Abstract
Several human and nonhuman adenovirus (AdV) vectors including bovine AdV type 3 (BAdV-3) were developed as gene delivery vectors to supplement and/or elude human AdV (HAdV)-specific neutralizing antibodies (vector immunity). Here we evaluated the vaccine immunogenicity and efficacy of BAdV-3 vector (BAd-H5HA) expressing hemagglutinin (HA) of a H5N1 influenza virus in a dose escalation study in mice with the intranasal (IN) or intramuscular (IM) route of inoculation in comparison with the HAdV type C5 (HAdV-C5) vector (HAd-H5HA) expressing HA of a H5N1 influenza virus. Dose-related increases in the immune responses were clearly noticeable. A single IM inoculation with BAd-H5HA resulted in enhanced cellular immune responses compared with that of HAd-H5HA and conferred complete protection following challenge with a heterologous H5N1 virus at the dose of 3 × 107 plaque-forming units (PFUs), whereas a significant amount of influenza virus was detected in the lungs of mice immunized with 1 × 108 PFUs of HAd-H5HA. Similarly, compared with that of HAd-H5HA, a single IN inoculation with BAd-H5HA produced significantly enhanced humoral (HA-specific immunoglobulin [IgG] and its subclasses, as well as HA-specific IgA) and cellular immune responses, and conferred complete protection following challenge with a heterologous H5N1 virus. Complete protection with BAd-H5HA was observed with the lowest vaccine dose (1 × 106 PFUs), but similar protection with HAd-H5HA was observed at the highest vaccine dose (1 × 108 PFUs). These results suggest that at least 30-fold dose sparing can be achieved with BAd-H5HA vector compared with HAd-H5HA vaccine vector.
Keywords: adenoviral vector, bovine adenoviral vector, human adenoviral vector, enhanced immunogenicity and protection, avian influenza, route of immunization, dose sparing, universal influenza vaccine
Introduction
Adenovirus (AdV) vector-based vaccines have been shown to elicit balanced humoral and cell-mediated immune (CMI) responses1, 2 by activating innate immunity through both Toll-like receptor (TLR)-dependent as well as TLR-independent pathways.3, 4 Due to the high prevalence of AdV in humans, the development of AdV-specific neutralizing antibodies, known as “pre-existing vector immunity,”5, 6, 7 is one of the potential concerns for several human AdV (HAdV) vector-based vaccine delivery systems. To address this concern, a number of less prevalent HAdVs or nonhuman AdVs have been developed as vaccine delivery vectors.8, 9 These nonhuman AdV vectors are based on bovine AdV (BAdV), simian AdV (SAdV), porcine AdV (PAdV), ovine AdV (OAdV), canine AdV (CAdV), avian AdV (AAdV), and murine AdV (MAdV).8, 9
It has been demonstrated that there were no reductions in humoral and CMI responses against the vaccine immunogen and the resultant protection efficacy of a BAdV type 3 (BAdV-3) vector-based H5N1 influenza vaccine even in the presence of exceptionally high levels of pre-existing HAdV vector immunity.10 In addition, pre-existing HAdV-neutralizing antibodies in humans did not cross-neutralize BAdV-3,11 and HAdV-specific CMI responses did not cross-react with BAdV-3.4 Bio-distribution, pathogenesis, transduction, and persistence studies in animal models and cell lines have suggested that the safety aspects of BAdV vectors are similar to that of HAdV vectors.12, 13 It has been illustrated that the cell internalization of BAdV-3 is independent of the HAdV type C5 (HAdV-C5) receptors [Coxsackievirus-AdV receptor (CAR) and αvβ3 or αvβ5 integrin],14 but it is indicated that the α(2,3)-linked and α(2,6)-linked sialic acid receptors serve as major receptors for BAdV-3 internalization.15 It appears that BAdV-based vectors may serve as excellent vaccine vectors for humans without any concerns of pre-existing HAdV vector immunity.
Vaccine formulation features that are important for developing effective pre-pandemic influenza vaccine strategies include the development of balanced humoral and CMI responses that could offer cross-protection, safety, and efficacy with a single dose, dose sparing for vaccine delivery to a large number of individuals, and the capacity to produce a large number of vaccines at short notice. In our previous studies, we have demonstrated that AdV vector-based vaccines could elicit potent humoral and CMI responses in mice conferring cross-protection depending on the immunogen(s) of choice.16, 17 Because AdV vectors have been evaluated for their efficacy as gene delivery vehicles in many clinical trials in humans,18, 19, 20, 21 it is well understood how to produce a clinical grade of purified AdV vector lots in exceptionally large quantities under good laboratory practice (GLP) conditions in certified cell lines in a short time span.1, 22, 23, 24
In this study, we have compared the immunogenicity and efficacy of BAdV-3 vector (BAd-H5HA) expressing hemagglutinin (HA) of a H5N1 influenza virus in a mouse model with that of HAdV-C5 vector (HAd-H5HA) expressing the same HA. The vaccine doses [1 × 106, 3 × 106, 1 × 107, 3 × 107, or 1 × 108 plaque-forming units (PFUs)] and the routes of immunization [intranasal (IN) or intramuscular (IM)] were evaluated to determine whether the BAdV vector system will serve as a better vaccine vector compared with the HAdV vector. Overall, significantly higher levels of humoral and CMI responses accompanied with higher protection efficacy were observed with the BAd-H5HA vector compared with that of HAd-H5HA. The best protection efficacy with a significantly lower vaccine dose was observed in the mouse group inoculated IN with BAd-H5HA. These results suggest that the BAdV-3-based vector system is a better vaccine delivery vehicle for developing pre-pandemic influenza vaccines.
Results
HA Expression Levels by HAd-H5HA or BAd-H5HA in Infected Cells
HAd-ΔE1E3-, HAd-H5HA-, BAd-ΔE1E3-, or BAd-H5HA-infected BHH3 cells were analyzed to determine the levels of H5 HA expression by immunoblotting using an HA-specific rabbit polyclonal antibody. Roughly equal expression levels of β-actin demonstrate equal loading in each lane (Figure 1). Similar densities of HA-specific bands at approximately 72 kDa indicated that HAd-H5HA or BAd-H5HA resulted in a similar level of HA expression (Figure 1).
Figure 1.
