Abstract
In naïve cells, the endoplasmic reticulum (ER) and the ER-resident Vesicle-associated membrane protein-Associated Proteins (VAP) are common components of sites of membrane contacts that mediate the nonvesicular transfer of lipids between organelles. There is increasing recognition that the hijacking of VAP by intracellular pathogens is a novel mechanism of host–pathogen interaction. Here, we summarize our recent findings showing that the Chlamydia inclusion membrane protein IncV tethers the ER to the inclusion membrane by binding to VAP via the molecular mimicry of two eukaryotic FFAT motifs. We extend the discussion to other microorganisms that have evolved similar mechanisms.
Keywords: Chlamydia, Coxiella, RNA virus, inclusion membrane protein, IncV, FFAT
Commentary to: Murray, R., Flora, E., Bayne, C., & Derré, I. (2017). IncV, a FFAT motif-containing Chlamydia protein, tethers the endoplasmic reticulum to the pathogen-containing vacuole. Proceedings of the National Academy of Sciences of the United States of America, 114(45), 12039–12044. doi:10.1073/pnas.1709060114.
In naïve cells, the nonvesicular transfer of lipids occurs at sites of membrane contact between the endoplasmic reticulum (ER) and various organelles. Several intracellular vacuolar pathogens were recently shown to hijack functional and structural proteins of these sites of membrane contact to establish direct contact with the ER and promote their intracellular replication. The best characterized example, so far, is Chlamydia trachomatis.
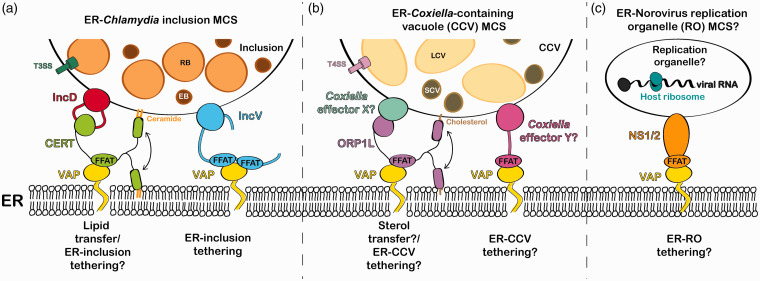
C. trachomatis is an obligate intracellular bacterium that establishes its intracellular niche within a membrane-bound vacuole called the inclusion. The bacteria use a type III secretion system to deliver effector Inc proteins into the inclusion membrane and mediate the interaction of the inclusion with cellular factors and organelles (Moore & Ouellette, 2014). Our lab has uncovered the role of Inc proteins in the formation of direct contact between the inclusion membrane and the ER (i.e., ER-Inclusion Membrane Contact Sites, MCS; Derré, Swiss, & Agaisse, 2011; Murray, Flora, Bayne, & Derré, 2017). Our original study showed that, at ER-Inclusion MCS, the Inc protein IncD recruits the host CERamide Transfer protein (CERT), which interacts with the ER-resident Vesicle-associated membrane protein-Associated Proteins (VAP; Figure 1(a)).
Figure 1.
Models of VAP-dependent ER contact with pathogen-containing vacuoles. (a) The Chlamydia Inc protein IncD interacts with the host ceramide transfer protein CERT which interacts with ER-resident protein VAP via a FFAT motif. The IncD–CERT–VAP complex may facilitate ceramide transfer to the inclusion membrane. The Chlamydia Inc protein IncV interacts with VAP through the molecular mimicry of FFAT motifs and tethers the ER to the inclusion. (b) ORP1L is recruited to the Coxiella-containing vacuole (CCV) and interacts with VAP via a FFAT motif. Unidentified Coxiella effector proteins could mediate ORP1L recruitment to the CCV and VAP-dependent ER-CCV tethering by FFAT motif mimicry. (c) The norovirus nonstructural protein NS1/2 interacts with VAP by mimicking a FFAT motif, which could mediate the formation of sites of membrane contact between the ER and the viral replication organelle. RB = Chlamydia reticulate body; EB = Chlamydia elementary body; T3SS = type III secretion system; LCV = Coxiella large cell variant; SCV = Coxiella small cell variant; T4SS = type IV secretion system.
Depletion of CERT and VAP led to decreased bacterial replication; therefore, it was proposed that the IncD-CERT–VAP complex plays a role in the acquisition of host lipids essential for bacterial development to the inclusion (Derré et al., 2011; Elwell et al., 2011).
