also affect longer-term feeding development and nutritional outcome. In the general population, breastfeeding is associated with less overweight and obesity and a higher IQ [1]. Also a higher protein intake in infancy may be associated with a later risk of obesity [2]. Approaches to the delivery of early care, support and education in PKU may affect parental acceptance of the condition and their attitude towards dietary management. On diagnosis of PKU, parents are expected to manage the dietary treatment immediately, and so are forced into a situation of coping (what must be done here and now) rather than being given time to adjust to the diagnosis of PKU in their infant [3]. Feeding infants with PKU is undoubtedly challenging. The goal of nutritional management is to maintain blood Phe levels between 120 and 360 μmol/L as recommended by the PKU European guidelines [4]. Timing of treatment commencement is determined by the age of newborn screening. If the age of newborn screening is later, in infants with classical PKU it is probable that blood Phe concentrations will be higher, and in turn it will take longer to stabilise blood Phe control, although this remains unreported in screened infants. Later newborn screening will require rapid reduction of blood Phe, possibly leading to temporary cessation of breastfeeding and overall, this may cause additional parental anxiety associated with feeding. Also, in slightly later diagnosed infants, well-established feeding routines pre-diagnosis may be more challenging to change, and the introduction of a strong tasting Phe-free infant amino acid formula (providing non Phe amino acids with other essential nutrients) may be difficult. The European guidelines recommend that treatment should commence by 10 days of age, however it is unknown how many European centres are able to achieve this target [4].
Dietary management in infancy is based on lowering and titrating dietary Phe intake from breast milk or standard infant formula so that blood Phe concentrations are maintained within target range whilst achieving optimal growth and development. There appears no universal approach to feeding infants with PKU, with health professionals adopting many different practices. For example, when partial breast milk is given, techniques may include: 1) a measured amount of expressed breast milk prior to a Phe-free infant formula [5], 2) a pre-measured amount of Phe-free infant formula prior to breastfeeding until satiety [[6], [7], [8]], 3) a pre-measured amount of Phe-free infant formula prior to ‘time-controlled’ breastfeeding, and 4) alternate breast and Phe-free infant formula feeds [[9], [10], [11]]. When a measured amount of standard infant formula is given, this may be administered pre or post Phe-free infant formula or both feeds may be mixed together [10, 12]. In addition, whilst it is known that breastfeeding management strategies vary among countries and between treatment centres within countries [7, [13], [14], [15], [16]], it is unknown if the type of strategy will impact on the duration of breastfeeding in PKU.
In our survey, we aimed to study dietary treatment methods in infants with PKU across European centres.
2. Material and methods
A cross sectional, survey monkey® questionnaire was sent to European IMD health professionals (dietitians, nutritionists and medical doctors) who were either members of the ‘The Society for the Study of Inborn Errors of Metabolism’ (SSIEM) or who have participated in previous European surveys assessing dietary practices [[17], [18], [19]]. The questionnaire was composed of 31 open and multiple-choice questions asking questions about the infant age at newborn screening and dietary feeding practices at diagnosis and during the first 12 months of life. The questionnaire was written in English but linguistic support (Portuguese, French, Spanish and Italian) was provided by the first author [AP] to clarify any queries. Only one answer per centre was accepted from health professionals and participants were instructed to answer each question according to their general clinical practice rather than with individual patient cases.
The questionnaire responses were analysed by individual centre in addition to the geographical region of each centre. The results were analysed in two parts 1) processes related to early feeding and 2) weaning processes (introduction of low protein foods, normal foods and change in protein substitutes).
In part 1, the following information was collected: number of infants diagnosed with PKU each year, infant age at newborn-screening, age of starting diet, hospital or home care at diagnosis, frequency of blood Phe monitoring, breastfeeding prevalence at diagnosis and post diagnosis, and duration of breastfeeding. Breastfeeding was defined as the only source of feed intake before diagnosis. Post diagnosis of PKU breastfeeding was defined as breast milk given with Phe-free infant formula.
Detail about the different methods of administering Phe-free infant formula with breast milk or standard infant formula was collected. Data on total protein requirements was also collected. Part 2 results will be published separately.