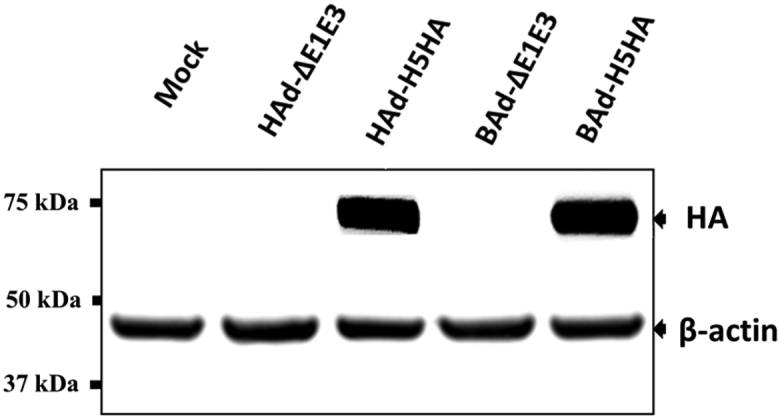
HA Expression Levels by HAd-H5HA or BAd-H5HA in Infected Cells
BHH3 cells were either mock infected or infected with HAd-ΔE1E3, HAd-H5HA, BAd-ΔE1E3, or BAd-H5HA at an MOI of 10 PFUs per cell. At 48 hr post-infection, the cells were harvested and processed for immunoblotting. HA expression was detected using an HA-specific polyclonal antibody. The expression levels of β-actin were monitored to demonstrate equal loading. The molecular weight markers in kDa are shown on the left.
Induction of Enhanced Humoral Immune Responses with BAd-H5HA Compared with HAd-H5HA
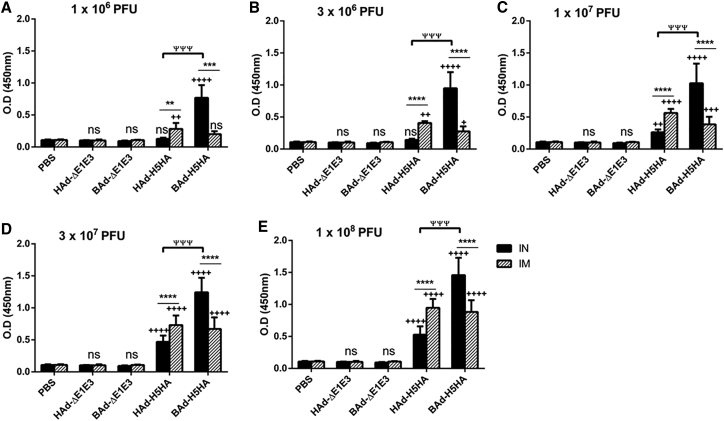
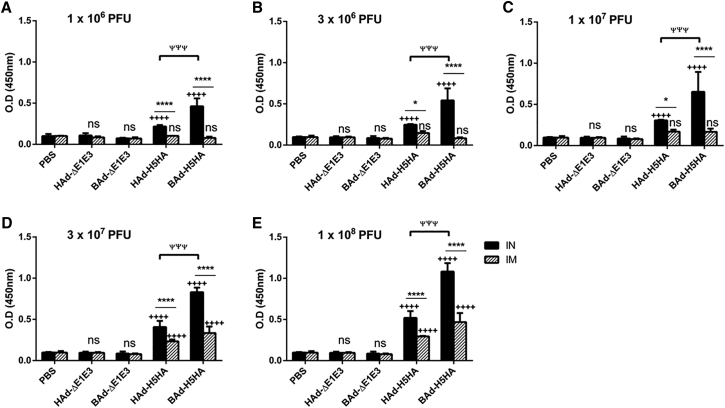
The mouse groups inoculated IN or IM once with 1 × 106 (Figure 2A), 3 × 106 (Figure 2B), 1 × 107 (Figure 2C), 3 × 107 (Figure 2D), or 1 × 108 (Figure 2E) PFUs of HAd-H5HA or BAd-H5HA elicited dose-dependent increases in anti-HA immunoglobulin G (IgG) antibody levels. These levels in the IM-inoculated HAd-H5HA groups were similar to or slightly better than those observed in the similarly inoculated BAd-H5HA groups. In the IN-inoculated BAd-H5HA groups, however, anti-HA IgG antibody levels were significantly higher than those in the similarly or IM-inoculated HAd-H5HA groups, or the IM-inoculated BAd-H5HA groups. The control groups inoculated IN or IM with PBS, HAd-ΔE1E3, or BAd-ΔE1E3 did not yield anti-HA IgG antibody levels above background (Figure 2).
Figure 2.
HA-Specific Serum IgG Antibody Responses in Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, serum samples were collected, diluted to 1:500, and the development of HA-specific IgG antibody responses were monitored by ELISA. Data are represented as the mean ± SD of the optical density (OD) readings. Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ). ** or ++, significant at p < 0.01; ***, +++, or ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
We have shown previously that animals immunized with AdV vectors expressing HA produce high levels of hemagglutination inhibition (HI) and virus-neutralizing (VN) antibody titers against homologous influenza virus strains.16, 17, 25, 26, 27 Therefore, here we only tried to determine HI and VN titers against an antigenically distinct influenza virus (VN/1203/RG) that was used for the challenge studies, and we noticed that none of the vaccinated groups developed any detectable HI and VN titers above the empty vector controls (data not shown). These findings were not unexpected because similar results were observed in our previous studies.17, 28
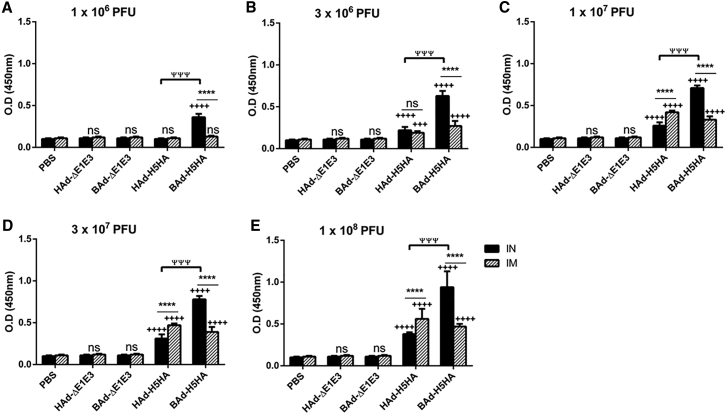
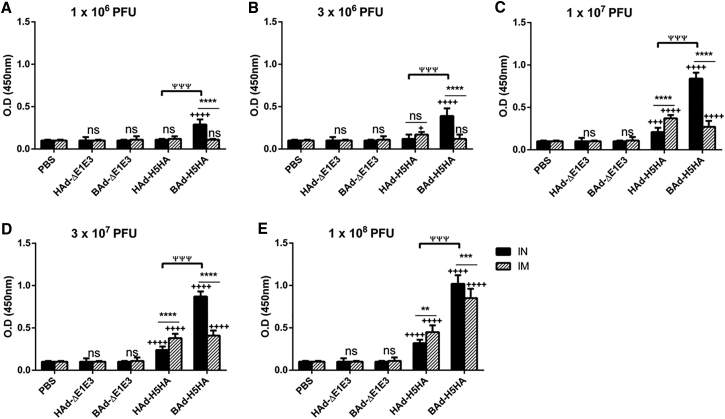
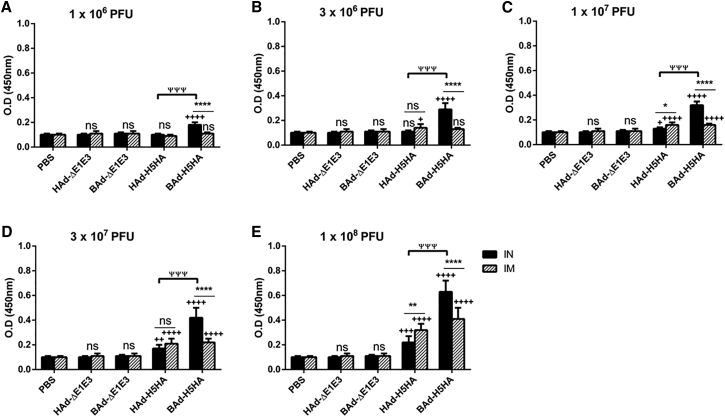
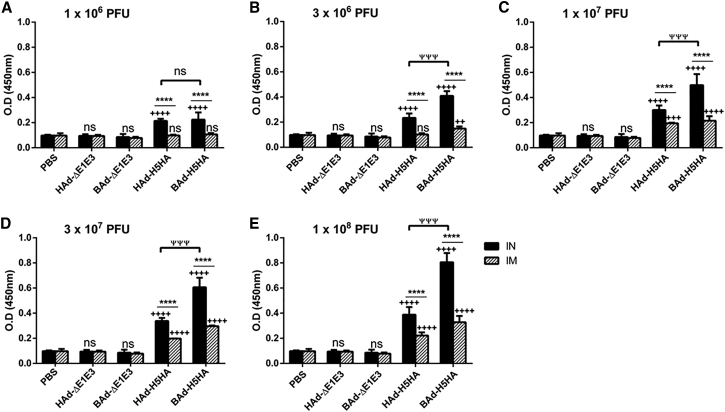
To determine whether the route of immunization or the vector type will influence the levels of IgG subclasses, the serum samples collected from the mouse groups inoculated IN or IM once with 1 × 106 (Figures 3A, 4A, and 5A), 3 × 106 (Figures 3B, 4B, and 5B), 1 × 107 (Figures 3C, 4C, and 5C), 3 × 107 (Figures 3D, 4D, and 5D), or 1 × 108 (Figures 3E, 4E, and 5E) PFUs of HAd-H5HA or BAd-H5HA were analyzed for anti-HA IgG1 (Figure 3), IgG2a (Figure 4), and IgG2b (Figure 5) levels by ELISA, respectively. As expected, there were dose-dependent increases in anti-HA IgG1, IgG2a, and IgG2b antibody levels. These levels in the IM-inoculated HAd-H5HA groups were similar to or slightly better or lower than those observed in the IM-inoculated BAd-H5HA groups. However, in the IN-inoculated BAd-H5HA groups, anti-HA IgG1, IgG2a, and IgG2b antibody levels were significantly higher than those in the IN- or IM-inoculated HAd-H5HA groups or the IM- inoculated BAd-H5HA groups (Figures 3, 4, and 5). Control groups inoculated IN or IM with PBS, HAd-ΔE1E3, or BAd-ΔE1E3 did not yield anti-HA IgG1 (Figure 3), IgG2a (Figure 4), or IgG2b (Figure 5) antibody levels above background.
