Abstract
Multinodular and Vacuolating Neuronal Tumor (MVNT) has been included in the most recent (2016) World Health Organization Classification of Tumors of the Central Nervous System as unique cytoarchitectural pattern of gangliocytoma. We present a case of a MVNT incidentally discovered in a 22-year old male, who presented with seizures after a head injury. Conventional MRI revealed a left parietal lesion with characteristic tiny, coalescent, well-defined, non-enhancing nodules, located in the juxtacortical white matter with partial involvement of an otherwise normal adjacent cortex and characterized by slight relative increase of the cerebral blood volume (CBV), compared to the contralateral white matter (lesional CBV/contralateral CBV = 1.112) and mild increase of choline and reduction of NAA (lesional choline/creatine ratio =1.36 and choline/NAA ratio=0.77, compared to 0.87 and 0.51, respectively). The patient fully responded to treatment with phenytoin and a follow-up MRI, six months later, showed the lesion without any substantial difference.
Keywords: Multinodular and vacuolating neuronal tumor, Magnetic resonance imaging, Diffusion-weighted imaging, Perfusion imaging, Magnetic resonance spectroscopy
Introduction
Multinodular and vacuolating neuronal tumor (MVNT) has been included in the most recent (2016) World Health Organization Classification of Tumors of the Central Nervous System as a unique cytoarchitectural pattern of gangliocytoma, although it remains unclear whether it is a true neoplastic process or a dysplastic hamartomatous/malformative lesion [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. Magnetic resonance imaging (MRI) findings are rather characteristic showing multiple, tiny, discrete or coalescent, sharply marginated, round or ovoid, nonenhancing nodules, located within the deep cortical ribbon and the superficial white matter, without remarkable mass effect [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. We present a case of a patient with a MVNT tumor discovered incidentally after head injury and discuss its morphologic, diffusion, hemodynamic, and metabolic properties.
Case report
A 22-year-old male presented with a 2-month history of seizures, started immediately after a head injury. His previous history was seizure free. Neurologic evaluation, along with electroencephalogram, indicated left temporal epilepsy. Conventional MRI revealed a left parietal lesion comprised of tiny, coalescent, well-defined nodules, hyperintense on T2 and FLAIR, and hypointense on T1 sequences, located in the juxtacortical white matter with partial involvement of an otherwise normal adjacent cortex. The lesion was characterized by increased diffusivity and lack of hemorrhagic elements or gadolinium enhancement (Fig. 1). Dynamic susceptibility contrast MR perfusion revealed a slight relative increase of the lesional cerebral blood volume (CBV) compared to the contralateral white matter (lesional CBV/contralateral CBV = 1.112; Fig. 2). MR spectroscopy showed mild increase of choline and reduction of NAA leading to increase in choline/creatine ratio (1.36) and choline/NAA ratio (0.77), compared to 0.87 and 0.51 (respective values of the contralateral brain)(Fig. 3). Furthermore, head CT, at the time of the traumatic insult, had revealed a left temporal hemorrhagic contusion, which appeared as a gliotic area with hemorrhagic deposition on the subsequent conventional MRI and considered to be responsible for the epileptic episodes (Fig. 4). The patient fully responded to treatment with phenytoin and has been seizure free ever since. Six months later a follow-up MRI was performed and showed the left parietal lesion, without any substantial structural, diffusion, hemodynamic, or metabolic changes (Fig. 5). A written informed consent was obtained from the patient after being briefed on study and publication details.
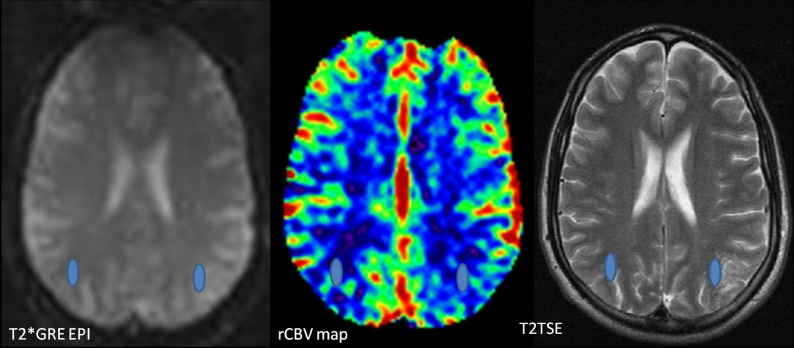
Fig. 1.
Conventional MRI revealed a left juxtacortical parietal lesion, with partial involvement of the adjacent cortex, which comprised of tiny, coalescent, well-defined nodules, hyperintense on T2 and FLAIR and hypointense on T1 sequences, with increased diffusivity and lack of gadolinium enhancement.
Fig. 2.