3. Results
3.1. Participants
Ninety-five centres from 21 European countries responded to the survey monkey® questionnaire. Centres were grouped into the following geographical regions to analyse the results:
-
•
Southern Europe (n = 30 centres): Portugal (n = 7 centres), Italy (n = 6 centres), Spain (n = 9 centres), Greece (n = 2 centres) and Turkey (6 centres);
-
•
Western Europe A (n = 16 centres): Belgium (n = 6 centres), France (n = 5 centres) and The Netherlands (n = 5 centres);
-
•
Western Europe B (n = 15 centres): Germany (n = 13 centres) and Austria (n = 2 centres);
-
•
Northern Europe (n = 24 centres): UK (n = 17 centres), Sweden (n = 3 centres), Norway (n = 1 centre), Denmark (n = 1 centre), Finland (n = 1 centre) and Ireland (n = 1 centre);
-
•
Eastern Europe (n = 10 centres): Latvia (n = 1 centre), Poland (n = 6 centres), Slovakia (n = 1 centre), Hungary (n = 1 centre) and Estonia (n = 1 centre).
The number of new infants diagnosed with PKU annually in each centre varied widely but over half of the respondents only had 1 to 3 new cases per year. The number of new infants diagnosed per year in centres were: 1–3 cases per annum, 52% (n = 49/95); 4–10 cases, 31% (n = 29/95); 11–20 cases, 11% (n = 10/95); 21–30 cases, 1% (n = 1/95); and ≥ 30 cases, 2% (n = 2/95).
New born screening occurred by day 3 of infant age in over 50% of centres: day 1, 2%, (n = 2/95) centres; day 2, 24%, (n = 23/95); day 3, 32% (n = 30/95); day 4–6 days, 38% (n = 36/95); day 7–10, 3% (n = 3/95); and > day 10, 1% (n = 1/95) centres.
3.2. Dietary practices after diagnosis
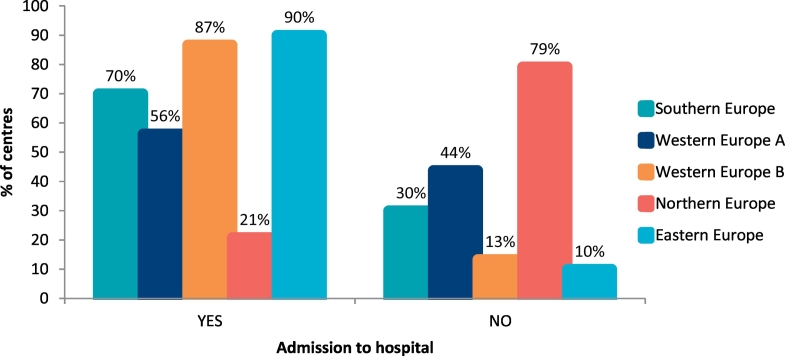
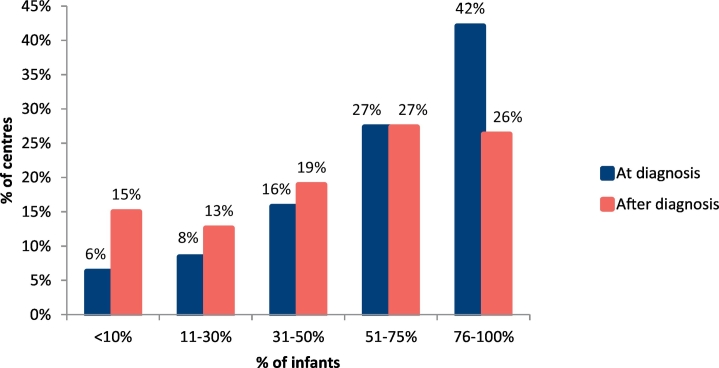
At diagnosis, infants were admitted to hospital in 61% of metabolic centres (n = 58/95 centres). The centres more likely to admit patients to hospital at the time of diagnosis were in Eastern Europe, Western Europe and Southern Europe (Fig. 1). Over 60% of centres (Southern Europe: 53%; Western Europe A: 69%; Western Europe B: 93%; Northern Europe: 63%; Eastern Europe: 30%) had commenced a low Phe diet by 10 days of age (<3 days, 1% [n = 1/95]; 4–10 days, 61% [n = 58/95]; 11–14 days, 31% [n = 29/95]; 15–20 day, 6% [n = 6/95]; >21 days, 1% [n = 1/95] of centres). Only 42% of centres were likely to have 76% or more of their infants on breast feeds at the time of diagnosis, with breastfeeding being highest in Southern and Western Europe (Fig. 2, Table 1). The numbers of infants who continued breastfeeding fell markedly and only 26% of centres maintained 76% or more of their infants on breast feeds beyond the diagnostic time (Fig. 2, Table 2). Overall, breastfeeding duration was short with a mean of: 4 weeks in 9% (n = 9/95), 5–17 weeks in 26% (n = 25/95), 18–26 weeks in 34% (n = 32/95), 27–52 weeks in 24% (n = 23/95) and > 1y, 6% (n = 6/95) of centres. Different geographical results are presented in Table 3. Only 17% of centres (n = 16/95) pre-measured expressed breast milk.