Figure 3.
HA-Specific Serum IgG1 Antibody Responses in Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, serum samples were collected, diluted to 1:500, and the development of HA-specific IgG1 antibody responses was monitored by ELISA. Data are represented as the mean ± SD of the optical density (OD) readings. Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ). +++ or ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
Figure 4.
HA-Specific Serum IgG2a Antibody Responses in Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, serum samples were collected, diluted to 1:50, and the development of HA-specific IgG2a antibody responses was monitored by ELISA. Data are represented as the mean ± SD of the optical density (OD) readings. Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ).+, significant at p < 0.05; **, significant at p < 0.01; ***, +++, or ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
Figure 5.
HA-Specific Serum IgG2b Antibody Responses in Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, serum samples were collected, diluted to 1:50, and the development of HA-specific IgG2b antibody responses was monitored by ELISA. Data are represented as the mean ± SD of the optical density (OD) readings. Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ). * or +, significant at p < 0.05; ** or ++, significant at p < 0.01; +++ or ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
Furthermore, to ascertain whether the route of immunization or the vector type will influence the development of HA-specific IgA responses at the mucosal level, the lung washes (Figure 6), as well as nasal washes (Figure 7) from the mouse groups, inoculated IN or IM once with 1 × 106 (Figures 6A and 7A), 3 × 106 (Figures 6B and 7B), 1 × 107 (Figures 6C and 7C), 3 × 107 (Figures 6D and 7D), or 1 × 108 (Figures 6E and 7E) PFUs of HAd-H5HA or BAd-H5HA, were analyzed for anti-HA IgA levels by ELISA. As expected, there were dose-dependent increases in anti-HA IgA antibody levels in the lung washes as well as nasal washes. IgA antibody levels in the lung washes and nasal washes in the IM-inoculated BAd-H5HA or HAd-H5HA groups were detected only with doses of 1 × 107 PFUs and onward (Figures 6 and 7). IgA antibody levels in the lung washes and nasal washes of the IM-inoculated BAd-H5HA groups were similar to or slightly higher than those in the IM-inoculated HAd-H5HA, whereas IgA antibody levels in the lung washes and nasal washes in the IN-inoculated BAd-H5HA or HAd-H5HA groups were detected with the lowest dose of 1 × 106 PFU and onward. In the IN-inoculated BAd-H5HA groups, anti-HA IgA antibody levels in the lung washes (Figure 6) and nasal washes (Figure 7) were significantly higher than those in the IN- or IM-inoculated HAd-H5HA groups or the IM-inoculated BAd-H5HA groups. The control groups inoculated IN or IM with PBS, HAd-ΔE1E3, or BAd-ΔE1E3 did not yield anti-HA IgA antibody levels above background (Figures 6 and 7).
Figure 6.
HA-Specific IgA Antibody Responses in Lung Washes of Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFU of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, nasal wash samples were collected, diluted to 1:5, and the development of HA-specific IgA antibody responses was monitored by ELISA. Data are represented as the mean ± SD of the optical density (OD) readings. Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ). *, significant at p < 0.05; ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
Figure 7.