In Murray et al. (2017), we recently showed that the Inc protein IncV interacts with VAP via mimicry of two FFAT (two phenylalanines [FF] in an Acidic Tract) motifs conserved in the eukaryotic proteins that interact with VAP (Figure 1(a)). FFAT motifs are defined as a core of seven amino acids flanked by regions enriched in acidic residues called the acidic tract (Murphy & Levine, 2016). Each position in the core motif tolerates substitutions, except for Position 2, which must be a phenylanine or tyrosine residue. We identified two FFAT motif cores in IncV and showed that IncV interacts with wild-type VAP but not with a FFAT-binding mutant of VAP. In agreement with the notion that the IncV–VAP interaction is mediated by IncV FFAT motifs, mutating the amino acids at Position 2 of both IncV FFAT motif cores to alanine residues disrupted the IncV–VAP interaction. Our results are also consistent with a role of the IncV–VAP complex in ER tethering, since overexpression of IncV in the inclusion membrane or ectopic targeting of IncV to the plasma membrane dramatically increased both VAP and ER recruitment to these membranes, in a FFAT motif-dependent manner. Altogether, our data demonstrate that the IncV–VAP interaction is sufficient to drive the formation of ER-Inclusion MCS and suggest that IncV tethering properties are independent of the membrane environment of the inclusion membrane.
Interestingly, IncV FFAT motifs contain the core motif, but not the typical acidic tract found in eukaryotic proteins, which is thought to provide an initial, non-specific electrostatic interaction with the electropositive groove of VAP before the core motif binds stably in a hydrophobic pocket (Furuita, Jee, Fukada, Mishima, & Kojima, 2010). In IncV, the FFAT motif cores are flanked by serine-rich regions, which could potentially be phosphorylated to mimic acidic tracts. Dynamic phosphorylation may offer temporal regulation of the assembly/disassembly of ER-Inclusion MCS. Finally, although an incV mutant displayed reduced VAP recruitment to the inclusion, it retained the ability to form ER-inclusion MCS, suggesting that there are redundant mechanisms to bring the ER in close contact with the inclusion. It is tempting to speculate that the IncD–CERT–VAP complex could also act as a tether and that the general mechanism of ER-Inclusion MCS formation relies on the direct and indirect recruitment of VAP by Chlamydia Inc proteins IncV and IncD, respectively.
The hijacking of cellular machinery present at sites of membrane contact extends to another obligate intracellular bacteria: Coxiella burnetii. The Coxiella-containing vacuole (CCV) also contacts the ER (ER-CCV MCS), though the mechanism mediating the formation of these contacts remains unknown (Justis et al., 2017; Figure 1(b)). Interestingly, these ER-CCV MCS are enriched in Oxysterol-binding protein Related Protein-1 Long variant (ORP1L), a FFAT motif-containing sterol transfer protein enriched at ER-endosome sites of membrane contact through its interaction with VAP (Rocha et al., 2009). Like C. trachomatis, C. burnetii encodes a secretion system, which is required for the formation of ER-CCV MCS. Thus, a C. burnetti effector protein might mediate the recruitment of ORP1L to the CCV. In that case, ORP1L could transfer cholesterol from the ER to the CCV, analogous to the CERT-dependent ceramide transfer at C. trachomatis ER-Inclusion MCS. C. burnetii might also encode FFAT motif-containing effector(s), like the Chlamydia Inc protein IncV, that could play a role in tethering the ER to the CCV.
Viruses have also been shown to hijack VAP. The RNA virus norovirus is replication defective in VAP knockout cells suggesting that the ER is required for norovirus replication (Mccune et al., 2017). Although noroviruses form membranous replication organelle (RO) near the ER, it is unclear whether these membranes directly contact the ER. Intriguingly, as described for C. trachomatis IncV, the norovirus-encoded nonstructural protein NS1/2 binds to VAP via a FFAT motif mimic (Figure 1(c)). It would be interesting to determine if the NS1/2–VAP interaction is used by norovirus to tether the ER to the RO. Remarkably, Aichi virus RO forms VAP-dependent sites of membrane contact with ER (Ishikawa-Sasaki, Nagashima, Taniguchi, & Sasaki, 2018) and points of contact between the Golgi-derived rhinovirus RO and the ER are enriched in VAP and FFAT motif-containing lipid transfer proteins (Roulin et al., 2014). However, the viral proteins involved in these processes remain unknown. Finally, viral proteins from hepatitis C and Tomato bushy stunt viruses also interact with VAP to promote viral replication, though the interactions seem to occur via FFAT-independent mechanisms (Nagy, Strating, & van Kuppeveld, 2016).
