Abstract
Childhood ADHD is associated with impairments in peer, family, and academic functioning. Although impairment is required for diagnosis, children with ADHD vary significantly in the areas in which they demonstrate clinically significant impairment. However, relatively little is known about the mechanisms and processes underlying these individual differences. The current study examined neurocognitive predictors of heterogeneity in peer, family, and academic functioning in a well-defined sample of 44 children with ADHD ages 8–13 (M = 10.31, SD = 1.42; 31 boys, 13 girls; 81% Caucasian). Reliable change analysis indicated that 98% of the sample demonstrated objectively defined impairment on at least one assessed outcome measure; 65% were impaired in two or all three areas of functioning. ADHD children with quantifiable deficits in academic success and family functioning performed worse on tests of working memory (d = 0.68 to 1.09), whereas children with impaired parent-reported social functioning demonstrated slower processing speed (d = 0.53). Dimensional analyses identified additional predictors of peer, family, and academic functioning. Working memory abilities were associated with individual differences in all three functional domains, processing speed predicted social functioning, and inhibitory control predicted family functioning. The current results add to a growing literature implicating neurocognitive abilities not only in explaining behavioral differences between ADHD and non-ADHD groups, but also in the substantial heterogeneity in ecologically valid functional outcomes associated with the disorder.
Keywords: ADHD, social, family, academic, functioning, heterogeneity
Attention-deficit/hyperactivity disorder (ADHD) is a complex, chronic, and heterogeneous disorder of brain, behavior, and cognition that affects approximately 5% of school-aged children (American Psychiatric Association [APA], 2013; Polanczyk, Willcutt, Salum, Kieling, & Rohde, 2014), at an annual cost of illness of $42 billion in the U.S. (Pelham, Foster, & Robb, 2007). Although long treated as error variance, heterogeneity in symptoms and impairments are being increasingly recognized as important considerations for refining our understanding of ADHD pathogenesis and improving treatment outcomes (Kofler et al., 2013; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005). To this end, a growing body of research has identified factors associated with within-group heterogeneity in ADHD behavioral symptom presentation, including demographic characteristics such as gender (Gaub & Carlson, 1997) and age (Halperin, Trampush, Miller, Marks, & Newcorn, 2008), informant and setting factors (Valo & Tannock, 2010; Whalen et al., 1978), medical and behavioral treatment (van der Oord, Prins, Oosterlaan, & Emmelkamp, 2008), and neurocognitive abilities (Chhabildas, Pennington, & Willcutt, 2001; Halperin et al., 2008; Nigg, Blaskey, Huang-Pollock, & Rappley, 2002; Sarver, Rapport, Kofler, Raiker, & Friedman, 2015). In contrast, relatively less is known about the mechanisms and processes associated with heterogeneity in daily functioning among children with ADHD. This relative paucity of research is surprising given that functional impairments may be better predictors of long-term clinical outcomes than core ADHD behavioral symptoms (Pelham, Fabiano, & Massetti, 2005). The goal of the current study is to examine factors associated with heterogeneity in peer, family, and academic functioning in a well-defined sample of children with ADHD, with a particular focus on neurocognitive abilities that (a) are also characterized by significant within-group heterogeneity among children with ADHD (Fair, Bathula, Nikolas, & Nigg, 2012; Rajendran, O’Neill, Marks, & Halperin, 2015), and (b) have been shown previously to help explain the disorder’s behavioral symptoms and functional deficits relative to typically developing children at the group level (Rapport, Orban, Kofler, & Friedman, 2013).
Childhood ADHD is associated most frequently with impairments in three primary areas: peer, family, and academic functioning (Pelham et al., 2005). Interestingly, although impairment is required for diagnosis (APA, 2013), children with ADHD vary significantly in the areas in which they demonstrate clinically significant impairment. For example, an estimated 50% to 80% of children with ADHD exhibit peer relational (social) problems (de Boo & Prins, 2007; Huang-Pollock, Mikami, Pfiffner, & McBurnett, 2009). Stated differently, these figures suggest that approximately 20% to 50% of these children are not viewed as experiencing clinically significant social problems. Similarly, rates of academic underachievement and learning difficulties are estimated to occur in 33% to 63% of children with ADHD across academic domains (Mayes & Calhoun, 2006). Impaired family functioning occurs in 62% to 87% of cases based on meta-analysis (Theule, Wiener, Tannock, & Jenkins, 2013) , and includes a variety of difficulties involving parental perceptions of lower attachment, warmth, and connectedness in the parent-child relationship (Keown & Woodward, 2002), impaired parent-child communication (Cussen, Sciberras, Ukoumunne, & Efron, 2012; Keown & Woodward, 2002), and lower levels of parental confidence (Johnston & Mash, 2001) and parental involvement (Rogers, Wiener, Marton, & Tannock, 2009). Collectively, the significant variation in impairment rates among children with ADHD highlights the heterogeneity in functional consequences for these children and underscores the importance of understanding predictors of cross-domain impairment risk. However, with the exceptions reviewed below, relatively little is known about the mechanisms and processes underlying this heterogeneity (Nigg, 2005). This gap in turn constrains our ability to understand and ultimately predict the extent to which individual children with ADHD are likely to develop impairments in each functional area.
Neurocognitive heterogeneity is a particularly appealing candidate to explain functional heterogeneity among children with ADHD for at least three reasons. First, the neurocognitive functions implicated in ADHD have been linked developmentally with a wide array of academic (Barry, Lyman, & Klinger, 2002; Thorell, 2007) and social/peer outcomes (Clark, Prior, & Kinsella, 2002; Dennis, Brotman, Huang, & Gouley, 2007; Holmes, Kim-Spoon, & Deater-Deckard, 2016). For example, developmental research suggests strong links between children’s working memory abilities and their social (Alloway et al., 2005) and academic functioning (Thorell, 2007). In particular, phonological working memory shows strong cross-sectional and longitudinal continuity with academic success in reading (Cain, Oakhill, & Bryant, 2004; Sarver et al., 2012; Sesma, Mahone, Levine, Eason, & Cutting, 2009), whereas visuospatial working memory may predict math productivity better than phonological working memory (Maybery & Do, 2003; Sarver et al., 2012). Similarly, inhibition has been linked with social functioning (Gewirtz, Stanton-Chapman, & Reeve, 2009; Nigg, 1999) as well as math (Thorell, 2007; Wåhlstedt, Thorell, & Bohlin, 2009), English and science achievement (St. Clair-Thompson & Gathercole, 2006), and processing speed predicts academic performance in reading, math, and written expression in non-ADHD samples (Mayes & Calhoun, 2007).
Second, clinical research suggests that many but not all children with ADHD have deficits in any given aspect of neurocognitive functioning. For example, meta-analytic effect sizes indicate that up to 80% of children with ADHD may exhibit working memory deficits (Kasper, Alderson, & Hudec, 2012), approximately 0% to 38% have inhibition deficits (Alderson, Rapport, & Kofler, 2007; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), and 41% to 45% demonstrate slowed processing speed (Frazier, Demaree, & Youngstrom, 2004; Kofler et al., 2013) based on comparison to typically developing groups (Zakzanis, 2001)1. In comparison, approximately 27% to 47% underperform on IQ tests (Frazier et al., 2004) that depend on these executive and other cognitive functions (Dennis et al., 2009). Within-group methods are generally consistent with meta-analytic estimates, and suggest clusters of children with ADHD that differ according to the specific test battery administered. For example, extant studies have identified ADHD subgroups according to working memory storage/rehearsal (short-term memory), processing speed and variability, temporal processing, and arousal (Fair et al., 2012), combinations of short-term memory, vigilance, set shifting, inhibition, and visual-motor integration (Biederman et al., 2004), or choice impulsivity, inhibition, and temporal processing, but not short-term memory (Sonuga-Barke, Bitsakou, & Thompson, 2010).
Third, experimental studies suggest that specific neurocognitive functions may account for ADHD-related deficits in behavioral symptoms and at least some aspects of functioning at the group level. Much of this research has focused on working memory, and suggests that experimentally increasing working memory demands evokes differential decreases in objectively-measured attentive behavior (Kofler, Rapport, Bolden, Sarver, & Raiker, 2010) and increases in gross motor activity (hyperactivity; Rapport et al., 2009; Kofler, Sarver, & Wells, in press) for ADHD relative to typically developing groups. In addition, cross-sectional mediation models suggest minimal differences between ADHD and typically developing groups with regard to impulsive responding (Raiker, Rapport, Kofler, & Sarver, 2012), inhibitory control (Alderson, Rapport, Hudec, Sarver, & Kofler, 2010), delay aversion (Patros et al., 2015), and response variability (Kofler et al., 2014) after accounting for working memory. Importantly, working memory deficits also appear important for explaining between-group differences in social functioning (Bunford et al., 2015; Kofler et al., 2011) and math performance (Antonini et al., 2016), suggesting an important role of this cognitive ability in ecologically valid, functional outcomes for children with ADHD. To our knowledge, however, no studies of childhood ADHD have simultaneously examined the role of multiple neurocognitive functions (e.g., inhibition, processing speed) in explaining impairments in social or family functioning.
To summarize, the impetus for examining the link between neurocognitive abilities and functional heterogeneity in ADHD comes from converging lines of research indicating that (a) neurocognitive abilities predict important functional outcomes in non-ADHD samples (Holmes et al., 2016; Thorell, 2007), (b) many but not all children with ADHD exhibit deficits in specific neurocognitive abilities and each area of functioning (Pelham et al., 2005; Rapport et al., 2013), and (c) neurocognitive deficits may explain behavioral and functional impairments in ADHD at the group level (Chacko, Kofler, & Jarrett, 2014).