HA-Specific IgA Antibody Responses in Nasal Washes of Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, lung wash samples were collected, diluted to 1:10, and the development of HA-specific IgA antibody responses was monitored by ELISA. Data are represented as the mean ± SD of the optical density (OD) readings. Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ). ++, significant at p < 0.01; +++ or ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
Induction of Enhanced CMI Responses with BAd-H5HA Compared with HAd-H5HA
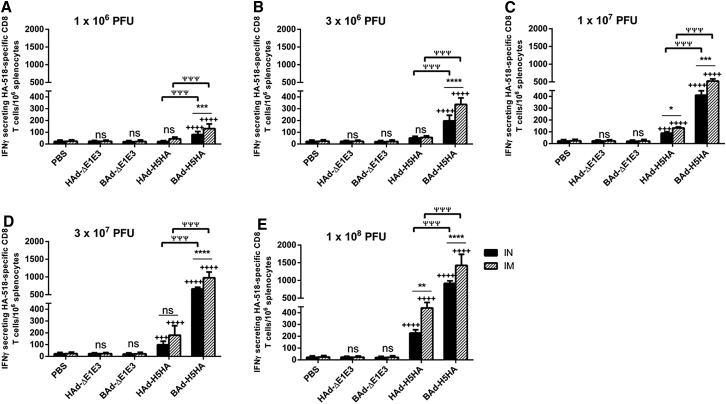
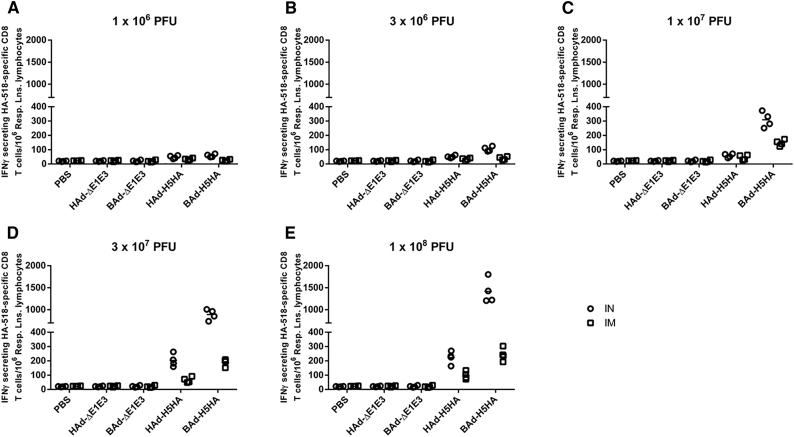
CMI responses against influenza viruses are important for virus clearance following infection and play an important role in heterologous as well as heterosubtypic protection against influenza viruses.17, 29 To verify whether the route of immunization or the vector type will impact the development of the CMI responses against influenza in vaccinated mice, we analyzed splenocytes (Figure 8), pooled respiratory area lymph node (RLN) cells (Figure 9), pooled inguinal lymph node (ILN) cells (Figure 10), and pooled lung lymphocytes (Figure 11) from AdV vector-inoculated groups for HA-specific CMI responses following in vitro stimulation with HA518 peptide using an interferon-gamma (IFNγ)-specific enzyme-linked immunospot (ELISpot) assay. The numbers of IFNγ-secreting HA518-specific CD8 T cells from the mouse groups inoculated IN or IM once with 1 × 106 (Figures 8A, 9A, 10A, and 11A), 3 × 106 (Figures 8B, 9B, 10B, and 11B), 1 × 107 (Figures 8C, 9C, 10C, and 11C), 3 × 107 (Figures 8D, 9D, 10D, and 11D), or 1 × 108 (Figures 8E, 9E, 10E, and 11E) PFUs of HAd-H5HA or BAd-H5HA are shown. There were dose-dependent increases in the numbers of IFNγ-secreting HA518-specific CD8 T cells in splenocytes (Figure 8), pooled RLN cells (Figure 9), pooled ILN cells (Figure 10), and pooled lung lymphocytes (Figure 11) of the vaccinated groups compared with the empty vector (Ad-ΔE1E3) or PBS control groups. There were significantly higher numbers of IFNγ-secreting HA518-specific CD8 T cells in splenocytes of the IM- or IN-inoculated BAd-H5HA groups compared with the IM- or IN-inoculated HAd-H5HA groups (Figure 8), and the numbers were consistently higher in the IM-inoculated vaccine groups compared with that of the IN-inoculated vaccine groups.
Figure 8.
HA518 Epitope-Specific IFNγ-Secreting CD8+ T Cells in the Spleens of Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, the spleens were collected, and the splenocytes were evaluated for HA-specific cell-mediated immune responses using IFNγ-ELISpot assay. The data represent mean ± SD of the number of spot-forming units (SFUs). Statistically significant responses are shown as compared with the PBS group (+), IN versus IM route of inoculation in the same group (*), or BAd-H5HA vector versus HAd-H5HA vector (ψ). *, significant at p < 0.05; **, significant at p < 0.01; ***, +++, or ψψψ, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analysis was done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; ns, no significance at p > 0.05.
Figure 9.
HA518 Epitope-Specific IFNγ-Secreting CD8+ T Cells in the Respiratory Lymph Nodes (RLNs) of Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, the RLNs were collected, and the pooled RLN cells were evaluated for HA-specific cell-mediated immune responses using the IFNγ-ELISpot assay. The data represent the individual replicate numbers of spot-forming units (SFUs) from pooled samples. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus.
Figure 10.
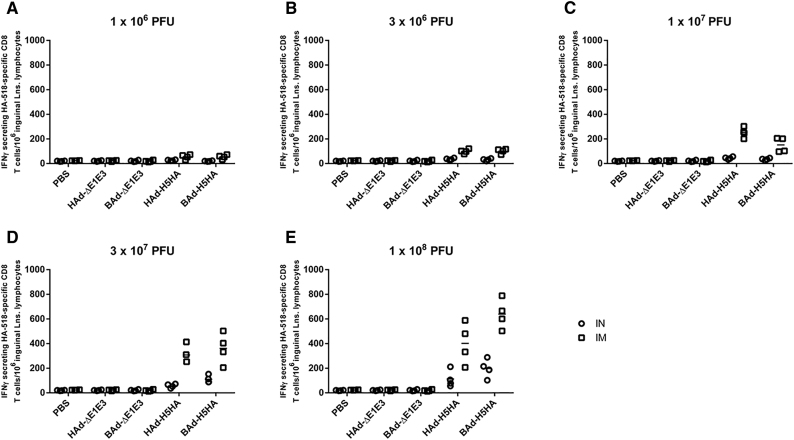
HA518 Epitope-Specific IFNγ-Secreting CD8+ T Cells in the Inguinal Lymph Nodes (ILNs) of Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, the ILNs were collected, and the pooled ILN cells were evaluated for HA-specific cell-mediated immune responses using IFNγ-ELISpot assay. The data represent the individual replicate numbers of spot-forming units (SFUs) from pooled samples. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus.
Figure 11.