Fig. 1.
Percentage of centres who admitted infants to hospital on diagnosis of PKU.
Fig. 2.
Percentage of centres breastfeeding infants with PKU at time of diagnosis and post diagnosis.
Table 1.
Infants that were breast fed at the time of PKU diagnosis in the different European geographical regions.
| Geographical region | % of centres with specific % of infants with PKU that were breast-fed AT TIME OF DIAGNOSIS |
||||
|---|---|---|---|---|---|
| <10% | 11–30% | 31–50% | 51–75% | 76–100% | |
| Southern Europe | 10% (3/30) | 7% (2/30) | 7% (2/30) | 16% (5/30) | 60% (18/30) |
| N = 30 | |||||
| Western Europe A | 0 | 13% (2/16) | 19% (3/16) | 44% (7/16) | 25% (4/16) |
| N = 16 | |||||
| Western Europe B | 0 | 0 | 0 | 47% (7/15) | 53% (8/15) |
| N = 15 | |||||
| Northern Europe | 12% (3/24) | 12% (3/24) | 38% (9/24) | 12% (3/24) | 26% (6/24) |
| N = 24 | |||||
| Eastern Europe | 0 | 10% (1/10) | 10% (1/10) | 40% (4/10) | 40% (4/10) |
| N = 10 | |||||
Table 2.
Infants that were breast fed post PKU diagnosis in the different European geographical regions.
| Geographical region | % of centres with specific % of infants with PKU that CONTINUE to breastfeed AFTER diagnosis |
||||
|---|---|---|---|---|---|
| <10% | 11–30% | 31–50% | 51–75% | 76–100% | |
| Southern Europe | 23% (7/30) | 10% (3/30) | 10% (3/30) | 20% (6/30) | 37% (11/30) |
| N = 30 | |||||
| Western Europe A | 13% (2/16) | 19% (3/16) | 25% (4/16) | 13% (2/16) | 31% (5/16) |
| N = 16 | |||||
| Western Europe B | 0 | 0 | 13% (2/15) | 67% (10/15) | 20% (3/15) |
| N = 15 | |||||
| Northern Europe | 17% (4/24) | 17% (4/24) | 29% (7/24) | 17% (4/24) | 20% (5/24) |
| N = 24 | |||||
| Eastern Europe | 10% (1/10) | 20% (2/10) | 20% (2/10) | 40% (4/10) | 10% (1/10) |
| N = 10 | |||||
Table 3.
Duration of infant breastfeeding by European geographical region (%).
| Geographical region | Duration of breastfeeding |
||||
|---|---|---|---|---|---|
| 4 weeks | 5–17 weeks | 18–26 weeks | 27–52 weeks | >1y | |
| Southern Europe | 23% (7/30) | 27% (8/30) | 23% (7/30) | 10% (3/30) | 17% (5/30) |
| N = 30 | |||||
| Western Europe A | 6% (1/16) | 50% (8/16) | 25% (4/16) | 19% (3/16) | 0 |
| N = 16 | |||||
| Western Europe B | 0 | 13% (2/15) | 47% (7/15) | 40% (6/15) | 0 |
| N = 15 | |||||
| Northern Europe | 4% (1/24) | 21% (5/24) | 42% (10/24) | 33% (8/24) | 0 |
| N = 24 | |||||
| Eastern Europe | 0 | 20% (2/10) | 40% (4/10) | 30% (3/10) | 10% (1/10) |
| N = 10 | |||||
In breastfeeding infants, 53% (n = 50/95 centres) of centres gave a measured amount of Phe-free infant formula before each breast feed to satiety (without any time limited breast feeds); 11% (n = 10/95) gave a pre-measured amount of Phe-free infant formula before time limited breast feeds and 6% (n = 6/95) gave breast feeds followed by a measured amount of Phe-free infant formula from a bottle. Twenty-three per cent (n = 22/95 centres) gave alternate breast feeds with phe-free infant formula and 7% (n = 7/95 centres) used different practices.