To this end, emerging evidence suggests that neurocognitive abilities may help explain heterogeneity in social (Miller & Hinshaw, 2010), academic (Biederman et al., 2004; Preston, Heaton, McCann, Watson, & Selke, 2009), and global functioning (Cheung et al., 2015) among children and adolescents with ADHD. Specifically, individual differences in IQ among children with ADHD predict their reading, math (Alloway & Stein, 2014), and spelling success (Preston et al., 2009). Beyond this most general cognitive estimate (Dennis et al., 2009), individual differences in working memory components predict concurrent reading, math, and overall academic achievement (Alloway & Stein, 2014; Mayes & Calhoun, 2007; Rogers, Hwang, Toplak, Weiss, & Tannock, 2011) and longitudinally predict their reading abilities into young adulthood (Miller, Nevado-Montenegro, & Hinshaw, 2012). Similarly, children with ADHD with faster processing speed show higher attainment in reading (Jacobson et al., 2011), math, and written expression (Mayes & Calhoun, 2007). Further, subgroups of children with ADHD defined by the quantity of their neurocognitive deficits differ in academic attainment and grade retention (Biederman et al., 2004). In contrast, to our knowledge no ADHD study has examined the extent to which individual differences in behavioral inhibition predict academic heterogeneity, examined the relation between neurocognitive task performance and family functioning, or simultaneously examined the impact of multiple neurocognitive abilities on functional outcomes.
The current study is the first to examine neurocognitive predictors of heterogeneity in academic, peer, and family functioning among children with ADHD, while also considering several known risk factors and correlates of academic and social difficulties in ADHD such as age, socioeconomic status, ADHD subtype/presentation, medication status, and gender. We selected global cognitive functioning (IQ) and four primary neurocognitive functions – phonological working memory, visuospatial working memory, behavioral inhibition, and processing speed – given the large bodies of research on these abilities in ADHD and developmental evidence linking each with one or more of the functional outcomes as described above. We predicted that a majority of children with ADHD would exhibit quantifiable, objectively defined deficits in each area of functional impairment (peer, family, academic), and that children with deficits in each area would demonstrate identifiable neurocognitive profiles. We expected dimensional analyses to be consistent with these between-group findings (functional impairment vs. no impairment), such that working memory abilities would predict individual differences in social problems (Bunford et al., 2015; Kofler et al., 2011), and each of the neurocognitive constructs would predict individual differences in academic functioning given the developmental and clinical findings reviewed above. No predictions regarding family functioning were offered due to the paucity of research.
Method
Participants
The sample comprised 44 children aged 8 to 13 years (M = 10.31, SD = 1.42; 31 boys, 13 girls) from the Southeastern United States, who were consecutive referrals to a children’s learning clinic (CLC) through community resources for a psychoeducational assessment and participation in a behavioral (N=37) or cognitive training (N=7) treatment study. Pre-treatment data was used in the current study. Working memory performance data was reported for a subset of the current sample in Kofler et al. (in press) to examine conceptually unrelated hypotheses. Psychoeducational evaluations were provided to the parents of all participants. All parents and children gave informed consent/assent, and the university’s Institutional Review Board approved the study prior to the onset of data collection.
Group Assignment
All children and their parents participated in a detailed, semi-structured clinical interview using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS; Kaufman et al., 1997). The K-SADS (2013 Update) assesses onset, course, duration, severity, and impairment of current and past episodes of psychopathology in children and adolescents based on DSM-5 criteria. Its psychometric properties are well established, including inter-rater agreement of .93 to 1.00, test-retest reliability of .63 to 1.00, and concurrent (criterion) validity between the K-SADS and psychometrically established parent rating scales (Kaufman et al., 1997).
K-SADS interviews were supplemented with parent and teacher ratings scales from the Behavior Assessment System for Children (BASC-2; Reynolds & Kamphaus, 2004) and Child Symptom Inventory (CSI-IV; Gadow, Sprafkin, & Salisbury, 2004). Children with any ADHD subtype/ presentation were eligible given evidence of the instability of ADHD subtypes (Valo & Tannock, 2010) and previous research implicating neurocognitive processes in both inattentive (Kofler et al., 2010) and hyperactive (Rapport et al., 2009) symptom clusters.
Forty-four children met the following criteria and were included in the ADHD group: (1) an independent diagnosis by the CLC’s directing clinical psychologist using DSM-5 criteria for ADHD based on K-SADS interviews; (2) parent ratings of at least 1.5 SDs above the mean on the Attention Problems and/or Hyperactivity clinical syndrome scales of the BASC-2 parent form, or exceeding the criterion score for the parent version of the ADHD-Inattentive and/or ADHD-Hyperactive/Impulsive subscales of the CSI; and (3) teacher ratings of at least 1.5 SDs above the mean on the Attention Problems and/or Hyperactivity clinical syndrome scales of the BASC-2 teacher form, or exceeding the criterion score for the teacher version of the ADHD-Inattentive and/or ADHD-Hyperactive/Impulsive subscales of the CSI. Four children with ADHD failed to meet the teacher cut-off criteria, likely due to behavior well controlled on medication. In these cases, previous psychoeducational evaluations were available that documented cross-setting behavioral symptoms and impairment. In accordance with DSM-5, all children had current impairments based on K-SADS parent interview.
Of the 44 children with ADHD (13 girls), 18 met “AND” criteria for Combined, 23 for Inattentive, and 3 for Hyperactive/Impulsive Presentation. The “AND” criteria required the child to meet symptom thresholds based on both parent and teacher report (Willcutt et al., 2012). For example, the Combined presentation was specified for children who met/exceeded symptom thresholds for both Inattentive and Hyperactive/Impulsive symptom clusters for both informants. To improve generalizability (Wilens et al., 2002), children with comorbidities were included. Comorbidities reflect clinical consensus best estimates based on parent and child K-SADS interviews, child psychoeducational testing, and multiple parent, child, and teacher norm-referenced questionnaires. In all cases, K-SADS interview indicated that the onset of ADHD symptoms preceded the onset of comorbid symptoms, and that the child’s inattention and/or hyperactive/impulsive symptoms could not be better accounted for by the comorbid condition. Comorbidities included oppositional defiant disorder (11%), depressive disorders (16%), and anxiety disorders (18%). None of the children screened positive for specific learning disorders in reading, math, or oral language based on DSM-5-recommended standard scores > 1.5 SD below the normative sample mean (APA, 2013, p. 69) on the Kaufman Test of Educational Achievement, Second or Third Edition (age norms; Kaufman & Kaufman, 2004, 2014); one child screened positive for deficits in written language. Child race/ethnicity included Caucasian non-Hispanic (81%), Hispanic English-speaking (7%), Asian (5%), African American (2%), and mixed racial/ethnic (5%) backgrounds.
Children were excluded from the study if they presented with (a) gross neurological, sensory, or motor impairment, (b) history of a seizure disorder, (c) psychosis, (d) autism spectrum disorder, (e) FSIQ score less than 80, or (f) non-stimulant medications that could not be withheld for testing. Twenty-two of the 44 children with ADHD were currently prescribed psychostimulants; medication was withheld for a minimum of 24 hr prior to both research testing sessions given evidence of psychostimulant effects on processing speed and other non-executive aspects of neurocognitive task performance (cf. Rapport et al., 2013).
Procedures
All children participated in two consecutive Saturday testing sessions following the baseline psychoeducational assessment. Neurocognitive tasks were administered as part of a larger battery of laboratory tasks that required the child’s presence for approximately 3 hr per session. All tasks were counterbalanced across testing sessions to minimize order effects. Children were seated in a caster-wheel swivel chair approximately 0.66 meters from the computer monitor for all tasks. Performance was monitored at all times by the examiner, who was stationed just out of the child’s view to provide a structured setting while minimizing performance improvements associated with examiner demand characteristics (Gomez & Sanson, 1994). All children received brief (2–3 min) breaks after each task, and preset longer (10–15 min) breaks after every 2–3 tasks to minimize fatigue.
Neurocognitive Performance
Phonological and visuospatial working memory.
The phonological and visuospatial working memory tasks developed by Rapport et al. (2008) were used for the current study. Previous studies of ADHD and typically developing children indicate large magnitude differences in these tasks (Kofler et al., 2014; Patros et al., 2015; Rapport et al., 2008), and performance on these tasks predicts ADHD-related impairments in objectively-measured activity level (Rapport et al., 2009), attentive behavior (Kofler et al., 2010), impulsivity (Raiker et al., 2012; Patros et al., 2015), inhibitory control (Alderson et al., 2010), and social dysfunction (Kofler et al., 2011). Evidence for reliability and validity of these working memory tasks includes high internal consistency (α = 0.82 to 0.97), 1–3 week test-retest reliability of .76 to .90 (Sarver et al., 2015), and demonstration of the expected magnitude of relations (Swanson & Kim, 2007) with established measures of short-term memory (Raiker et al., 2012). Six trials were administered at each set size (3, 4, 5, or 6 stimuli) based on re-analysis of data demonstrating that all 6-trial versions correlate ≥ .90 with the corresponding 12-trial versions reported in Kofler et al. (in press). The 24 total trials (6 trials at each set size) were randomized, and then grouped into 2 blocks of 12 trials each, such that the stimulus set size for a given trial was not predictable based on the preceding trial. Mixed presentation was selected given evidence that it results in higher central executive working memory demands due to memory set unpredictability relative to sequential presentation (Conway et al., 2005; Kofler et al., in press). Five practice trials were administered before each working memory task; children were required to achieve 80% correct before advancing to the full task. Children received short breaks between each 12-trial block (approximately 1–2 min). Task duration was approximately 2.5 (visuospatial) to 3.5 (phonological) minutes per block for the phonological and visuospatial tasks described below.
Phonological (PH) working memory task.