For the T2* DSC-perfusion MRI a 2D single-shot multislice Gradient Echo Echo Planar Imaging (GREEPI) sequence was used. Relative CBV values of the lesion and the contralateral white matter were estimated by using the T2TSE images for coregistration with the GRE-EPI images. The ROIs were placed at the bolus peak of the GRE-EPI images, to avoid vascular structures, and automatically transferred to the CBV map, using dedicated software (NordicIce, NordicNeuroLab, Bergen, Norway).
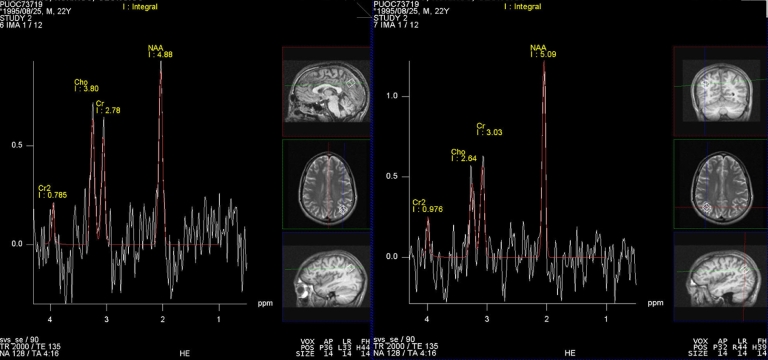
Fig. 3.
MR spectroscopy showed mild increase of choline/creatine ratio (1.36) and choline/NAA ratio (0.77) comparing to 0.87 and 0.51 (respective values of the contralateral brain).
Fig. 4.
A post traumatic head CT had revealed a left temporal hemorrhagic contusion. Two months later the same lesion appeared hyperintense on FLAIR, with hypointense foci on GRE, indicating of a gliotic area with chronic hemorrhagic elements deposition.
Fig. 5.
A follow-up MRI was performed 6 months later and revealed the left parietal lesion with identical imaging characteristics on T2 and T1 sequences, before and after Gadolinium administration, GRE sequence and ADC map.
Discussion
MNVTs are rare entities that have been recently recognized, by WHO, as distinctive neuronal lesions, although they were first reported by Huse et al, in 10 patients, in 2013 [1]. Since then, 51 additional cases have been reported worldwide [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], including a case series of 33 patients by Nunes et al [9]. MVNT is frequently associated with seizures or seizure equivalents and occur mainly in adults older than 35 years. It presents as a cluster of nodules, normally numerous and of small size, located juxtacortically on the inner surface of an otherwise normal-appearing cortex and most frequently on the parietal or temporal lobe. Gadolinium enhancement, restricted diffusion, diffuse infiltration, mass effect, or edema is not normally observed [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11].
Histologically, MVNT consists of several, frequently numerous, discrete and well-demarcated nodules, found in the cerebral hemispheres, mainly within the deep cortical ribbon and superficial subcortical white matter. The lesion originates from ganglion cells, which normally present dysplastic and vacuolated, oriented perpendicular to the cortical surface. Extracellular vacuolation is also common. Occasionally, the neuronal cells group together in a perivascular arrangement. Hypomyelination of the lesions may be observed and the adjacent brain parenchyma appears normal or astrogliotic. Expression of the HuC/HuD and Olig2 neuronal antigens is typical, indicating neurogenesis at an earlier stage of neuronal development. Labeling with conventional tumor immunomarkers has not been successful in distinguishing the lesion, which is considered to represent a benign, dysplastic early neuronal immunophenotype, favoring a malformating lesion over a neoplasm [1], [2], [3], [4], [8], [9], [10].
Conventional MRI is the primary imaging modality for the diagnosis of MVNT and reveals highly characteristic imaging features. The juxtacortical, coalescent, tiny, nonenhancing nodules appear hypo/isotense on T1w and hyperintense on T2w and FLAIR sequences, indicating a high protein or solid component within the vacuolated lesions. The absence of cortical involvement and prominent mass effect and the intra- or perilesional tumor nodules support the diagnosis of MVNT, instead of focal cortical dysplasia, or dysembryoplastic neuroepithelial tumor (DNET), while hyperintensity on FLAIR excludes the possibility of enlarged perivascular spaces. In accordance to our case, in 3 reported cases that employed MR spectroscopy a relatively increased choline/NAA peak ratio has, also, been found [1], [3], [4], [11]. In 2 reported cases that used perfusion MRI MVNTs are characterized by no increase or even decreased CBV values, respectively [6], [10].