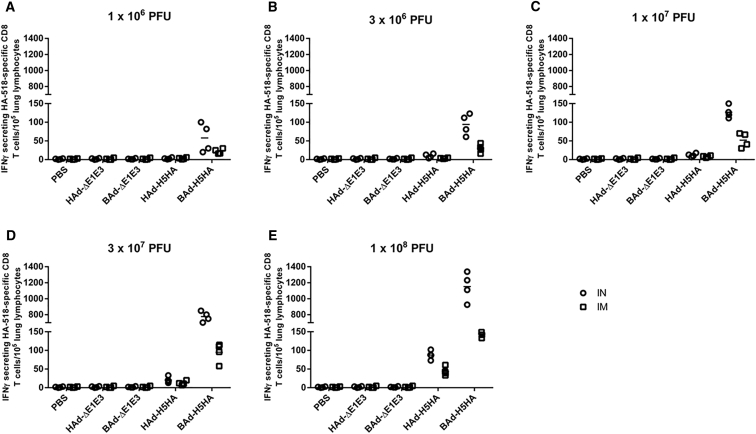
HA518 Epitope-Specific IFNγ-Secreting CD8+ T Cells in the Lungs of Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after inoculation, the lungs were collected, and the pooled lung lymphocytes were evaluated for HA-specific cell-mediated immune responses using IFNγ-ELISpot assay. The data represent the individual replicate numbers of spot-forming units (SFUs) from pooled samples. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus.
There were substantially higher numbers of IFNγ-secreting HA518-specific CD8 T cells in the RLNs of the IM- or IN-inoculated BAd-H5HA groups compared with those of the IM- or IN-inoculated HAd-H5HA groups (Figure 9), and the numbers were consistently higher in the IN-inoculated vaccine groups compared with those of the IM-inoculated vaccine groups. Whereas in ILNs of the IM-inoculated vaccine groups, higher numbers of IFNγ-secreting HA518-specific CD8 T cells were detected compared with those of the empty vector (Ad-ΔE1E3) or PBS control groups, or the IN-inoculated vaccine groups (Figure 10), and the numbers were consistently higher in the IM-inoculated vaccine groups compared with those of the IN-inoculated vaccine groups. Furthermore, considerably elevated numbers of IFNγ-secreting HA518-specific CD8 T cells in the lung lymphocytes of the IM- or IN inoculated BAd-H5HA groups compared with those of the IM- or IN-inoculated HAd-H5HA groups, respectively, were visualized (Figure 11), and the numbers were consistently higher in the IN-inoculated vaccine groups compared with those of the IM-inoculated vaccine groups.
Development of Enhanced Protection in Mice Immunized with BAd-H5HA Compared with HAd-H5HA
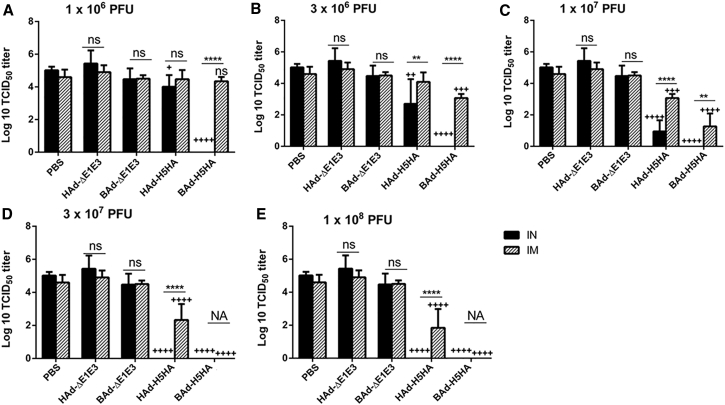
Because the influenza virus VN/1203/RG, which was used as a challenge virus to evaluate the efficacy of protection, does not cause consistent morbidity or mortality in mice,16, 17 significant reductions in lung viral titers in vaccinated animals following challenge are a useful measure of the virus clearance and vaccine protective efficacy. The mouse groups were immunized IN or IM once with 1 × 106 (Figure 12A), 3 × 106 (Figure 12B), 1 × 107 (Figure 12C), 3 × 107 (Figure 12D), or 1 × 108 (Figure 12E) PFUs of HAd-H5HA or BAd-H5HA, and subsequently challenged with 100 mouse infectious dose 50 (MID50) of VN/1203/RG. For the IN route of inoculation, the lowest vaccine dose of 1 × 106 PFUs of BAd-H5HA conferred complete protection following challenge (Figure 12A). The lowest vaccine dose for IN-inoculated HAd-H5HA that yielded complete protection following challenge was 3 × 107 PFUs (Figure 12E). For the IM route of inoculation, the lowest vaccine dose for BAd-H5HA that conferred complete protection following challenge was 3 × 107 PFUs (Figure 12D), but for the IM-inoculated HAd-H5HA animal group, the log lung virus titer with a dose of 1 × 108 PFUs was 1.84 ± 1.14 compared with 4.90 ± 0.42 and 4.6 ± 0.46 log lung virus titers in the empty vector (HAd-ΔE1E3) or PBS control groups, respectively (Figure 12E). Even at a vaccine dose of 3 × 108 PFUs per animal for IM-inoculated HAd-H5HA animal group, the log lung virus titer of 0.84 ± 0.5 was observed following challenge (data not shown).
Figure 12.
Lung Influenza Virus Titers in Mice Immunized Once with BAd-H5HA or HAd-H5HA
Mice were immunized intramuscularly (IM) or intranasally (IN) once with 1 × 106 (A), 3 × 106 (B), 1 × 107 (C), 3 × 107 (D), or 1 × 108 (E) PFUs of BAd-H5HA or HAd-H5HA. For all dose groups, mice inoculated IM or IN with PBS or 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as negative or internal controls, respectively. Four weeks after immunization, mice were challenged with 100 MID50 of A/Vietnam/1203/2004(H5N1)-PR8/CDC-RG influenza virus. Three days after the challenge, mice were euthanized and the lungs were collected to determine lung virus titers. The data are shown as mean Log10 TCID50 ± SD, and the detection limit was 0.5 Log10 TCID50/mL. Statistically significant responses are shown as compared with the PBS group (+) or IN versus IM route of inoculation in the same group (*). +, significant at p < 0.05; ** or ++, significant at p < 0.01; +++, significant at p < 0.001; and **** or ++++, significant at p < 0.0001. The statistical analyses were done by Bonferroni post-test and two-way ANOVA using GraphPad Prism 6. BAd-ΔE1E3, BAd empty vector; BAd-H5HA, bovine adenoviral vector expressing hemagglutinin (HA) of A/Hong Kong/156/97(H5N1) influenza virus; HAd-ΔE1E3, HAd empty vector; HAd-H5HA, human adenoviral vector expressing HA of A/Hong Kong/156/97(H5N1) influenza virus; NA, not applicable; ns, no significance at p > 0.05.