The phonological working memory task is similar to the Letter-Number Sequencing subtest on the Wechsler Intelligence Scale for Children-Fifth Edition (WISC-V; Wechsler, 2014), and assesses phonological working memory based on Baddeley’s (2007) model. Children were presented a series of jumbled numbers and a letter at a rate of 1 stimuli/second. The letter was never presented in the first or last position of the sequence to minimize potential primacy and recency effects, and was counterbalanced across trials to appear an equal number of times in the other serial positions (i.e., position 2, 3, 4, or 5). Children were instructed to recall the numbers in order from smallest to largest, and to say the letter last (e.g., 4 H 6 2 is correctly recalled as 2 4 6 H). Two trained research assistants, shielded from the participant’s view, recorded oral responses independently (interrater reliability was 99.50%).
Visuospatial (VS) working memory task.
Children were shown nine squares arranged in three offset vertical columns on a computer monitor. The columns were offset from a standard 3×3 grid to minimize the likelihood of phonological coding of the stimuli (e.g., by equating the squares to numbers on a telephone pad). A series of 2.5 cm diameter dots (3, 4, 5, or 6) were presented sequentially in one of the nine squares during each trial such that no two dots appeared in the same square on a given trial. All but one dot was black; the exception being a red dot that never appeared as the first or last stimulus in the sequence. Each dot was displayed for 800 ms followed by a 200 ms interstimulus interval. Children were instructed to respond by pressing the corresponding squares on a modified computer keyboard, and to re-order the dot locations by indicating the serial position of the black dots in the order presented followed by the serial position of the red dot last.
Dependent variables: Working memory task performance.
Performance data were collected for each trial for each participant. The randomized trials were collated during post-processing to allow estimation of performance at each stimulus set size (3, 4, 5, 6). Partial-credit unit scoring (i.e., stimuli correct per trial) was used to index overall working memory performance at each set size as recommended (Conway et al., 2005).
Behavioral inhibition and processing speed.
Stop-signal task.
The stop-signal task and administration instructions are identical to those described in Schachar et al. (2000) and Alderson, Rapport, Sarver, and Kofler, (2008). Psychometric evidence includes high internal consistency and 3-week test-retest reliability (.72), as well as convergent validity with other inhibitory control measures (Soreni, Crosbie, Ickowicz, & Schachar, 2009). Go-stimuli are displayed for 1000 ms as uppercase letters X and O positioned in the center of a computer screen (500 ms interstimulus interval; total trial duration = 1500 ms). Xs and Os appear with equal frequency throughout the experimental blocks. A 1000 Hz auditory tone (i.e., stop-stimulus) is presented randomly on 25% of trials. Stop-signal delay (SSD) – the latency between presentation of go- and stop-stimuli – is initially set at 250 ms, and dynamically adjusted ± 50 ms contingent on participant performance. Successfully inhibited stop-trials are followed by a 50 ms increase in SSD, and unsuccessfully inhibited stop-trials are followed by a 50 ms decrease in SSD. The algorithm is designed to approximate successful inhibition on 50% of the stop-trials. In the current study, inhibition success was 53.9%, 54.3%, 51.0%, and 51.3% across the four experimental blocks. All participants completed two practice blocks and four consecutive experimental blocks of 32 trials per block (24 go-trials, 8 stop-trials per block).
Dependent variables: Inhibition.
Stop-signal delay (SSD) at each of the four blocks served as the primary indices of behavioral inhibition. SSD was selected based on conclusions from recent meta-analytic reviews that SSD was the most direct measure of behavioral inhibition in stop-signal tasks that utilize dynamic stop-signal delays, given that SSDs change systematically according to inhibitory success or failure (Alderson et al., 2007; Ilieva, Hook, & Farah, 2015; Lijffijt, Kenemans, Verbaten, & van Engeland, 2005).
Dependent variables: Processing speed.
Mean choice reaction time (MRT) to correct go trials during each of the four stop signal blocks served as the primary indices of processing speed. Anticipatory responses (RTs < 150 ms) were excluded as recommended (e.g., Schmiedek, Oberauer, Wilhelm, Süß, & Wittmann, 2007).
Global Intellectual Functioning (IQ).
All children were administered the Wechsler Abbreviated Scales of Intelligence-Second Edition (WASI-II; Wechsler, 2011; n = 35), WISC-IV (Wechsler, 2003; n = 2), or WISC-V (Wechsler, 2014; n = 7) to obtain an overall estimate of intellectual functioning. Full Scale IQ (FSIQ) was not analyzed because FSIQ performance depends heavily on the neurocognitive constructs described above (Ackerman, Beier, & Boyle, 2005; Dennis et al., 2009). Following Rapport et al. (2008) and Kofler et al. (2013), we computed a residual FSIQ score by covarying the working memory, inhibition, and processing speed factor scores, described below, out of FSIQ (R2 = .30, p = .006). This residual FSIQ score represents cognitive functions important for IQ test performance other than these neurocognitive constructs, and was examined as a potential predictor in the analyses described below. Importantly, our method of removing the influence of working memory from IQ assumes that working memory influences IQ rather than vice versa. This assumption is based on a large and compelling cognitive literature showing working memory as an important predictor of global IQ (cf. Engle et al., 1999; Giofre et al., 2013; Tourva et al., 2016), and specific developmental evidence that age-related improvements in working memory lead directly to improvements in IQ (Tourva et al., 2016). Thus, we propose that it is reasonable to conclude that the shared variance between working memory and IQ is, in large part, attributable to working memory’s influence on IQ rather than vice versa.
We considered using the General Ability Index (GAI) rather than FSIQresidual given the conceptual interpretation of GAI as an IQ estimate that is free of the influence of working memory and processing speed (Wechsler, 2014). However, the construct validity of this interpretation appears limited. Specifically, the WISC-V Technical and Interpretive Manual (Table 5.1, page 74) indicates that GAI correlates .61 with WMI, indicating significant influence of working memory on this ‘process free’ estimate (the WMI-FSIQ correlation of .72 is similarly high, despite interpretive manual recommendation to conceptualize GAI as IQ without the influence of WM). Similarly, the WASI-2 FSIQ, which is conceptually GAI because it is comprised of the same VCI and PRI subtests used to calculate GAI on the WISC-IV, correlates .88 with the WISC-IV FSIQ (Wechsler, 2011, p. 131), again suggesting that the conceptual distinction between GAI and FSIQ is limited. We note also that according to the Wechsler manuals, GAI was not created or verified via factor analysis like FSIQ, but is rather a conceptually derived estimate (Wechsler, 2014, page 16). The statistical overlap between WMI and GAI suggests limited utility of this index for estimating process-free IQ abilities, and suggests that its raw inclusion in the model would result in removing significant variance attributable to working memory from working memory (cf. Dennis et al., 2007; Rapport et al., 2008).
Neurocognitive Dimension Reduction.
Control for the task impurity problem.
To address the task impurity problem pervasive within neurocognitive measurement (Snyder, Miyake, & Hankin, 2015), we used a dimension reduction approach to isolate reliable variance associated with each neurocognitive construct and approximate the removal of all random and task-specific, non-construct error (Conway et al., 2005). Because no task is process pure, generalizability of results requires experimenters to use multiple measures and create factor scores to estimate common variance associated with each construct (for review and specific examples, see Shipstead, Redick, & Engle, 2010). Following Kofler et al. (2013), this involved creating a factor score for each neurocognitive construct using a principal components factor analysis on the 16 neurocognitive performance variables (4 blocks each for PHWM, VSWM, SSD, and MRT; 78.01% of variance accounted for; construct-specific factor loadings r = .68 to .92; Supplementary Table 1). The ratio of participants (44) to factors (4) was deemed acceptable (Hogarty, Hines, Kromrey, Ferron, & Mumford, 2005). By design, the intercorrelations among the derived Phonological Working Memory, Visuospatial Working Memory, Behavioral Inhibition, and Processing Speed variables were rall = .00 (p > .99). Higher scores reflect better working memory and inhibition but slower processing speed.
Peer, Family, and Academic Functioning
Nationally standardized, psychometrically sound, and widely used instruments were used to obtain estimates of overall peer, family, and academic functioning. Parents and teachers were asked to consider the child’s behavior when off medication.
Peer (social) functioning.
The BASC-2 (Reynolds & Kamphaus, 2004) parent and teacher forms are 160- and 139-item scales, respectively, that assess internalizing and externalizing behavior problems in children ages 2–21. Raw scores are converted to age- and gender-specific T-scores based on the national standardization sample (N = 1,800 per form). The parent and teacher Social Skills subscales each contain 9 items that index children’s peer/social functioning (6-week test-retest = .84-.86; α = .87-.92). Parent and teacher social skills composite scores served as the primary indices of social functioning at home and school, respectively. Higher scores reflect better social functioning.
Family functioning.
The Parenting Relationship Questionnaire (PRQ; Kamphaus & Reynolds, 2006) is a 71-item parent report scale that assesses family functioning across seven domains (national standardization N = 4,130). T-scores are obtained for each factor according to age and gender; no total PRQ score is computed. Four subscales were selected that were thought to be most relevant to the parent-child relationship: Parent-Child Attachment, Parent-Child Communication, Parent-Child Involvement, and Parenting Confidence (4–5 week test-retest = .76-.84; α = .82-.88). Higher scores reflect better perceived attachment, involvement, communication, and parenting efficacy.
Academic functioning.
The Academic Performance Rating Scale (APRS; DuPaul, Rapport, & Perriello, 1991) was completed by each child’s teacher to assess academic functioning (2 week test-retest = .93-.95; α = .94-.95). The APRS contains subscales that reflect Academic Productivity and Academic Success. The Academic Productivity scale is comprised of 12 items that assess academic efficiency (e.g., percentage of classwork completed correctly) and consistency, following group instructions, and completing work in a timely manner. The Academic Success subscale contains seven items that assess the quality of reading and spoken work, how quickly children learn new material, and how well they retain new information. T-scores were obtained by comparing performance to the standardization sample (N = 487) according to age and gender. Higher scores reflect better academic functioning.