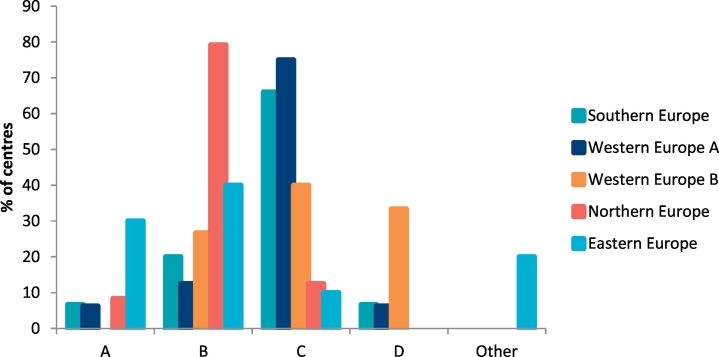
When a standard infant formula feed was given for the Phe source, 8% (n = 8/95 centres) of centres gave a measured amount of Phe-free infant formula first followed by measured amount of standard infant formula, whereas 37% (n = 35/95) gave a measured amount of standard infant formula followed by Phe-free infant formula to satiety. Forty-four per cent of centres (n = 42/95) advised mixing Phe-free formula together with standard infant formula, 8% (n = 8/95 centres) alternated feeds of standard infant formula with Phe-free infant formula and 2% (n = 2/95 centres) used different practices. Fig. 3 indicates the different practices by geographical regions.
Fig. 3.
Description by different European geographical regions of how Phe–free infant formula is given when a standard formula is used to provide the phenylalanine source.
Abbreviations: A: give a measured amount of Phe-free infant formula first followed by a measured amount of standard infant formula; B: give a measured amount of standard infant formula followed by Phe-free infant formula to satiety; C: mix Phe-free infant formula with standard infant formula; D: alternate feeds of standard infant formula and Phe-free Infant formula.
The total protein requirements (including both natural protein and protein equivalent from protein substitute) prescribed by centres in the first 12 months of life were: 1.5 g/kg/day, 1% (n = 1/95); 2 g/kg/day, 24% (n = 23/95); 2.5 g/kg/day, 36% (n = 34/95); 3 g/kg/day, 36% (n = 34/95 centres); 3.5 g/kg/day, 1% (n = 1/95 centres) and undefined 2% (n = 2/95 centres). Table 4 presents the amounts prescribed by different geographical regions.
Table 4.
Total protein prescribed (g/kg/day) to infants with PKU by different European geographical regions (%).
| Geographical region | Total protein prescription |
|||||
|---|---|---|---|---|---|---|
| 1.5 g/kg/day | 2.0 g/kg/day |
2.5 g/kg/day | 3.0 g/kg/day | 3.5 g/kg/day | other | |
| Southern Europe | 3% (1/30) | 13% (4/30) | 43% (13/30) | 37% (11/30) | 4% (1/30) | 0 |
| N = 30 | ||||||
| Western Europe A | 0 | 38% (6/16) | 56% (9/16) | 6% (1/16) | 0 | 0 |
| N = 16 | ||||||
| Western Europe B | 0 | 73% (11/15) | 20% (3/15) | 0 | 0 | 7% (1/15) |
| N = 15 | ||||||
| Northern Europe | 0 | 4% (1/24) | 21% (5/24) | 75% (17/24) | 0 | 4% (1/24) |
| N = 24 | ||||||
| Eastern Europe | 0 | 10% (1/10) | 40% (4/10) | 50% (5/10) | 0 | 0 |
| N = 10 | ||||||
The majority of centres (85% [n = 81/95]) started weaning with phe-free solids between 17 and 26 weeks, 3% of centres (n = 3/95) before 17 weeks and 12% (n = 11/95) after 26 weeks of age.
During the first year of life following blood Phe stabilization, blood Phe levels were monitored by centres: twice weekly by 16% (n = 15/95); weekly by 69% (n = 66/95); once every 2 weeks by 11% (n = 10/95) and monthly by 4% (n = 4/95) of centres.
4. Discussion
This is the largest survey conducted on infant feeding practices in PKU throughout Europe. Methods used to feed infants with PKU varied widely across centres. Our study indicated that over 60% of centres had started dietary treatment by the time infants were 10 days of age. It is encouraging that many centres met the European PKU guidelines [4]. Earlier newborn screening prevents severe neurological impairment as well as permitting timely implementation of the Phe restricted diet, although it is unclear how this impacts on long term feeding.
The need to admit infants into hospital at the time of diagnosis is associated with availability of local resources, traditional local practices or early testing for BH4 responsiveness. It is unknown if hospital admission is associated with earlier stabilization of blood Phe levels, alleviation or heightening of parental anxiety.