Discussion
Several studies using AdV vector-based influenza vaccines have been conducted both in animal models25, 26, 30 and as clinical trials in humans19, 31 to explore the potential of this vector system. Previously we have shown that AdV vector-based vaccines can provide complete protection against challenge with homologous as well as heterologous (antigenically distinct) influenza virus strains.25 Moreover, AdV vector vaccines containing multiple HAs from different HA subtypes or expressing nucleoprotein (NP) of H5N1 influenza conferred either complete protection or significant reduction in lung virus titers, respectively, following challenge with pre-pandemic H5, H7, or H9 influenza viruses.16, 17
The purpose of this study was to determine some of the parameters that are important for a pre-pandemic influenza vaccine including route of immunization, dose sparing, protection from a single dose, and protection against a heterologous influenza virus strain. This study was based on the hypothesis that a combination of HA-specific CMI responses and cross-reactive (although not necessarily cross-neutralizing) humoral immune responses will provide heterologous protection against an antigenically distinct H5N1 influenza virus. In addition, this study also focused on determining the vector type and route of immunization for enhanced immunogenicity conferring efficient protection.
Overall, there were no significant differences in the serum anti-HA IgG, IgG1, IgG2a, and IgG2b or mucosal IgA responses in mouse groups inoculated IM with either BAd-H5HA or HAd-H5HA. However, mice inoculated with BAd-H5HA had higher numbers of IFNγ-secreting HA518-specific CD8 T cells in the spleen, lung, and RLNs than the groups inoculated with HAd-H5HA. In contrast, there were no significant differences in the numbers of IFNγ-secreting HA518-specific CD8 T cells in the ILNs of the mouse groups inoculated IM with either BAd-H5HA or HAd-H5HA. An intravenous bio-distribution study in mice has demonstrated that the BAdV vector genome persists longer and with higher copy numbers in the spleen and lungs than that of the HAdV vector.12 Similar bio-distribution following the IM route of immunization may be responsible for the higher level of CMI responses with the BAd-H5HA vector. Additional studies will be required to determine the mechanism(s) of these observations.
The HK/156 HA expressed in the AdV vectors, and the HA in the challenged virus VN/1203/RG are antigenically different, so it was not surprising that the serum samples from either BAd-H5HA- or HAd-H5HA-inoculated animal groups did not have detectable levels of HI or VN titers against the challenge virus. We purposely opted for antigenically distinct HAs as an immunogen and a challenge virus to test our hypothesis that a combination of CMI responses and non-neutralizing antibodies will provide heterologous protection. The mouse group inoculated IM with 3 × 107 PFUs of BAd-H5HA were completely protected from the challenge with VN/1203/RG, whereas detectable levels (0.84 ± 0.5 log) of lung virus titers were observed in the mouse group IM-inoculated with 3 × 108 PFUs of HAd-H5HA. These observations suggest that significantly higher levels of CMI responses and non-neutralizing antibodies elicited with BAd-H5HA may be responsible for enhanced protection at a significantly lower dose.
In the mouse groups inoculated IN with BAd-H5HA, levels of serum anti-HA IgG, IgG1, IgG2a, and IgG2b, and levels of mucosal anti-HA IgA were significantly higher than the groups inoculated IM with BAd-H5HA or inoculated IM or IN with HAd-H5HA. Also, the numbers of IFNγ-secreting HA518-specific CD8 T cells in the spleen, lung, and RLNs were much higher in BAd-H5HA groups than the HAd-H5HA groups. Additional experiments are required to determine whether the high levels of humoral (systemic and mucosal) and CMI responses are due to better transduction and/or the levels and duration of persistence of the BAdV vector genomes following immunization with BAd-H5HA. Because BAdV-3 utilizes the α(2,3)-linked and α(2,6)-linked sialic acid receptors as the major receptors for virus internalization,15 whereas HAdV-C5 uses CAR32, 33, 34 for virus entry in the susceptible cells, we expected that BAd-H5HA will better transduce the respiratory tract following IN inoculation compared with HAd-H5HA. Moreover, the levels and duration of persistence of the BAdV vector genome copy numbers in the lungs were found to be significantly higher than that of the HAdV vector in an intravenous bio-distribution study.12 There is a possibility that a similar situation may occur following the IN inoculation.
Complete protection from challenge with an antigenically distinct H5N1 influenza virus VN/1203/RG was observed even in the mouse group inoculated IN with 1 × 106 PFUs of BAd-H5HA, whereas a similar level of protection with HAd-H5HA was obtained only with a much higher vector dose of 3 × 107 PFUs. These observations suggest that BAdV vector-based IN vaccine delivery system has considerable promise for dose sparing because an approximately 30-fold lower vaccine dose of BAd-H5HA compared with HAd-H5HA conferred complete protection. Dose sparing not only lowers vaccine costs, but also increases the capacity to produce a large number of doses especially in an event similar to the influenza pandemic. Of course, additional studies will be needed to determine the long-term efficacy of cross-protective humoral and CMI responses induced by BAdV-3-based vectors.
The results described in this manuscript suggest that the BAdV-3-based vector system has many advantages over HAdV systems as a vaccine delivery vehicle for developing pre-pandemic influenza vaccines. Further studies in another animal model of influenza, such as ferrets, will be essential to fully explore the potential of this vaccine delivery system. Additional studies will be required to determine the best combinational antigens or immunogenic domains that could elicit broadly cross-protective immune responses when delivered through the BAdV vector system for developing a universal influenza vaccine for pandemic preparedness and for offering a better vaccine option against seasonal influenza viruses.
Materials and Methods
Cell Lines, AdV Vectors, and Influenza Viruses
BHH3 (bovine-human hybrid clone 3),35 BHH2C (bovine-human hybrid clone 2C),35 293 (human embryonic kidney cells expressing HAdV-C5 E1 proteins),36 and MDCK (Madin-Darby canine kidney) cell lines were propagated as monolayer cultures in minimum essential medium (MEM) (Life Technologies from Thermo Fisher Scientific, Waltham, MA, USA) containing either 10% reconstituted fetal bovine serum or fetal calf serum (Hyclone from Thermo Fisher Scientific) and gentamycin (50 μg/mL).
The construction and characterization of BAd-H5HA [BAdV-3 E1 and E3 deleted vector expressing HA of A/Hong Kong/156/97(H5N1) (HK/156)],10 BAd-ΔE1E3 (BAdV-3 E1 and E3 deleted empty vector),11 HAd-H5HA [HAdV-C5 E1 and E3 deleted vector expressing HA of HK/156],25 and HAd-ΔE1E3 (HAdV-C5 E1 and E3 deleted empty vector)37 have been described previously. BAd-H5HA and BAd-ΔE1E3 were grown and titrated in BHH3 cells as described previously,10 and HAd-H5HA and HAd-ΔE1E3 were grown in 293 cells and titrated in BHH2C cells as described previously.17 These vectors were purified by cesium chloride density gradient ultracentrifugation as described previously.38
A/Vietnam/1203/2004(H5N1)-PR8/CDC-RG [VN/1203/RG] that was created by reverse genetics (RG) in the A/PR/8/1934(H1N1) [PR8] background with a deletion in the polybasic cleavage site of the HA gene segment was grown in embryonated hen eggs and titrated in the eggs and/or MDCK. The HA gene in the vaccine vectors was from HK/156, which is antigenically distinct from the HA gene in the challenge virus VN/1203/RG.