Socioeconomic Status
Socioeconomic status.
Socioeconomic status (SES) was estimated using the Hollingshead (1975) scoring based on caregiver(s)’ education and occupation.
Data Analysis Overview
The analytic plan was executed in two tiers. The first Tier examined functional heterogeneity in ADHD by quantifying the extent to which our sample exhibited impairments in each functional area relative to published age and gender norms, and examining between-group differences in neurocognitive abilities across Impaired vs. Not Impaired subgroups for each functional outcome. Following Sarver and colleagues (2015), this involved applying the Jacobson & Truax (1991) model of reliable change to each child’s norm-referenced scores on each of the peer, family, and academic outcomes. This method was selected over static cut points (e.g., 1 SD below the mean) because it improves precision by explicitly accounting for measurement unreliability (Jacobson & Truax, 1991). Children were classified as Impaired or Not Impaired on each functional outcome based on whether their norm-referenced score was reliably below the normative sample (i.e., difference exceeded chance at p < .05). This classification was based on computation of the Reliable Change Index (RCI), or the ratio of the difference between the child’s score and the test mean divided by standard error (computed using each measure’s reported test-retest reliability and the SD of the normative sample; Rule B; Jacobson & Truax, 1991) individually for each child for each outcome. Reported test-retest reliability across all tests/subscales was .76 to .95. The RCI is tested against the z distribution; impairment is defined as a score that is significantly worse than the test mean given the test’s SD and reported reliability. We then compared the neurocognitive performance of children defined as Impaired vs. Not Impaired on each functional outcome using bias-corrected, bootstrapped Cohen’s d effect sizes. Inspection of the RCI data indicated that the impairment cut-offs centered around 1 SD below the normative sample mean across measures; statistical significance was obtained at different cut points across measures dependent on each measure’s test-retest reliability (i.e., for tests with lower reliability, scores further from the mean were required to conclude with p < .05 certainty that the child’s score was more likely to come from the dysfunctional/impaired population than the functional population). To further probe individual differences in functioning and capitalize on the increased power of continuous vs. dichotomous variables, the second Tier used a dimensional approach to examine neurocognitive predictors of norm-referenced T-scores for peer, family, and academic functioning among children with ADHD.
Bootstrapping
All analyses were completed utilizing a bias-corrected bootstrapping procedure to minimize Type II error as recommended by Shrout and Bolger (2002). Bootstrapping is appropriate for total sample sizes as low as 20 (Efron & Tibshirani, 1993); the bias-corrected, bootstrapped 95% confidence intervals were used to estimate effect magnitude and determine statistical significance for all comparisons. SPSS version 22 was used for all analyses, and 10,000 samples were derived from the original sample (N = 44) by a process of resampling with replacement (Shrout & Bolger, 2002).
Results
Power Analysis
Given the relatively small sample size, we conducted a power analysis using GPower (v3.1; Faul, Erdfelder, Lang, & Buchner, 2007) to determine our sensitivity for detecting effects. The between-group analyses (Impaired vs. Not Impaired) are powered to detect large effects (d ≥ .80) based on our sample size of 44 for power = .80 and α = .05; we therefore report bias-corrected, bootstrapped Cohen’s d effect sizes and interpreted 95% confidence intervals rather than p-values given their robustness to distributional characteristics (Fritz & MacKinnon, 2007). Minimal guidance was available for a priori selection of expected effect sizes due to the paucity of studies defining heterogeneity based on functional outcomes rather than cognitive or behavioral symptoms in ADHD. However, effects of this magnitude were considered reasonable based on meta-analyses indicating large magnitude relations between ADHD and neurocognitive abilities (e.g., working memory; Kasper et al., 2012), and between ADHD and each area of functional impairment as reviewed above. In addition, we supplemented the between-group analyses with linear regression to capitalize on the increased power associated with continuous relative to dichotomous variables. Power analysis for regression indicated we are adequately powered to reliably detect effects of ρ2 = .27 for power = .80, α = .05, and 6 predictors (PHWM, VSWM, BI, PS, IQ, and 1 covariate as described below) based on our sample size of 44.
Preliminary Analyses
Means and SDs for each outcome variable are shown in Table 1. All variables were screened for univariate/multivariate outliers and tested against p < 0.001. No significant outliers were found. One-sample t-tests revealed that the BASC-2 parent and teacher Attention Problems scores (for both Combined and Inattentive presentations) and Hyperactivity scores (for the ADHD-Combined group) were significantly elevated relative to the scale’s T-score mean of 50 as expected (all p < .0005; Table 1). Age, SES, gender, ADHD subtype/presentation, and medication status were not significantly related to any of the peer, family, or academic outcomes (all 95% CI substantially overlap 0.0; all p ≥ .20), with the following exceptions: Child age was related to parent-child attachment (r = .38, 95% CI = .11 to .60, p = .02), communication (r = .42, 95% CI = .10 to .67, p = .009), and involvement (r = .47, 95% CI = .20 to .72, p = .002); SES was related to all teacher-reported outcomes including social functioning (r = .37, 95% CI = .09 to .58, p = .02), academic success (r = .40, 95% CI = .11 to .63, p = .01), and academic productivity (r = .30, 95% CI = .01 to .54, p = .07) , and medication status was related to parent involvement (r = −.31, 95% CI = −.01 to −.60, p = .06). These variables were therefore included as covariates in the models predicting outcomes with which they were correlated; all others reflect simple model results with no covariates.
Table 1.
Demographic, behavioral, neurocognitive, and functional outcome variables
| Variable | ADHD-Inattentive Presentation | ADHD-Combined/ Hyperactive Presentation | Overall Sample | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| N (Boys/Girls) | 23 (12/11) | 21 (19/2) | 44 (31/13) | |||
| Age | 10.71 | 1.52 | 9.87 | 1.18 | 10.31 | 1.42 |
| SES | 50.63 | 9.98 | 49.70 | 10.76 | 50.19 | 10.24 |
| FSIQ | 106.78 | 13.93 | 110.75 | 16.16 | 108.48 | 14.82 |
| BASC-2 Attention Problems (T-score) | ||||||
| Parent | 67.43 | 8.10 | 67.19 | 6.87 | 67.32 | 7.45 |
| Teacher | 63.61 | 8.16 | 61.14 | 8.59 | 62.43 | 8.36 |
| BASC-2 Hyperactivity (T-score) * | ||||||
| Parent * | 65.74 | 14.29 | 77.52 | 9.55 | 71.36 | 13.50 |
| Teacher * | 53.30 | 8.24 | 64.90 | 14.65 | 58.84 | 13.00 |
| Academic Performance Rating Scale (T-score) | ||||||
| Academic Success | 48.08 | 9.09 | 50.12 | 10.45 | 49.06 | 9.70 |
| Academic Productivity | 43.59 | 6.82 | 44.33 | 9.11 | 43.95 | 7.91 |
| BASC Social Functioning (T-score) | ||||||
| Parent | 41.91 | 8.38 | 41.67 | 9.15 | 41.80 | 8.66 |
| Teacher | 44.87 | 10.13 | 46.67 | 11.03 | 45.73 | 10.48 |
| PRQ Family Functioning (T-score) | ||||||
| Attachment | 49.32 | 7.56 | 44.33 | 11.20 | 47.08 | 9.58 |
| Communication | 41.14 | 11.43 | 38.00 | 13.08 | 39.73 | 12.14 |
| Involvement | 50.14 | 8.26 | 48.00 | 9.04 | 49.18 | 8.58 |
| Parenting Confidence | 45.05 | 8.33 | 43.39 | 7.65 | 44.30 | 7.97 |
| Working Memory (Stimuli Correct/Trial) | ||||||
| PH 3 | 2.92 | 0.12 | 2.89 | 0.22 | 2.91 | 0.17 |
| PH 4 | 3.39 | 0.72 | 3.42 | 0.51 | 3.40 | 0.62 |
| PH 5 | 3.28 | 1.23 | 3.46 | 1.06 | 3.37 | 1.15 |
| PH 6 | 2.70 | 1.37 | 2.89 | 1.55 | 2.79 | 1.44 |
| VS 3 | 2.31 | 0.54 | 2.08 | 0.61 | 2.20 | 0.58 |
| VS 4 | 2.83 | 0.70 | 2.39 | 0.97 | 2.62 | 0.86 |
| VS 5 | 2.73 | 0.95 | 2.53 | 1.23 | 2.63 | 1.08 |
| VS 6 | 2.41 | 1.17 | 2.21 | 1.45 | 2.32 | 1.30 |
| Inhibition and Processing Speed (Milliseconds) | ||||||
| SSD 1 | 245.38 | 78.75 | 267.43 | 74.78 | 258.13 | 76.14 |
| SSD 2 | 242.39 | 86.99 | 270.07 | 86.92 | 255.35 | 85.07 |
| SSD 3 | 247.28 | 80.92 | 255.26 | 86.33 | 253.04 | 81.52 |
| SSD 4 | 238.04 | 95.06 | 276.32 | 80.57 | 256.27 | 87.93 |
| MRT 1 | 585.83 | 96.34 | 602.06 | 99.49 | 591.31 | 95.63 |
| MRT 2 | 581.59 | 75.02 | 580.22 | 176.29 | 597.13 | 90.01 |
| MRT 3 | 584.72 | 113.38 | 614.93 | 77.62 | 599.61 | 97.04 |
| MRT 4 | 565.99 | 137.36 | 621.42 | 63.23 | 592.09 | 109.98 |
| Derived, ‘Process Pure’ Factor Scores | ||||||
| Behavioral Inhibition | −0.20 | 1.01 | 0.24 | 0.96 | 0.00 | 1.00 |
| Visuospatial Working Memory | 0.17 | 0.83 | −0.21 | 1.17 | 0.00 | 1.00 |
| Phonological Working Memory | −0.06 | 1.01 | 0.07 | 1.00 | 0.00 | 1.00 |
| Processing Speed | 0.02 | 0.91 | −0.02 | 1.12 | 0.00 | 1.00 |
| FSIQresidual | −1.66 | 12.13 | 2.13 | 13.53 | 0.00 | 12.74 |
Note.