Although the use of breastfeeding in PKU is well established, the percentage of centres breastfeeding was highly variable between regions: Southern, Western (Germany and Austria) and Eastern Europe had the highest incidence of breastfeeding at the time of diagnosis, but the numbers fell in both Eastern and Western European countries following diagnosis. Some countries in Northern Europe (mainly UK) had a lower rate of breastfeeding at diagnosis but infants were a little older at diagnosis with a mean diagnostic age of >10 day.
In PKU, breastfeeding maintenance is more complex than in the general population. The availability of home support with reliable and consistent advice is important, but at diagnosis mothers have to make immediate decisions about feeding choice. Blood Phe levels above 1000 μmol/L at diagnosis may require cessation of breastfeeding for 24 to 48 h, and any difficulty in re-establishing breast feeds, may lead to breastfeeding failure. Mothers are commonly overwhelmed and distressed; they are uncertain and anxious about the amount of breast milk consumed and the potential harm this may cause to the baby.
With breastfeeding, the most common practice was to administer a pre-measured amount of Phe-free formula prior to breast-feeds. Surprisingly, alternating feeds of breast milk and Phe-free infant formula was practiced in 23% of centres. There are few reports describing the use of this method [6, 10, 11]. It will probably work well in early infancy when Phe requirements are high, but as this decreases with age, it may be necessary to further limit the number of daily breast feeds offered and replace this with additional Phe-free formula. This may lead to uneven distribution of Phe intake causing increased fluctuation in blood Phe concentrations. Prospective, controlled studies examining this technique are required to study 24 h blood Phe variation but the simplicity of this technique is very attractive.
Some centres ask mothers to only express breast milk so that it can be pre-measured, but there are now many longitudinal retrospective studies on large numbers of infants that show that giving pre-measured Phe-free infant formula before breastfeeding to satiety is safe and satisfactory blood Phe control and growth is achievable [8, 20, 21].
Only 30% of breastfeeding women continued to breast feed beyond 6 months. This was highly variable from 19% centres in Western Europe A to 40% in Western Europe B and Eastern Europe. Overall this data confirms previous findings, which clearly and consistently indicate that breastfeeding is commonly for a short duration in PKU [8, 10, 11, 14, 15, [22], [23], [24], [25]]. Interestingly, even though Northern Europe is the region with a lower percentage of breastfeeding infants at diagnosis, their percentage of continuation is similar to other regions (33%) and by 6 months of age, breastfeeding rates (34%) were close to the normal population [26].
For infants who were given standard infant formula the most popular method was mixing standard infant formula together with Phe-free infant formula, although this technique is under researched [11, 12]. It is certainly more convenient for parents but when Phe sources are introduced from food, a lower volume of standard infant formula is mixed with the Phe-free infant protein substitute leading to a stronger taste of the latter and possibly lower acceptance by the infant. There are few longitudinal studies examining different methods of bottle feeding in PKU and the impact on blood Phe control as well as later adherence to protein substitute.
The amount of total protein prescribed (97% prescribed ≥2 g/kg/day) was higher than the European guidelines (2017) [4]. In the European guidelines, it is recommended an additional 40% more protein from Phe-free amino acids is given compared with the WHO/FAO/UNU 2007 safe levels of protein intake [27], but this figure is empirically based. There have been reports of poor growth in young children with PKU [4], and Hoeksma demonstrated that a higher intake of natural protein intake, rather than the total protein intake was an important factor in head circumference growth [28]. There are also reports to indicate that higher doses of protein substitute improve natural protein tolerance in young children [4]. We consider further evidence is required before centres reduce doses of protein substitute in infancy according to the new European guidelines (2017) [4].
This study has several limitations. It was cross-sectional rather than prospective. Data about blood Phe control measurements were not collected. Data was collected about general practice rather than individual prescriptions. Only one questionnaire was completed by each PKU centre and it was possible that different professionals may have had different practices within the same centre. Moreover, different access to special formula, experience of professionals and ability to do home blood sampling may have influenced practices.
5. Conclusion
Even though PKU is the most commonly studied inherited metabolic disorder, infant feeding practices varied widely across Europe. Interestingly, little attention was paid to this subject in the PKU European Guidelines (2017) [4] and there are few reports comparing different feeding techniques with blood Phe control, stability of blood Phe levels and growth. Controlled prospective studies are needed to assess how different practices influence these outcomes in order to define the optimal infant feeding practices in PKU.
Author's contributions
AP and AM developed the questionnaire, interpreted data, analysed data and wrote the first draft of the manuscript. All the remaining authors were involved in data collection, interpretation of data and critical revision of the paper for important intellectual content and final approval of the version before publication.
Funding
Funding was not needed to develop this study.