Immunogenicity and Protection Studies in Mice
All animal experiments were conducted following the approvals and guidelines of the Institutional Animal Care and Use Committee (IACUC) and the Institutional Biosafety Committee (IBC). All immunization and protection studies in mice were performed in a US Department of Agriculture (USDA)-approved BSL-2+ facility with the approvals of the IACUC and the IBC. Six-to-eight-week-old BALB/c mice (Harlan Sprague Dawley, Indianapolis, IN, USA) served as the subjects for immunization and protection studies following approved guidelines.
The mouse groups (10 animals/group) were mock inoculated with PBS (pH 7.2) or inoculated IN or IM with 1 × 106, 3 × 106, 1 × 107, 3 × 107, or 1 × 108 PFUs of BAd-H5HA or HAd-H5HA. The mouse groups inoculated IN or IM with 1 × 108 PFUs of BAd-ΔE1E3 or HAd-ΔE1E3 served as vector controls. Four weeks post-inoculation, five animals per group were anesthetized with 50 μL ketamine-xylazine solution (90 mg/kg ketamine and 10 mg/kg xylazine in PBS) by intraperitoneal injections, the blood samples were collected via retro-orbital puncture, nasal washes were collected by washing the nasal passage with 0.5 mL of PBS, and the lung washes were prepared after homogenizing one lung from each animal in 1 mL of PBS as described previously.39 The serum samples, nasal washes, and lung washes were used to evaluate the humoral immune responses. The second lung lobe was processed to collect CD3+ T cells from the lung cells using MagniSort Mouse CD3 Positive Selection Kit following the manufacturer’s instructions (Affymetrix eBioscience, San Diego, CA, USA) and used to monitor CMI responses. The spleens, RLNs, and ILNs were also collected to evaluate CMI responses.
The remaining five animals per group were challenged IN with 100 MID50 of VN/1203/RG. Three days post-challenge, the animals were euthanized under ketamine-xylazine anesthesia as described earlier, and the lungs were collected for determination of the lung virus titers as described previously.17
Immunoblotting
HA expression levels in BHH3 cells infected with either HAd-H5HA or BAd-H5HA were determined by immunoblotting following a previously described protocol.10, 40 An H5 HA-specific polyclonal rabbit antibody was used in immunoblotting.
ELISA
ELISA was performed as described earlier.41, 42 96-well ELISA plates (eBioscience) were coated with purified HA protein (0.5 μg/mL) of HK/156 (MyBioSource, San Diego, CA, USA) and incubated overnight at 4°C. Following blocking with 1% BSA in PBS, diluted serum samples (1:500 dilution for IgG and IgG1 and 1:50 for IgG2a and IgG2b), 1:5 diluted nasal washes, or 1:10 diluted lung washes were added and incubated at room temperature for 2 hr. During the standardization process, various dilutions of each type of sample were tested to establish the best dilution. The horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b, or IgA antibodies (Invitrogen from Thermo Fisher Scientific) at a recommended dilution for each antibody was added and incubated at room temperature for 2 hr. The color development was obtained with a BD OptEIA ELISA TMB substrate (Thermo Fisher Scientific). The reaction was stopped with a 2N sulfuric acid solution, and the optical density readings were obtained at 450 nm using a SpectraMax i3x microplate reader (Molecular Devices, Sunnyvale, CA, USA).
ELISpot Assays
The ELISpot assays were performed as described previously.25 The splenocytes, lung lymphocytes, RLNs, and ILNs were used for IFNγ ELISpot assays after stimulating the cells with HA518 (IYSTVASSL) peptide (H-2Kd-restricted CTL epitope for HA). The number of spot-forming units (SFUs) was counted using AID ELiSpot reader 8.0 (Autoimmun Diagnostika, Germany).
Statistical Analyses
One- and two-way ANOVAs with Bonferroni post-test were performed to determine statistical significance. The statistical significance was set at p < 0.05.
Author Contributions
Conceptualization, S.K.M., S.S.; Data Curation, E.E.S., A.O.H., R.K., S.V.V, W.C.; Formal Analysis, E.E.S., A.O.H., S.S., S.K.M.; Funding Acquisition, S.K.M., S.S, I.Y.; Investigation, E.E.S., A.O.H., R.K., W.C., I.Y., S.G., S.S., S.K.M.; Methodology, E.E.S., A.O.H., R.K., W.C.; Project Administration, S.K.M., S.S.; Resources, S.K.M., S.S., I.Y., S.G.; Supervision, S.K.M., S.S.; Validation, E.E.S., A.O.H., R.K., W.C., I.Y., S.G., S.S., S.K.M.; Writing – Original Draft: E.E.S., S.K.M.; Writing – Review and Editing: E.E.S., R.K., A.O.H., W.C., I.Y., S.G., S.S., S.K.M.
Conflicts of Interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. None of the authors have any conflicts of interest.
Acknowledgments
This work was supported by Public Health Service grant AI059374 from the National Institute of Allergy and Infectious Diseases and by the Hatch Funds. We thank Donna Schrader and Sara Penka for their assistance in preparing the manuscript. E.E.S. was supported by an Egyptian Government Scholarship for PhD studies at Purdue University and was on study leave from the College of Veterinary Medicine, Benha University.
Contributor Information
Suryaprakash Sambhara, Email: ssambhara@cdc.gov.
Suresh K. Mittal, Email: mittal@purdue.edu.