= ADHD subtypes/presentations differ at p < .05. APRS = Academic Performance Rating Scale (T-scores); BASC-2 = Behavior Assessment System for Children (T-scores); PRQ = Parent Relationship Questionnaire (T-scores); FSIQ = Full Scale Intelligence Quotient (Standard Scores); PH = Phonological Working Memory (Stimuli Correct/Trial); VS = Visuospatial Working Memory (Stimuli Correct/Trial); MRT = Mean Response Time (milliseconds); SSD = Stop-signal Delay (milliseconds).
Tier 1. Functional heterogeneity subgroup classification
Descriptive statistics.
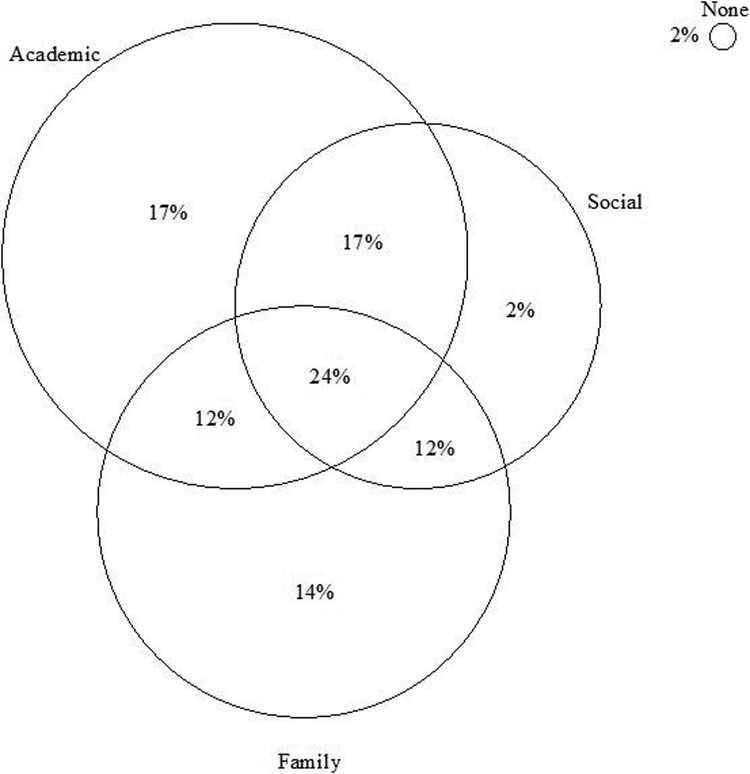
Following Sarver and colleagues (2015), each child was classified as Impaired or Not Impaired on each functional outcome using the Jacobson and Truax (1991) model of reliable change. As shown in Figure 1, the current sample displayed substantial heterogeneity in each functional outcome. Specifically, 98% (n = 43 of 44) of the sample displayed impairment in at least one measured domain, and 65% were impaired in two (41%) or all three domains (24%). The 2% characterized as Not Impaired reflected one participant who fell just below the criterion for academic impairment (z = 1.80, p = .07). Within functional domains, the proportion of ADHD children classified as Impaired was 70% for teacher-reported academic functioning, 62% for parent-reported family functioning, and 55% for teacher- or parent-reported social functioning.
Figure 1.
Visual heuristic showing the proportion of children with ADHD classified as Impaired in each functional area based on the Jacobson & Truax (1991) model of reliable change. Circle sizes are proportionate to the percentage of children identified as Impaired in each domain. Percentages are rounded to the nearest whole number.
Collectively, these descriptive analyses confirmed significant functional heterogeneity in the current sample that was similar to previous studies in terms of the proportion of children with ADHD classified as impaired in each domain (de Boo & Prins, 2007; Mayes & Calhoun, 2006). Of primary interest was the extent to which children with impairments in each functional domain demonstrated an identifiable neurocognitive profile. We therefore compared children defined as Impaired vs. Not Impaired on each functional outcome, and interpreted the bias-corrected, bootstrapped 95% confidence intervals of the Cohen’s d effect size for each between-group comparison as described above.
Academic functioning (teacher report).
Comparisons of Impaired vs. Not Impaired children revealed that children defined by impairments in academic success exhibited large magnitude phonological working memory deficits (d = 0.86, 95% CI = 0.28 to 1.44, p = .007). Similarly, children defined based on impairments in academic productivity demonstrated medium magnitude impairments in phonological working memory (d = 0.54, 95% CI = −0.02 to 1.10, p = .08); however, the possibility of no effect for this comparison remained due to the narrow inclusion of 0.0 in the confidence interval. We observed also a medium magnitude effect on global IQ for children with deficits in academic success (d = 0.57, 95% CI = −0.12 to 1.21, p = .09); however, the 95% confidence interval included 0.0, suggesting the possibility of no effect. Effect sizes were small-to-minimal for all other comparisons (all d ≤ 0.30, all 95% CIs centered around 0.0).
Social functioning (parent and teacher report).
Children defined as socially impaired based on parent report demonstrated slower processing speed (d = 0.53, 95% CI = 0.07 to 1.25, p = .04). In addition, children with parent-reported social impairment demonstrated medium magnitude impairments in phonological working memory (d = 0.53, 95% CI = −0.17 to 1.22, p = .12); however, the possibility of no effect for this comparison remained due to the narrow inclusion of 0.0 in the confidence interval. Children with teacher-defined social impairments showed small-to-medium deficits in inhibitory control (d = 0.44, 95% CI = −0.28 to 1.09, p = .20) and global IQ (d = 0.44, 95% CI = −0.18 to 1.06, p = .19) with 95% confidence intervals that leave open the possibility of no effect. Effect sizes were small-to-minimal for all other comparisons (all d ≤ 0.21, all 95% CIs centered around 0.0).
Family functioning (parent report).
Children defined based on impairments in parent-child attachment demonstrated large magnitude deficits in visuospatial working memory (d = 1.09, 95% CI = 0.47 to 1.66, p = .001). A medium magnitude effect size was noted also for phonological working memory (d = 0.45, 95% CI = −0.52 to 1.49, p = .34); however, the 95% confidence interval substantially overlapped 0.0, suggesting a high likelihood of no effect. Children whose parents reported significantly impaired parenting confidence demonstrated large magnitude visuospatial working memory deficits (d = 1.05, 95% CI = 0.01 to 2.07, p = .048) and medium magnitude deficits in phonological working memory (d = 0.68, 95% CI = 0.04 to 1.33, p = .04). Effect sizes were small-to-minimal for all other comparisons (all d ≤ 0.44, all 95% CIs centered around 0.0). No neurocognitive deficits were detected for children with impaired parent-child communication (all d ≤ 0.32, all 95% CIs centered around 0.0).
Tier 2. Dimensional analyses
Academic functioning (teacher report).
The bias-corrected, bootstrapped regression model was significant for academic success (R2 = .49, p < .005). Better-developed visuospatial working memory (partial R2 = .12, B = 3.01, 95% CI = 0.76 to 5.52, p = .03), phonological working memory (partial R2 = .24, B = 3.98, 95% CI = 1.89 to 6.35, p = .001), and IQ (partial R2 = .26, B = 0.32, 95% CI = 0.11 to 0.50, p = .002) predicted higher academic success. Processing speed showed similar relations with academic success (partial R2 = .11, B = 2.84, 95% CI = −0.19 to 5.68, p = .07); however, the possibility of no effect for this predictor remained due to the narrow inclusion of 0.0 in the confidence interval. Better-developed phonological working memory predicted higher academic productivity (partial R2 = .16, B = 3.17, 95% CI = 0.89 to 5.51, p = .01), but the omnibus test for academic productivity was nonsignificant (R2 = .21, p = .14). Inhibition did not predict either academic outcome (both p > .15, both 95% CI substantially overlap 0.0, both R2 < .05).
Social functioning (parent and teacher report).
The neurocognitive variables significantly predicted teacher-reported social functioning (R2 = .43, p = .005), such that better-developed visuospatial working memory (partial R2 = .12, B = 4.02, 95% CI = 1.05 to 7.00, p = .03) and processing speed (partial R2 = .16, B = 4.53, 95% CI = 1.18 to 8.34, p = .03) predicted better social functioning. Higher SES predicted better teacher-reported social functioning (partial R2 = .26, B = 0.56, 95% CI = 0.23 to 0.91, p = .002); inhibition, phonological working memory, and IQ did not predict teacher-reported social functioning (all 95% CIs centered around 0.0, all R2 < .04, all p > .29). In contrast, only phonological working memory predicted parent-reported social functioning (partial R2 = .11, B = 3.07, 95% CI = 0.17 to 6.42, p = .06), but the omnibus test for parent-reported social functioning was nonsignificant (R2 = .12, p = .44).
Family functioning (parent report).