Availability of data and materials
The datasets used and/or analysed during this current study are available from the corresponding author upon request.
Ethics approval and consent to participate
This was not applicable as this survey collected information about health professional's practice rather than individual patient data.
Consent for publication
Health professionals were made aware that the results of the survey would be published and gave their consent when participating.
Competing interests
Alex Pinto has received an educational grant from Cambrooke Therapeutics and grants from Vitaflo, Nutricia, Merck Serono and Biomarin to attend scientific meetings. Anne Daly has undertaken evaluation work for the nutritional companies – Vitaflo Ltd., Nutricia Ltd. and Metax. Sharon Evans is a research dietitian funded by Nutricia; financial support from Nutricia and Vitaflo to attend study days and conferences. Júlio César Rocha is member of the European Nutrition Expert Panel (Biomarin) and member of an Advisory Board for Applied Pharma Research. Anita MacDonald has received research funding and honoraria from Nutricia, Vitaflo International and Merck Serono. She is a member of the European Nutrition Expert Panel (Biomarin), member of Sapropterin Advisory Board (Biomarin), member of the Advisory Board entitled ELEMENT (Danone-Nutricia), and member of an Advisory Board for Arla and Applied Pharma Research. Rita Carvalho received grants to attend scientific meetings from Biomarin and Jaba Recordati. Liesbeth van der Ploeg received grants from Nutricia and from Vitaflo to attend scientific meetings on the field of metabolic diseases. Agnieszka Chrobot declares grants to attend scientific meetings from Nutricia, Vitaflo and Mead Johnson. Kamilla Straczek received honoraria from Nutricia Metabolics, Vitaflo and Mead-Johnson. Maria Giżewska received honoraria and was a consultant for: Nutricia International/Danone, Merck-Serono, Mead Johnson, BioMarin and Vitaflo. Alice Rossi has received funding from Biomarin, Nutricia, Piam Farmaceutici and Vitaflo to attend scientific meetings and courses. She is also a member of the European Nutritionist Expert Panel in PKU (Biomarin). Katharina Dokoupil has received honoraria from Nutricia, Vitaflo, Merck Serono and Dr. Schär and is a member of the European Nutrition Expert Panel (Biomarin), member of a Nutricia Advisory Board and member of a Nestlé Advisory Board. Consuelo Pedrón-Giner has received support from Vitaflo to attend SSIEM meetings. Ulrike Och declares grants to attend scientific meetings from Biomarin and Dr. Schär. Barbara Cochrane has received research funding and honoria from Nutricia and Vitaflo International. The remaining authors declare no competing interests.
Acknowledgments
We thank Vitaflo for supporting the publication cost of this paper.
References
- 1.Bernardo H., Cesar V., W.H. Organization . 2013. Long-Term Effects of Breastfeeding: A Systematic Review. [Google Scholar]
- 2.Grote V., von Kries R., Closa-Monasterolo R., Scaglioni S., Gruszfeld D., Sengier A., Langhendries J.P., Koletzko B. Protein intake and growth in the first 24 months of life. J. Pediatr. Gastroenterol. Nutr. 2010;51(Suppl. 3) doi: 10.1097/MPG.0b013e3181f96064. (S117-118) [DOI] [PubMed] [Google Scholar]
- 3.Carpenter K., Wittkowski A. 2018. Parenting a Child with Phenylketonuria (PKU): An Interpretative Phenomenological Analysis (IPA) of the Experience of Parents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Wegberg A.M.J., MacDonald A., Ahring K., Belanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Gizewska M., Huijbregts S.C., Kearney S., Leuzzi V., Maillot F., Muntau A.C., van Rijn M., Trefz F., Walter J.H., van Spronsen F.J. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan L.L., Elder S.B. Breastfeeding the infant with PKU. J. Human Lactation. 1997;13:231–235. doi: 10.1177/089033449701300313. [DOI] [PubMed] [Google Scholar]