References
- 1.Vemula S.V., Mittal S.K. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin. Biol. Ther. 2010;10:1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahi Y.S., Bangari D.S., Mittal S.K. Adenoviral vector immunity: its implications and circumvention strategies. Curr. Gene Ther. 2011;11:307–320. doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J., Huang X., Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A., Tandon M., Ahi Y.S., Bangari D.S., Vemulapalli R., Mittal S.K. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010;17:634–642. doi: 10.1038/gt.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangari D.S., Mittal S.K. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Kostense S., Koudstaal W., Sprangers M., Weverling G.J., Penders G., Helmus N., Vogels R., Bakker M., Berkhout B., Havenga M., Goudsmit J. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 7.Nwanegbo E., Vardas E., Gao W., Whittle H., Sun H., Rowe D., Robbins P.D., Gambotto A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangari D.S., Mittal S.K. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal S.K., Ahi Y.S., Vemula S.V. Xenogenic adenoviral vectors A2. In: Curiel D.T., editor. Adenoviral Vectors for Gene Therapy. Second Edition. Academic Press; 2016. pp. 495–528. [Google Scholar]
- 10.Singh N., Pandey A., Jayashankar L., Mittal S.K. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol. Ther. 2008;16:965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangari D.S., Shukla S., Mittal S.K. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem. Biophys. Res. Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A., Bangari D.S., Tandon M., Pandey A., HogenEsch H., Mittal S.K. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 2009;386:44–54. doi: 10.1016/j.virol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A., Bangari D.S., Vemula S.V., Mittal S.K. Persistence and the state of bovine and porcine adenoviral vector genomes in human and nonhuman cell lines. Virus Res. 2011;161:181–187. doi: 10.1016/j.virusres.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangari D.S., Sharma A., Mittal S.K. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem. Biophys. Res. Commun. 2005;331:1478–1484. doi: 10.1016/j.bbrc.2005.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Bangari D.S., Sharma A., Mittal S.K. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology. 2009;392:162–168. doi: 10.1016/j.virol.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoelscher M.A., Singh N., Garg S., Jayashankar L., Veguilla V., Pandey A., Matsuoka Y., Katz J.M., Donis R., Mittal S.K., Sambhara S. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J. Infect. Dis. 2008;197:1185–1188. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vemula S.V., Ahi Y.S., Swaim A.M., Katz J.M., Donis R., Sambhara S., Mittal S.K. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS ONE. 2013;8:e62496. doi: 10.1371/journal.pone.0062496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gene Therapy Clinical Trials Worldwide. (2017). Vectors used in gene therapy clinical trials. http://www.abedia.com/wiley/vectors.php.
- 19.Gurwith M., Lock M., Taylor E.M., Ishioka G., Alexander J., Mayall T., Ervin J.E., Greenberg R.N., Strout C., Treanor J.J. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect. Dis. 2013;13:238–250. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osman M., Mistry A., Keding A., Gabe R., Cook E., Forrester S., Wiggins R., Di Marco S., Colloca S., Siani L. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017;11:e0005527. doi: 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesonen S., Kangasniemi L., Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol. Pharm. 2011;8:12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 22.Lusky M. Good manufacturing practice production of adenoviral vectors for clinical trials. Hum. Gene Ther. 2005;16:281–291. doi: 10.1089/hum.2005.16.281. [DOI] [PubMed] [Google Scholar]
- 23.Dormond E., Perrier M., Kamen A. From the first to the third generation adenoviral vector: what parameters are governing the production yield? Biotechnol. Adv. 2009;27:133–144. doi: 10.1016/j.biotechadv.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Silva A.C., Fernandes P., Sousa M.F.Q., Alves P.M. Scalable production of adenovirus vectors. In: Chillón M., Bosch A., editors. Volume 1089. Humana Press; 2014. pp. 175–196. (Adenovirus. Methods in Molecular Biology (Methods and Protocols)). [DOI] [PubMed] [Google Scholar]
- 25.Hoelscher M.A., Garg S., Bangari D.S., Belser J.A., Lu X., Stephenson I., Bright R.A., Katz J.M., Mittal S.K., Sambhara S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoelscher M.A., Jayashankar L., Garg S., Veguilla V., Lu X., Singh N., Katz J.M., Mittal S.K., Sambhara S. New pre-pandemic influenza vaccines: an egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin. Pharmacol. Ther. 2007;82:665–671. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vemula S.V., Amen O., Katz J.M., Donis R., Sambhara S., Mittal S.K. Beta-defensin 2 enhances immunogenicity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res. 2013;178:398–403. doi: 10.1016/j.virusres.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan A.O., Amen O., Sayedahmed E.E., Vemula S.V., Amoah S., York I., Gangappa S., Sambhara S., Mittal S.K. Adenovirus vector-based multi-epitope vaccine provides partial protection against H5, H7, and H9 avian influenza viruses. PLoS ONE. 2017;12:e0186244. doi: 10.1371/journal.pone.0186244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas P.G., Keating R., Hulse-Post D.J., Doherty P.C. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao W., Soloff A.C., Lu X., Montecalvo A., Nguyen D.C., Matsuoka Y., Robbins P.D., Swayne D.E., Donis R.O., Katz J.M. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 2006;80:1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Kampen K.R., Shi Z., Gao P., Zhang J., Foster K.W., Chen D.T., Marks D., Elmets C.A., Tang D.C. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Bergelson J.M. Adenovirus receptors. J. Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonberg-Holm K., Crowell R.L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976;259:679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- 34.Bergelson J.M., Cunningham J.A., Droguett G., Kurt-Jones E.A., Krithivas A., Hong J.S., Horwitz M.S., Crowell R.L., Finberg R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 35.van Olphen A.L., Mittal S.K. Development and characterization of bovine x human hybrid cell lines that efficiently support the replication of both wild-type bovine and human adenoviruses and those with E1 deleted. J. Virol. 2002;76:5882–5892. doi: 10.1128/JVI.76.12.5882-5892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 37.Noblitt L.W., Bangari D.S., Shukla S., Knapp D.W., Mohammed S., Kinch M.S., Mittal S.K. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 38.Pandey A., Singh N., Vemula S.V., Couëtil L., Katz J.M., Donis R., Sambhara S., Mittal S.K. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS ONE. 2012;7:e33428. doi: 10.1371/journal.pone.0033428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papp Z., Middleton D.M., Mittal S.K., Babiuk L.A., Baca-Estrada M.E. Mucosal immunization with recombinant adenoviruses: induction of immunity and protection of cotton rats against respiratory bovine herpesvirus type 1 infection. J. Gen. Virol. 1997;78:2933–2943. doi: 10.1099/0022-1317-78-11-2933. [DOI] [PubMed] [Google Scholar]
- 40.van Olphen A.L., Tikoo S.K., Mittal S.K. Characterization of bovine adenovirus type 3 E1 proteins and isolation of E1-expressing cell lines. Virology. 2002;295:108–118. doi: 10.1006/viro.2002.1389. [DOI] [PubMed] [Google Scholar]
- 41.Mittal S.K., Middleton D.M., Tikoo S.K., Babiuk L.A. Pathogenesis and immunogenicity of bovine adenovirus type 3 in cotton rats (Sigmodon hispidus) Virology. 1995;213:131–139. doi: 10.1006/viro.1995.1553. [DOI] [PubMed] [Google Scholar]
- 42.Mittal S.K., Aggarwal N., Sailaja G., van Olphen A., HogenEsch H., North A., Hays J., Moffatt S. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine. 2000;19:253–263. doi: 10.1016/s0264-410x(00)00170-5. [DOI] [PubMed] [Google Scholar]