The omnibus tests were significant for parenting confidence (R2 = .39, p = .007), parent-child attachment (R2 = .34, p = .04), and parental involvement (R2 = .46, p = .008). Better-developed inhibitory control predicted greater parent-reported attachment (partial R2 = .13, B = 3.02, 95% CI = 0.74 to 5.62, p = .03) and parenting confidence (partial R2 = .26, B = 4.24, 95% CI = 1.89 to 6.18, p < .0005). In addition, better-developed phonological working memory predicted greater parenting confidence (partial R2 = .20, B = 3.07, 95% CI = 0.37 to 5.38, p = .02). Older age predicted more difficulties with parent-child attachment (partial R2 = .15, B = 1.89, 95% CI = 0.34 to 3.22, p = .03) and parental involvement (partial R2 = .23, B = 2.50, 95% CI = 0.45 to 4.21, p = .01); psychostimulant medication predicted lower reported parent-child involvement (partial R2 = .21, B = 6.70, 95% CI = 0.81 to 12.72, p = .05). The omnibus test for parent-child communication was nonsignificant (R2 = .25, p = .18). Processing speed and visuospatial working memory failed to predict any family outcomes (all p > .12, all 95% CI substantially overlap 0.0, all R2 < .07).
Discussion
The current study was the first to examine neurocognitive predictors of heterogeneity in each of the three primary areas of functional impairment associated with ADHD (Pelham et al., 2005). Overall, results add to our understanding of individual differences in neurocognitive abilities among children with ADHD, and reveal that this variation appears to play important roles in peer, family, and academic functioning. Specifically, working memory abilities were associated with ADHD-related heterogeneity in all three functional domains, processing speed predicted teacher-reported social functioning, and inhibitory control predicted caregiver perceptions of family functioning. These findings were generally consistent with the developmental literature (Holmes et al., 2016) and previous comparisons of ADHD and typically developing groups (Chhabildas et al., 2001; Rapport et al., 2013; Rucklidge & Tannock, 2002), and extend previous findings by demonstrating that specific neurocognitive abilities are important for understanding heterogeneity in functional impairments among children with ADHD.
Among the neurocognitive predictors, working memory abilities accounted for significant individual differences across several functional indicators, and were the only assessed neurocognitive abilities to predict outcomes across both informants and all three areas of functioning. This pattern implicates working memory dysfunction as a liability for broad-based functional impairment, and is consistent with previous studies linking individual differences in working memory components with academic attainment among children with ADHD (Alloway & Stein, 2014), as well as studies identifying cross-sectional (Mayes & Calhoun, 2007; Rogers et al., 2011) and longitudinal associations (Miller et al., 2012; Sarver et al., 2012) between working memory storage/rehearsal subcomponents and individual differences in specific academic domains. The current study extends these findings, and suggests that working memory may also be important for understanding heterogeneity in family and social functioning among children with ADHD. That is, children with ADHD who are better able to mentally store and process information are perceived by teachers and parents as more socially adept and more effectively parented. Conversely, underdeveloped working memory likely makes it extraordinarily difficult to engage in the give-and-take, listen-and-wait behaviors required for adept social interactions (Kofler et al., 2011). This explanation is consistent also with the observation that a majority of DSM-5 hyperactivity/impulsivity items refer to intrusive verbal behavior and the inability to maintain thoughts and forestall action (e.g., interrupts conversations, blurts out).
The current results were highly consistent with previous studies demonstrating strong continuity between working memory and social problems in ADHD (Bunford et al., 2015; Kofler et al., 2011), and provide new data suggesting that children’s working memory abilities may influence parental perceptions regarding relationship quality and their ability to effectively parent their ADHD child. Combined with the finding that better developed inhibitory control predicts improved family functioning, these results are generally consistent with developmental models suggesting that child cognitive/intellectual assets may facilitate positive interactions with caring adults (Lerner, Phelps, Forman, & Bowers, 2009), which in turn may shape early executive function development (Cuevas et al., 2014) and buffer against adverse outcomes for at-risk children (Eccles & Gootman, 2002). The current study extends these findings by identifying specific neurocognitive abilities that influence parent-child interactions for children with ADHD. Alternatively, the distinct neurocognitive profiles associated with family vs. social (peer) impairments may suggest that the abilities and behaviors required for successful parent-child interactions differ somewhat from those required for successful peer interactions. That is, parents may have expectations for their children that require better developed working memory and inhibitory control (e.g., following multistep directions, inhibiting unwanted behaviors), whereas successful interactions with same-aged peers may rely to a greater extent on rapid processing of social information (Phillips et al., 2007). This hypothesis is consistent with the current finding that somewhat more children were classified as impaired in family functioning (62%) relative to social functioning (55%), as well as meta-analytic findings that working memory deficits may be more prevalent and/or of larger magnitude than processing speed deficits (Kasper et al., 2012; Kofler et al., 2013).
Inhibitory control was uniquely associated with family functioning. Its failure to predict academic functioning was surprising given our use of a psychometrically supported inhibition task (Alderson et al., 2007; Snyder et al., 2015) and previous developmental studies suggesting a small but significant role of inhibitory control in academic functioning (St. Clair-Thompson & Gathercole, 2006; Thorell, 2007; Wåhlstedt et al., 2009). In contrast, the current findings were consistent with previous ADHD studies that failed to find links between inhibition and ADHD symptoms (Alderson et al., 2010; Rucklidge & Tannock, 2002), as well as meta-analytic conclusions that inhibitory control may be intact in ADHD (Alderson et al., 2007; Lijffijt et al., 2005). Interestingly, the zeitgeist regarding inhibition appears to be shifting in both the clinical and cognitive literatures. Whereas inhibitory control was once considered a core executive function (Miyake et al., 2000) with promise for offering a unifying explanation of ADHD (Barkley, 1997), it may now be considered a ‘dead end’ in ADHD (Rommelse et al., 2007) and is no longer considered a core executive function in at least one influential model of human cognition (Miyake & Friedman, 2012). Notably, however, inhibitory control showed strong continuity with parental confidence and parent-child attachment in the current study, suggesting that it remains an important factor in understanding ADHD-related impairments even if inhibition deficits are not present at the group level (Alderson et al., 2007; Lijffijt et al., 2005).
Limitations
The unique contribution of the current study was its systematic examination of neurocognitive predictors of functional heterogeneity in a well-defined sample of children with ADHD. Several caveats merit consideration despite methodological refinements including our approach to isolating reliable variance associated with each neurocognitive function and examination of multiple impairment domains. Generalization of findings from highly controlled laboratory experiments are always limited to some extent, and no conclusions regarding neurocognitive deficits can be drawn due to the lack of a typically developing comparison group. However, ADHD-related impairments in neurocognitive abilities are well documented (Kasper et al., 2012), and impairments in each functional outcome (Pelham et al., 2005) were quantified objectively using norm referenced, psychometrically sound tests. In addition, significant predictors of each functional outcome were detected, suggesting adequate power and supporting our a priori effect estimation. However, the significant unexplained variance in each outcome indicates a clear need for future research that includes larger samples, as well as typically developing and clinical comparison groups to determine the extent to which the mechanisms associated with peer, family, and academic functioning differ across clinical and nonclinical populations.
In addition, several of the children with ADHD met criteria for comorbid behavioral and mood disorders; thus, the extent to which the findings generalize to children with ‘pure’ ADHD is unknown. The inclusion of these common comorbidities, however, is expected to improve generalizability given that the sample is more representative of the larger population of children with ADHD (for which the majority have at least one comorbid diagnosis; Wilens et al., 2002). Fifty percent of our ADHD sample was prescribed stimulant medication, which was broadly consistent with epidemiological estimates (39% to 69%; Froelich et al., 2007; Visser et al., 2014). Although medication status was generally unrelated to our study variables, it may have dampened effect size estimates when juxtaposing neurocognitive performance off medication with parent/teacher perceptions that may be influenced by medication. The mean IQ of our sample was higher than the national average by approximately 1/3rd to 2/3rd SD; thus, the extent to which the findings generalize to children with average or lower intellectual abilities remains unknown. Finally, future research may benefit from examining the influence of informant source on impairment estimates (Valo & Tannock, 2010), as well as impairment indicators beyond those represented herein (e.g., health impairment, quality of life, sociometric standing) to further specify the mechanisms and processes underlying these impairments and identify mechanistic subtypes (Fair et al., 2012).
Clinical and Research Implications
Collectively, results of the current study suggest that neurocognitive processes are particularly important for understanding heterogeneity in daily functioning among children with ADHD. In particular, children with impairments in academic and family functioning showed large magnitude working memory deficits, whereas children with social impairments demonstrated slowed processing speed. If replicated, these findings suggest differential assessment and intervention targets depending on each child’s functional impairment profile. That is, in addition to direct remediation of each identified functional area, improved efficacy may be realized by adding interventions that target the specific mechanisms associated with the child’s identified functional impairment(s) (Chacko et al., 2014). For example, children with academic impairments may be likely to benefit from interventions that facilitate academic success and productivity (e.g., class-wide peer tutoring; DuPaul & Weyandt, 2006) while concurrently targeting their underdeveloped working memory abilities. Similarly, we hypothesize that processing speed training may augment interventions that facilitate prosocial engagement (Mikami, Lerner, Griggs, McGrath, & Calhoun, 2010), and family-based interventions may see incremental benefits when combined with working memory and/or inhibitory control training. Unfortunately, extant medications and ‘working memory’ training programs generally fail to improve working memory (Melby-Lervåg & Hulme, 2016; Rapport et al., 2013; Rubia et al., 2014; Shipstead, Hicks, & Engle, 2012), suggesting this combined approach will have to wait until next-generation neurocognitive trainings and/or medications have been developed and shown to effectively improve the specific neurocognitive abilities they claim to target (Chacko et al., 2014). Nonetheless, the current results add to a growing literature implicating neurocognitive abilities not only in explaining behavioral differences between ADHD and non-ADHD groups, but also in the substantial heterogeneity in functional outcomes associated with the disorder.