- 6.Greve L.C., Wheeler M.D., Green-Burgeson D.K., Zorn E.M. Breast-feeding in the management of the newborn with phenylketonuria: a practical approach to dietary therapy. J. Am. Dietetic Assoc. 1994;94:305–309. doi: 10.1016/0002-8223(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald A., Depondt E., Evans S., Daly A., Hendriksz C., Chakrapani A.A., Saudubray J.M. Breast feeding in IMD. J. Inherit. Metab. Dis. 2006;29:299–303. doi: 10.1007/s10545-006-0332-x. [DOI] [PubMed] [Google Scholar]
- 8.Motzfeldt K., Lilje R., Nylander G. Breastfeeding in phenylketonuria. Acta Paediatrica. 1999;Supplement 88:25–27. doi: 10.1111/j.1651-2227.1999.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 9.Acosta P.B., Yannicelli S., Marriage B., Steiner R., Gaffield B., Arnold G., Lewis V., Cho S., Berstein L., Parton P., Leslie N., Korson M. Protein status of infants with phenylketonuria undergoing nutrition management. J. Am. Coll. Nutr. 1999;18:102–107. doi: 10.1080/07315724.1999.10718836. [DOI] [PubMed] [Google Scholar]
- 10.Kanufre V.C., Starling A.L., Leao E., Aguiar M.J., Santos J.S., Soares R.D., Silveira A.M. Breastfeeding in the treatment of children with phenylketonuria. Jornal de pediatria. 2007;83:447–452. doi: 10.2223/JPED.1672. [DOI] [PubMed] [Google Scholar]
- 11.van Rijn M., Bekhof J., Dijkstra T., Smit P.G., Moddermam P., van Spronsen F.J. A different approach to breast-feeding of the infant with phenylketonuria. Eur. J. Pediatr. 2003;162:323–326. doi: 10.1007/s00431-003-1182-2. [DOI] [PubMed] [Google Scholar]
- 12.Kose E., Aksoy B., Kuyum P., Tuncer N., Arslan N., Ozturk Y. The effects of breastfeeding in infants with phenylketonuria. J. Pediatr. Nurs. 2018;38:27–32. doi: 10.1016/j.pedn.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Ahring K., Belanger-Quintana A., Dokoupil K., Gokmen Ozel H., Lammardo A.M., MacDonald A., Motzfeldt K., Nowacka M., Robert M., van Rijn M. Dietary management practices in phenylketonuria across. Eur Cent. Clin. Nutr. (Edinburgh, Scotland) 2009;28:231–236. doi: 10.1016/j.clnu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Banta-Wright S.A., Press N., Knafl K.A., Steiner R.D., Houck G.M. Breastfeeding infants with phenylketonuria in the United States and Canada. Breastfeeding Med. 2014;9:142–148. doi: 10.1089/bfm.2013.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banta-Wright S.A., Shelton K.C., Lowe N.D., Knafl K.A., Houck G.M. Breast-feeding success among infants with phenylketonuria. J. Pediatr. Nursing. 2012;27:319–327. doi: 10.1016/j.pedn.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portnoi P., MacDonald A., Watling R., Clarke B.J., Barnes J., Robertson L., White F., Jarvis C., Laing S., Weetch E., Holliday K., Francis D., Netting M., Wall C. A survey of feeding practices in infants with phenylketonuria. J. Human Nutr. Diet. 1999;12:287–292. [Google Scholar]
- 17.Aguiar A., Ahring K., Almeida M.F., Assoun M., Belanger Quintana A., Bigot S., Bihet G., Blom Malmberg K., Burlina A., Bushueva T., Caris A., Chan H., Clark A., Clark S., Cochrane B., Corthouts K., Dalmau J., Dassy M., De Meyer A., Didycz B., Diels M., Dokupil K., Dubois S., Eftring K., Ekengren J., Ellerton C., Evans S., Faria A., Fischer A., Ford S., Freisinger P., Gizewska M., Gokmen-Ozel H., Gribben J., Gunden F., Heddrich-Ellerbrok M., Heiber S., Heidenborg C., Jankowski C., Janssen-Regelink R., Jones I., Jonkers C., Joerg-Streller M., Kaalund-Hansen K., Kiss E., Lammardo A.M., Lang K., Lier D., Lilje R., Lowry S., Luyten K., MacDonald A., Meyer U., Moor D., Pal A., Robert M., Robertson L., Rocha J.C., Rohde C., Ross K., Saruhan S., Sjoqvist E., Skeath R., Stoelen L., Ter Horst N.M., Terry A., Timmer C., Tuncer N., Vande Kerckhove K., van der Ploeg L., van Rijn M., van Spronsen F.J., van Teeffelen-Heithoff A., van Wegberg A., van Wyk K., Vasconcelos C., Vitoria I., Wildgoose J., Webster D., White F.J., Zweers H. Practices in prescribing protein substitutes for PKU in Europe: No uniformity of approach. Mol. Genet. Metab. 