Altogether, the examples presented here demonstrate the importance of sites of membrane contact for the development of the intracellular replication niche of bacteria and viruses and reinforce the notion that VAP hijacking could be a common pathogenesis-promoting strategy through the direct interaction of microbial protein displaying FFAT motifs or indirect interaction by recruiting cellular VAP-interacting proteins. These microbes also provide unique model systems in which to study the nuances of FFAT motif binding to VAP, which could shed light on the structural and functional properties of cellular components involved in VAP-dependent cellular processes.
Acknowledgments
The authors thank Dr. Hervé Agaisse, Dr. Maria Eugenia Cortina, Rachel Ende, and R. Clayton Bishop for their critical reading of this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH Grants R01AI101441 (to I. D.) and T32AI007046 (to R. S.).
References
- Derré I., Swiss R., Agaisse H. (2011). The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathogens, 7(6). doi:10.1371/journal.ppat.1002092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell C. A., Jiang S., Kim J. H., Lee A., Wittmann T., Hanada K., Engel J. N. (2011). Chlamydia trachomatis co-opts gbf1 and cert to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathogens, 7(9). doi:10.1371/journal.ppat.1002198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuita K., Jee J., Fukada H., Mishima M., Kojima C. (2010). Electrostatic interaction between oxysterol-binding protein and VAMP-associated protein a revealed by NMR and mutagenesis studies. Journal of Biological Chemistry, 285(17), 12961–12970. doi:10.1074/jbc.M109.082602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Sasaki K., Nagashima S., Taniguchi K., Sasaki J. (2018). A model of OSBP-mediated cholesterol supply to Aichi virus RNA replication sites involving protein-protein interactions among viral proteins, ACBD3, OSBP, VAP-A/B, and SAC1. Journal of Virology, 92(8). doi:10.1128/JVI.01952-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justis A. V., Hansen B., Beare P. A., King K. B., Heinzen R. A., Gilk S. D. (2017). Interactions between the Coxiella burnetii parasitophorous vacuole and the endoplasmic reticulum involve the host protein ORP1L. Cellular Microbiology, 19(1). doi:10.1111/cmi.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccune B. T., Tang W., Lu J., Eaglesham J. B., Thorne L., Krezel A. M., Virgin W. (2017). Noroviruses co-opt the function of host proteins VAPA and VAPB for replication via a phenylalanine–phenylalanine-acidic-tract-motif mimic in nonstructural viral protein NS1/2. mBio, 8(4), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. R., Ouellette S. P. (2014). Reconceptualizing the chlamydial inclusion as a pathogen-specified parasitic organelle: An expanded role for Inc proteins. Frontiers in Cellular and Infection Microbiology, 4(October), 1–10. doi:10.3389/fcimb.2014.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. E., Levine T. P. (2016). VAP, a versatile access point for the endoplasmic reticulum: Review and analysis of FFAT-like motifs in the VAPome. Biochimica et Biophysica Acta – Molecular and Cell Biology of Lipids, 1861(8), 952–961. doi:10.1016/j.bbalip.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Murray R., Flora E., Bayne C., Derré I. (2017). IncV, a FFAT motif-containing Chlamydia protein, tethers the endoplasmic reticulum to the pathogen-containing vacuole. Proceedings of the National Academy of Sciences, 114(45), 12039–12044. doi:10.1073/pnas.1709060114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P. D., Strating J. R. P. M., van Kuppeveld F. J. M. (2016). Building viral replication organelles: Close encounters of the membrane types. PLoS Pathogens, 12(10), 6–11. doi:10.1371/journal.ppat.1005912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha N., Kuijl C., Van Der Kant R., Janssen L., Houben D., Janssen H., Neefjes J. (2009). Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150Glued and late endosome positioning. Journal of Cell Biology, 185(7), 1209–1225. doi:10.1083/jcb.200811005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulin P. S., Lötzerich M., Torta F., Tanner L. B., Van Kuppeveld F. J. M., Wenk M. R., Greber U. F. (2014). Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host and Microbe, 16(5), 677–690. doi:10.1016/j.chom.2014.10.003 [DOI] [PubMed] [Google Scholar]