Supplementary Material
Acknowledgements:
This work was supported in part by a UVa Curry School of Education Foundation grant (PI: Kofler) from the Galant Family and an NIH grant (R34 MH102499-01, PI: Kofler). The sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The authors thank Erin Lunsford, Paula Aduen, Dr. Hillary Schaefer, and the CLC-V undergraduate research assistants.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to report.
1 Conservatively computed based on Cohen’s d effect sizes as the percentage of non-overlap between the ADHD and non-ADHD population distributions (i.e., the percentage of children with ADHD scoring outside the typically developing range) as recommended (Zakzanis, 2001).
References
- Ackerman PL, Beier ME, & Boyle MO (2005). Working memory and intelligence: The same or different constructs? Psychological Bulletin, 131, 30–60. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Hudec KL, Sarver DE, & Kofler MJ (2010). Competing core processes in ADHD: Do working memory deficiencies underlie behavioral inhibition deficits?. Journal of Abnormal Child Psychology, 38, 497–507. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, & Kofler MJ (2007). ADHD and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology, 35, 745–758. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Sarver DE, & Kofler MJ (2008). ADHD and behavioral inhibition: A re-examination of the stop-signal task. Journal of Abnormal Child Psychology, 36, 989–998. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, Adams AM, Willis C, Eaglen R, & Lamont E (2005). Working memory and phonological awareness as predictors of progress towards early learning goals at school entry. British Journal of Developmental Psychology, 23, 417–426. [Google Scholar]
- Alloway TP, & Stein A (2014). Investigating the link between cognitive skills and learning in non-comorbid samples of ADHD and SLI. International Journal of Educational Research, 64, 26–31. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Antonini TN, Kingery KM, Narad ME, Langberg JM, Tamm L, Epstein JN (2016). Neurocognitive and behavioral predictors of math performance in children with and without ADHD. Journal of Attention Disorders, 20, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2007). Working memory, thought, and action. Oxford, UK: Oxford University Press. [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121, 65–94. [DOI] [PubMed] [Google Scholar]
- Barry TD, Lyman RD, & Klinger LG (2002). Academic underachievement and ADHD: The negative impact of symptom severity on school performance. Journal of School Psychology, 40, 259–283. [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, … & Faraone SV (2004). Impact of executive function deficits and ADHD on academic outcomes in children. Journal of Consulting and Clinical Psychology, 72, 757–766. [DOI] [PubMed] [Google Scholar]
- Bunford N, Brandt N, Golden C, Dykstra J, Suhr J, & Owens J (2015). Attention-deficit/hyperactivity disorder symptoms mediate the association between deficits in executive functioning and social impairment in children. Journal of Abnormal Child Psychology, 43, 133–147. [DOI] [PubMed] [Google Scholar]
- Cain K, Oakhill J, & Bryant P (2004). Children’s reading comprehension ability: Concurrent prediction by working memory, verbal ability, and component skills. Journal of Educational Psychololgy, 96, 31–42. [Google Scholar]
- Chacko A, Kofler M, & Jarrett M (2014). Improving outcomes for youth with ADHD: A conceptual framework. Clinical Child and Family Psychology Review, 17, 368–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CHM, Rijdijk F, McLoughlin G, Faraone SV, Asherson P, & Kuntsi J (2015). Childhood predictors of adolescent and young adult outcome in ADHD. Journal of Psychiatric Research, 62, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabildas N, Pennington BF, & Willcutt EG (2001). A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology, 29, 529–540. [DOI] [PubMed] [Google Scholar]
- Clark C, Prior M, & Kinsella G (2002). The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. Journal of Child Psychology and Psychiatry, 43, 785–796. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, & Engle RW (2005). Working memory span tasks: Methodological review and user’s guide. Psychonomic Bulletin & Review, 12, 769–86. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Deater‐Deckard K, Kim‐Spoon J, Watson AJ, Morasch KC, & Bell MA (2014). What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental Science, 17, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussen A, Sciberras E, Ukoumunne OC, & Efron D (2012). Relationship between symptoms of attention-deficit/hyperactivity disorder and family functioning: A community-based sample. European Journal of Pediatrics, 171, 271–280. [DOI] [PubMed] [Google Scholar]
- de Boo GM, & Prins PJ (2007). Social incompetence in children with ADHD: Possible moderators and mediators in social-skills training. Clinical Psychology Review, 27, 78–97. [DOI] [PubMed] [Google Scholar]
- Dennis T, Brotman L, Huang K, & Gouley K (2007). Effortful control, social competence, and adjustment problems in children at risk for psychopathology. Journal of Clinical Child & Adolescent Psycholology,36, 442–54. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Rapport MD, & Perriello LM (1991). Teacher ratings of academic skills: The development of the academic performance rating scale. School Psychology Review, 20, 284–300. [Google Scholar]
- DuPaul GJ, & Weyandt LL (2006). School‐based intervention for children with ADHD. International Journal of Disability, Development and Education, 53, 161–176. [Google Scholar]
- Eccles JS, & Gootman JA (2002). Features of positive developmental settings. Community Programs to Promote Youth Development, 86–118. [Google Scholar]
- Efron B, & Tibshirani RJ (1993). An introduction to the bootstrap Monographs on Statistics and Applied Probability (57). New York and London: Chapman and Hall/CRC. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, & Conway ARA (1999). Working memory, short-term memory, and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General, 125, 309–331. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, & Nigg JT (2012). Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences, 109, 6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G* Power 3: A flexible statistical power analysis program. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, & Youngstrom EA (2004). Meta-analysis of intellectual and neuropsychological test performance in ADHD. Neuropsychology, 18, 543–555. [DOI] [PubMed] [Google Scholar]
- Fritz MS, & MacKinnon DP (2007). Required sample size to detect the mediated effect. Psychological Science, 18, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, & Kahn RS (2007) Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Archives of Pediatrics and Adolescent Medicine, 161, 857–864. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J, & Salisbury H (2004). Further validity evidence for the teacher version of the Child Symptom Inventory-4. School Psychology Quarterly, 19, 50–71 [Google Scholar]
- Gaub M, & Carlson CL (1997). Gender differences in ADHD: A meta-analysis and critical review. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Gewirtz S, Stanton‐Chapman T, & Reeve R (2009). Can inhibition at preschool age predict ADHD symptoms and social difficulties in third grade? Early Child Development and Care, 179, 353–368. [Google Scholar]
- Giofrè D, Mammarella IC, & Cornoldi C (2013). The structure of working memory and how it relates to intelligence in children. Intelligence, 41, 396–406. [Google Scholar]
- Gomez R, & Sanson A (1994). Effects of experimenter and mother presence on the attentional performance and activity of hyperactive boys. Journal of Abnormal Child Psychology, 22, 517–529. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, & Newcorn JH (2008). Neuropsychological outcome in adolescents/young adults with childhood ADHD: Profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry, 49, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarty KY, Hines CV, Kromrey JD, Ferron JM & Mumford KR (2005). The quality factor solutions in exploratory factor analysis: the influence of sample size, communality, and overdetermination. Educational and Psychological Measurement, 65, 202–226. [Google Scholar]
- Hollingshead AB, 1975. Four factor index of social status. Unpublished working paper, Department of Sociology, Yale University.