In our patient, radiological investigation included conventional MR sequences, along with diffusion-weighted imaging (DWI), DSC-MR perfusion imaging and MR spectroscopy. The tumor displayed increased diffusivity, compatible with the lack of mitotic activity in most of the reported cases, and the impression of diminished cellularity of the nodules compared to adjacent white matter [9]. Perfusion imaging revealed only a slight increase in cerebral blood volume (CBV), reflecting the absence of substantial microvascular proliferation [6]. Finally, MR spectroscopy showed a mild increase of choline/creatine and choline/NAA ratio, compatible with the disorganization of tissue architecture and the congregation of dysplastic immature cells [1], [2], [3], [4], [8], [9], [10].
In the case series of Nunes et al [9], 81 % of MNVT presented as incidental findings on MRI in asymptomatic patients and considered to be “leave me alone” lesions, which usually do not require biopsy or resection. In the majority of symptomatic cases surgical treatment proved to be therapeutic. Anticonvulsant treatment may be used in addition to surgery, in case of persistent symptoms, with good results. In our patient, the characteristic imaging features of the left parietal lesion, combined with the history of post traumatic temporal epilepsy led to the diagnosis of an incidental MTVN. Our patient received only anticonvulsant treatment with phenytoin and has been asymptomatic at the follow-up, with identical MRI findings 6 months after the initial diagnosis.
Conclusion
MNVT have been recently recognized as a distinct neuronal lesion, although its malformative or neoplastic origin is still debatable. Most of the reported MNVTs were found incidentally in asymptomatic patients with typical imaging features and need no further surgical biopsy or resection. To our knowledge, this is the first case employing different nonconventional MRI techniques to delineate the diffusion, hemodynamic and metabolic properties of this lesion, and further support its benign nature.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.07.016.
Appendix. Supplementary materials
References
- 1.Huse JT, Edgar M, Halliday J, Mikolaenko I, Lavi E, Rosenblum MK. Multinodular and vacuolating neuronal tumors of the cerebrum: 10 cases of a distinctive seizure-associated lesion. Brain Pathol. 2013;23(5):515–524. doi: 10.1111/bpa.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodi I, Curran O, Selway R, Elwes R, Burrone J, Laxton R. Two cases of multinodular and vacuolating neuronal tumour. Acta Neuropathol Commun. 2014;20(2):7. doi: 10.1186/2051-5960-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukushima S, Yoshida A, Narita Y, Arita H, Ohno M, Miyakita Y. Multinodular and vacuolating neuronal tumor of the cerebrum. Brain Tumor Pathol. 2015;32(2):131–136. doi: 10.1007/s10014-014-0198-9. [DOI] [PubMed] [Google Scholar]
- 4.Nagaishi M, Yokoo H, Nobusawa S, Fujii Y, Sugiura Y, Suzuki R. Localized overexpression of alpha-internexin within nodules in multinodular and vacuolating neuronal tumors. Neuropathology. Dec 2015;35(6):561–568. doi: 10.1111/neup.12217. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi M, Komori T, Nakata Y, Yagishita A, Morino M, Isozaki E. Multinodular and vacuolating neuronal tumor affecting amygdala and hippocampus: A quasi-tumor? Pathol Int. 2016;66(1):34–41. doi: 10.1111/pin.12366. [DOI] [PubMed] [Google Scholar]
- 6.Cathcart SJ, Klug JR, Helvey JT, White ML, Gard AP, McComb RD. Multinodular and vacuolating neuronal tumor. A rare seizure-associated entity. Am J Surg Pathol. 2017;41:1005–1010. doi: 10.1097/PAS.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 7.Lobo R, Srinivasan A. Case of the season: multinodular and vacuolating neuronal tumor. Semin Roentgenol. 2018;53:3–5. doi: 10.1053/j.ro.2017.11.001. org/ [DOI] [PubMed] [Google Scholar]
- 8.Thom M, Liu J, Bongaarts A, Reinten RJ, Paradiso B, Jäger HR. Multinodular and vacuolating neuronal tumors in epilepsy: dysplasia or neoplasia. Brain Pathol. 2018;28:155–171. doi: 10.1111/bpa.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes RH, Hsu CC, da Rocha AJ, do Amaral LLF, Godoy LFS, Watkins TW. Multinodular and vacuolating neuronal tumor of the cerebrum: a new “leave me alone” lesion with a characteristic imaging pattern. AJNR Am J Neuroradiol. 2017;38(10):1899–1904. doi: 10.3174/ajnr.A5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Quarante LH, Ruiz-Juretschke F, Sola Vendrell E, Gil de Sagredo Del Corral OL, Agarwal V, Garcia-Leal R. Multinodular and vacuolating neuronal tumor of the cerebrum. A rare entity. New case and review of the literature. Neurocirugia (Astur) 2018;29(1):44–55. doi: 10.1016/j.neucir.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Badat N, Savatovsky J, Charbonneaue F, Collin A, Lecler A. Multinodular vauolating and neuronal tumor of the cerebrum. Neurology. 2017;89:304–305. doi: 10.1212/WNL.0000000000004123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.