2015;115:17–22. doi: 10.1016/j.ymgme.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Daly A., Pinto A., Evans S., Almeida M.F., Assoun M., Belanger-Quintana A., Bernabei S.M., Bollhalder S., Cassiman D., Champion H., Chan H., Dalmau J., de Boer F., de Laet C., de Meyer A., Desloovere A., Dianin A., Dixon M., Dokoupil K., Dubois S., Eyskens F., Faria A., Fasan I., Favre E., Feillet F., Fekete A., Gallo G., Gingell C., Gribben J., Kaalund Hansen K., Ter Horst N.M., Jankowski C., Janssen-Regelink R., Jones I., Jouault C., Kahrs G.E., Kok I.L., Kowalik A., Laguerre C., Le Verge S., Lilje R., Maddalon C., Mayr D., Meyer U., Micciche A., Och U., Robert M., Rocha J.C., Rogozinski H., Rohde C., Ross K., Saruggia I., Schlune A., Singleton K., Sjoqvist E., Skeath R., Stolen L.H., Terry A., Timmer C., Tomlinson L., Tooke A., Vande Kerckhove K., van Dam E., van den Hurk T., van der Ploeg L., van Driessche M., van Rijn M., van Wegberg A., Vasconcelos C., Vestergaard H., Vitoria I., Webster D., White F.J., White L., Zweers H., MacDonald A. Dietary practices in propionic acidemia: a European survey. Mol. Genet. Metab. Rep. 2017;13:83–89. doi: 10.1016/j.ymgmr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto A., Alfadhel M., Akroyd R., Atik Altinok Y., Bernabei S.M., Bernstein L., Bruni G., Caine G., Cameron E., Carruthers R., Cochrane B., Daly A., de Boer F., Delaunay S., Dianin A., Dixon M., Drogari E., Dubois S., Evans S., Gribben J., Gugelmo G., Heidenborg C., Hunjan I., Kok I.L., Kumru B., Liguori A., Mayr D., Megdad E., Meyer U., Oliveira R.B., Pal A., Pozzoli A., Pretese R., Rocha J.C., Rosenbaum-Fabian S., Serrano-Nieto J., Sjoqvist E., Timmer C., White L., van den Hurk T., van Rijn M., Zweers H., Ziadlou M., MacDonald A. International practices in the dietary management of fructose 1–6 biphosphatase deficiency. Orphanet J. Rare Dis. 2018;13:21. doi: 10.1186/s13023-018-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demirkol M., Huner G., Kuru N., Donmez S., Baykal T., Seckin Y. Feasibility of breastfeeding in inborn errors of metabolism: experience in phenylketonuria. Ann. Nutr. Metab. 2001;45:497–498. [Google Scholar]
- 21.Huner G., Demirkol M. Presented at Turkish Society for PKU Istanbul, Turkey. 1996. Breast-feeding and phenylketonuria. [Google Scholar]
- 22.Agostoni C., Verduci E., Fiori L., Riva E., Giovannini M. Breastfeeding rates among hyperphenylalaninemic infants. Acta paediatrica (Oslo, Norway: 1992) 2000;89:366–367. [PubMed] [Google Scholar]
- 23.Cornejo V., Manriquez V., Colombo M., Mabe P., Jimenez M., De la Parra A., Valiente A., Raimann E. Phenylketonuria diagnosed during the neonatal period and breast feeding. Revista medica de Chile. 2003;131:1280–1287. [PubMed] [Google Scholar]
- 24.Huner G., Baykal T., Demir F., Demirkol M. Breastfeeding experience in inborn errors of metabolism other than phenylketonuria. J. Inherit. Metab. Dis. 2005;28:457–465. doi: 10.1007/s10545-005-0457-3. [DOI] [PubMed] [Google Scholar]
- 25.Segev A.S., Anikster U., Schwartz G. Paper presented at the Dietary Management of Inborn Errors of Metabolism, London, England. 2004. The incidence and duration of breastfeeding in the PKU clinic. [Google Scholar]
- 26.Statistics N. 2010. Infant Feeding Survey - UK; p. 2010. [Google Scholar]
- 27.World Health Organization Technical Report Series. 2007. Protein and amino acid requirements in human nutrition; pp. 1–265. (back cover) [PubMed] [Google Scholar]
- 28.Hoeksma M., Van Rijn M., Verkerk P.H., Bosch A.M., Mulder M.F., de Klerk J.B., de Koning T.J., Rubio-Gozalbo E., de Vries M., Sauer P.J., van Spronsen F.J. The intake of total protein, natural protein and protein substitute and growth of height and head circumference in Dutch infants with phenylketonuria. J. Inherit. Metab. Dis. 2005;28:845–854. doi: 10.1007/s10545-005-0122-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during this current study are available from the corresponding author upon request.