- Holmes CJ, Kim-Spoon J, & Deater-Deckard K (2016). Linking executive function and peer problems from early childhood through middle adolescence. Journal of Abnormal Child Psychology, 44, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock CL, Mikami AY, Pfiffner L, & McBurnett K (2009). Can executive functions explain relation between ADHD and social adjustment? Journal of Abnormal Child Psychology, 37, 679–91. [DOI] [PubMed] [Google Scholar]
- Ilieva I., Hook C., & Farah M (2015). Prescription stimulants’ effects on healthy inhibitory control, working memory, and episodic memory: A meta-analysis. Journal of Cognitive Neuroscience, 27, 1069–89. [DOI] [PubMed] [Google Scholar]
- Jacobson LA, Ryan M, Martin RB, Ewen J, Mostofsky SH, Denckla MB, & Mahone EM (2011). Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychology, 17, 209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NS, & Truax P (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59, 12–19. [DOI] [PubMed] [Google Scholar]
- Johnston C, & Mash EJ (2001). Families of children with attention-deficit/hyperactivity disorder: Review and recommendations for future research. Clinical Child and Family Psychology Review, 4, 183–207. [DOI] [PubMed] [Google Scholar]
- Kamphaus RW, & Reynolds CR (2006). PRQ: Parenting relationship questionnaire manual. NCS Pearson. [Google Scholar]
- Kasper LJ, Alderson RM, & Hudec KL (2012). Moderators of working memory deficits in children with ADHD: A meta-analytic review. Clinical Psychology Review, 32, 605–617. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). Kaufman Test of Educational Achievement (2nd ed.). Minneapolis, MN: NCS Pearson. [Google Scholar]
- Kaufman AS, & Kaufman NL (2014). Kaufman Test of Educational Achievement, Third Edition. Bloomington, MN: NCS Pearson. [Google Scholar]
- Keown LJ, & Woodward LJ (2002). Early parent-child relations and family functioning of preschool boys with pervasive hyperactivity. Journal of Abnormal Child Psychology, 30, 541–553. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Alderson RM, Raiker JS, Bolden J, Sarver DE, & Rapport MD (2014). Working memory and intraindividual variability as indicators in ADHD. Neuropsychology, 28, 459–71. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, & Raiker JS (2010). ADHD and working memory: The impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology, 38, 149–161. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, & Alderson RM (2011). Working memory deficits and social problems in children with ADHD. Journal of Abnormal Child Psychology, 39, 805–817. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, & Kolomeyer EG (2013). Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review, 33, 795–811. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Sarver DE, & Wells EL (in press). Working memory and hyperactivity in ADHD: Experimental evidence for a functional relation. Journal of Attention Disorders. [DOI] [PubMed] [Google Scholar]
- Lerner JV, Phelps E, Forman YE, & Bowers EP (2009). Positive youth development In Lerner RM & Steinberg LD (Eds.), Handbook of Adolescent Psychology (pp. 524–558). NJ; Wiley. [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, & van Engeland H (2005). A meta-analytic review of stopping performance in ADHD. Journal of Abnormal Psychology, 114, 216–222. [DOI] [PubMed] [Google Scholar]
- Maybery MT, & Do N (2003). Relationships between facets of working memory and performance on a curriculum-based mathematics test in children. Educational and Child Psychology, 20, 77–92. [Google Scholar]
- Mayes SD, & Calhoun SL (2006). Frequency of reading, math, and writing disabilities in children with clinical disorders. Learning and Individual Differences, 16, 145–157. [Google Scholar]
- Mayes SD, & Calhoun SL (2007). Learning, attention, writing, and processing speed in typical children and children with ADHD, autism, anxiety, depression, and oppositional-defiant disorder. Child Neuropsychology, 13, 469–493. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M, & Hulme C (2016). There is no convincing evidence that working memory training is effective: A reply to au et al. (2014) and karbach and verhaeghen (2014). Psychonomic Bulletin & Review, 23, 324–330. [DOI] [PubMed] [Google Scholar]
- Mikami AY, Lerner MD, Griggs MS, McGrath A, & Calhoun CD (2010). Parental influence on children with ADHD: II. Results of a pilot intervention training parents as friendship coaches for children. Journal of Abnormal Child Psychology, 38, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, & Hinshaw SP (2010). Does childhood executive function predict adolescent functional outcomes in girls with ADHD?. Journal of Abnormal Child Psychology, 38, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Nevado-Montenegro AJ, & Hinshaw SP (2012). Childhood executive function continues to predict outcomes in young adult females with and without childhood-diagnosed ADHD. Journal of Abnormal Child Psychology, 40, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science, 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Nigg JT (1999). The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM–IV combined type, extension, and qualification. Journal of Abnormal Child Psychology, 27, 393–402. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2005). Neuropsychologic theory and findings in ADHD: The state of the field and salient challenges for the coming decade. Biological Psychiatry, 57, 1424–1435. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, & Rappley MD (2002). Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy Child Adolescent Psychiatry, 41, 59–66. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke EJ (2005). Causal heterogeneity in ADHD: Do we need neuropsychologically impaired subtypes?. Biological Psychiatry, 57, 1224–30. [DOI] [PubMed] [Google Scholar]
- Patros CHG, Alderson RM, Lea SE, Tarle SJ, Kasper LJ, & Hudec KL (2015). (2015). Visuospatial working memory under-lies choice-impulsivity in boys with ADHD. Research in Developmental Disabilities, 38, 134–144. [DOI] [PubMed] [Google Scholar]
- Pelham WE Jr., Fabiano GA, & Massetti GM (2005). Evidence-based assessment of ADHD in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34, 449–476. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Foster EM, & Robb JA (2007). The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambulatory Pediatrics, 7, 121–131. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Tunstall M, & Channon S (2007). Exploring the role of working memory in dynamic social cue decoding using dual task methodology. Journal of Nonverbal Behavior, 31, 137–152. [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, & Rohde LA (2014). ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43, 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AS, Heaton SC, McCann SJ, Watson WD, & Selke G (2009). The role of multidimensional attentional abilities in academic skills of children with ADHD. Journal of Learning Disabilities, 42, 240–249. [DOI] [PubMed] [Google Scholar]
- Raiker JS, Rapport MD, Kofler MJ, & Sarver DE (2012). Objectively-measured impulsivity and ADHD: Testing competing predictions from the working memory and behavioral inhibition models of ADHD. Journal of Abnormal Child Psychology, 40, 699–713. [DOI] [PubMed] [Google Scholar]
- Rajendran K, O’Neill S, Marks DJ & Halperin JM (2015). Latent profile analysis of neuropsychological measures to determine preschoolers’ risk for ADHD. Journal of Child Psychology and Psychiatry, 56, 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, & Sims V (2008). Working memory deficits in boys with ADHD. Journal of Abnormal Child Psychology, 36, 825–837. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, & Alderson RM (2009). Hyperactivity in boys with ADHD: A ubiquitous core symptom or manifestation of working memory deficits?. Journal of Abnormal Child Psychology, 37, 521–534. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, & Friedman LM (2013). Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review. Clinical Psychology Review, 33, 1237–1252. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2004). BASC-2: Behavior Assessment System for Children: Second Edition, manual. Bloomington, MN: Pearson Assessments. [Google Scholar]
- Rogers M, Hwang H, Toplak M, Weiss M, & Tannock R (2011). Inattention, working memory, and academic achievement in adolescents referred for ADHD. Child Neuropsychology, 17, 444–58. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Wiener J, Marton I, & Tannock R (2009). Parental involvement in children’s learning: Comparing parents of children with and without attention-deficit/hyperactivity disorder (ADHD). Journal of School Psychology, 47, 167–185. [DOI] [PubMed] [Google Scholar]
- Rommelse NNJ, Altink ME, de Sonneville LMJ, Buschgens CJM, Buitelaar J, Oosterlaan J, & Sergeant JA (2007) Are motor inhibition and cognitive flexibility dead ends in ADHD?. Journal of Abnormal Child Psychology, 35, 957–967. [DOI] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, & Radua J (2014).Effects of stimulants on brain function in ADHD: A systematic review and meta-analysis. Biological Psychiatry, 76, 616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge JJ, & Tannock R (2002). Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry, 43, 988–1003. [DOI] [PubMed] [Google Scholar]
- Sarver DE, Rapport MD, Kofler MJ, Raiker JS, & Friedman LM (2015). Hyperactivity in ADHD: Impairing deficit or compensatory behavior?. Journal of Abnormal Child Psychology. [DOI] [PubMed] [Google Scholar]
- Sarver DE, Rapport MD, Kofler MJ, Scanlan SW, Raiker JS, Altro TA, & Bolden J (2012). Attention problems, phonological short-term memory, and visuospatial short-term memory: Differential effects on near-and long-term scholastic achievement. Learning and Individual Differences, 22, 8–19. [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, & Klim P (2000). Confirmation of an inhibitory control deficit in ADHD. Journal of Abnormal Child Psychology, 28, 227–235. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Süß HM, & Wittmann WW (2007). Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General, 136, 414–429. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Mahone EM, Levine T, Eason SH, & Cutting LE (2009). The contribution of executive skills to reading comprehension. Child Neuropsychology, 15, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Hicks KL, & Engle RW (2012). Cogmed working memory training: Does the evidence support the claims?. Journal of Applied Research in Memory and Cognition, 1, 185–193. [Google Scholar]
- Shipstead Z, Redick TS, & Engle RW (2010). Does working memory training generalize?. Psychologica Belgica, 50, 245–276. [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7, 422–445. [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, & Thompson M (2010). Beyond the dual pathway model. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 345–355. [DOI] [PubMed] [Google Scholar]
- Soreni N, Crosbie J, Ickowicz A, & Schachar R (2009). Stop signal and Conners’ CPT: Test-retest reliability of two inhibition measures in ADHD children. Journal of Attention Disorders, 13, 137–43. [DOI] [PubMed] [Google Scholar]
- St. Clair-Thompson HL, & Gathercole SE (2006). Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology, 59, 745–759. [DOI] [PubMed] [Google Scholar]
- Swanson L, & Kim K (2007). Working memory, short-term memory, and naming speed as predictors of children’s mathematical performance. Intelligence, 35, 151–168. [Google Scholar]
- Theule J, Wiener J, Tannock R, & Jenkins JM (2013). Parenting stress in families of children with ADHD: A meta-analysis. Journal of Emotional and Behavioral Disorders, 21, 3–17. [Google Scholar]
- Thorell LB (2007). Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? Journal of Child Psychology and Psychiatry, 48, 1061–1070. [DOI] [PubMed] [Google Scholar]
- Tourva A, Spanoudis G, & Demetriou A (2016). Cognitive correlates of developing intelligence: The contribution of working memory, processing speed and attention. Intelligence, 54, 136–146. [Google Scholar]
- Valo S, & Tannock R (2010). Diagnostic instability of DSM–IV ADHD subtypes: Effects of informant source, instrumentation, and methods for combining symptom reports. Journal of Clinical Child & Adolescent Psychology, 39, 749–760. [DOI] [PubMed] [Google Scholar]
- van der Oord S, Prins PJM, Oosterlaan J, & Emmelkamp PM (2008). Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: A meta-analysis. Clinical Psychology Review, 28, 783–800. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, … Blumberg SJ (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United states, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry, 53, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wåhlstedt C, Thorell LB, & Bohlin G (2009). Heterogeneity in ADHD: Neuropsychological pathways, comorbidity and symptom domains. Journal of Abnormal Child Psychology, 37, 551–564. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children-Fourth Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2014). Wechsler Intelligence Scale for Children-Fifth Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence-Second Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Whalen CK, Collins BE, Henker B, Alkus Stephen,R., Adams D, & Stapp J (1978). Behavior observations of hyperactive children and methylphenidate (Ritalin) effects in systematically structured classroom environments: Now you see them, now you don’t. Journal of Pediatric Psychology, 3, 177–187. [Google Scholar]
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, Spencer TJ (2002). Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 41, 262–268. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of ADHD: A meta-analytic review. Biological Psychiatry, 57, 1336–46. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R,… Lahey BB (2012). Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology,121, 991–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK (2001). Statistics to tell the truth, the whole truth, and nothing but the truth. Archives of Clinical Neuropsychology, 16, 653–667. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.