Abstract
The epithelial sodium channel (ENaC) and mitogen-activated protein kinase (MAPK) pathway have been reported to be associated with the progression of acute lung injury (ALI). Oxymatrine (OMT) alone or combined with other drugs can ameliorate paraquat- or oleic acid-induced lung injury. However, the effect of OMT on lipopolysaccharide (LPS)-induced ALI remains unknown. The aim of the present study was to evaluate whether OMT can attenuate LPS-induced ALI through regulation of the ENaC and MAPK pathway using an ALI mouse model. Histological assessment of the lung and inflammatory cell counts in bronchoalveolar lavage fluid (BALF) were performed by H&E and Wright-Giemsa staining. The lung wet/dry (W/D) weight ratio and the levels of tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), ENaC subunits, and the MAPK pathway members were determined. Isolated type II rat alveolar epithelial cells were incubated with OMT 30 min before LPS stimulation to investigate the activation of ENaC and the MAPK pathway. The results showed that OMT remarkably alleviated histopathologic changes in lung and pulmonary edema, reduced inflammatory cell counts in BALF, and decreased TNF-α and CRP levels in a dose-dependent manner. OMT significantly increased the three subunits of ENaC proteins in vivo and in vitro, while it decreased p-ERK/ERK, p-p38/p38, and p-JNK/JNK ratios in vivo. However, only the JNK pathway was markedly inhibited in vitro following pretreatment with OMT. Collectively, the results suggested that OMT might alleviate LPS-induced ALI by elevating ENaC proteins and inhibiting the JNK signaling pathway.
Keywords: acute lung injury, epithelial sodium channel, MAPK, oxymatrine
Introduction
Acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome (ARDS), are clinically severe respiratory disorders characterized by gas exchange impairment, hemorrhage, and pulmonary edema [38]. LPS is a major component of the outer membrane of Gram-negative bacteria, including Pseudomonas aeruginosa (P. aeruginosa). LPS stimulation can activate a variety of immune cells and induce the release of pro-inflammatory and immunoregulatory cytokines from the host, thereby affecting the immune function of the body [44]. When delivered into animals and humans, LPS can cause typical symptoms of lung infection, including pulmonary leukocyte accumulation, edema, severe inflammation, and mortality [29].
Oxymatrine (OMT), a quinolizidine alkaloid, is a bioactive ingredient of the traditional Chinese herb Sophorae flavescentis radix (the dried roots of Sophora flavescens Ait) [27]. OMT has been demonstrated to possess antiviral, anticancer, anti-inflammatory, and anti-fibrotic activities [11]. Studies revealed that OMT can alleviate LPS-induced mastitis and acute intestinal inflammation through regulation of the nuclear factor-κB (NF-κB) signaling pathway [13, 35]. A recent study demonstrated that the combination of OMT and sodium ferulate protects mice against ALI. However, the possible mechanism of OMT in LPS-induced ALI remains unclear.
The mitogen-activated protein kinase (MAPK) pathway participates in the release of inflammatory cytokines/mediators in the pathogenesis of inflammatory diseases, including ALI [3, 5]. A recent study reported that OMT can suppress inflammation responses in LPS-activated microglia through the MAPK pathway [9]. The MAPK pathway is also inactivated in the protection of OMT against sepsis-induced myocardial injury and intracerebral hemorrhage (ICH) [18, 37]. Xu et al. demonstrated that OMT can attenuate oleic acid-induced ALI by inhibiting the p38 MAPK pathway [34]. The above evidence suggests that the MAPK pathway may be involved in protection of OMT against LPS-induced ALI.
Pulmonary edema is a primary pathological feature of ALI/ARDS, and the epithelial sodium channel (ENaC) plays an important role in relieving pulmonary edema in ALI/ARDS [8, 14]. In lung tissue, ENaC could transport excess edematous fluid out of the alveolar cavity through the channel transport mechanism [10, 43], whereas abnormally low expression of ENaC leads to impaired fluid clearance [30]. Dagenais and colleagues demonstrated that P. aeruginosa and its secreted LPS reduce the expression and activity of ENaC in alveolar epithelial cells in vivo and in vitro [1, 6, 7, 28]. Moreover, inhibition of the MAPK pathway abrogated LPS-induced ENaC downregulation [28]. Thus, we speculate that OMT may prevent the downregulation of ENaC and protect against LPS-induced ALI through regulation of the MAPK pathway.
In the present study, an animal model of ALI was generated in C57BL/6 mice by intratracheal injection of LPS from P. aeruginosa. We evaluated the effect of OMT on pathological changes, inflammation in BALF and lung tissues, and pulmonary edema in vivo and clarified the possible mechanisms both in vivo and in vitro.
Materials and Methods
Ethics statement
Experimental procedures were approved by the Animal Care and Use Committee of China Medical University and performed in accordance with relevant guidelines (approval number: 2015PS361K).
Animals and groups
Male C57BL/6 mice (age, 8–10 weeks; weight, 18–22 g) were purchased from Charles River (Beijing, China) and randomly divided into 5 groups (the control group, LPS group, LPS + 12.5 mg/kg OMT group, LPS + 25 mg/kg OMT group, and LPS + 50 mg/kg OMT group) (18 mice/group). After anesthetization, the mice in the LPS group were intratracheally administered 50 µg LPS from P. aeruginosa serotype 10 (product no.: L9143) (Sigma-Aldrich Corp., St. Louis, MO, USA) in 70 µl PBS to establish the ALI model. The mice in the control group received the same volume of PBS in the same way as described above. The mice in the LPS + OMT groups were intraperitoneally injected with 12.5, 25, or 50 mg/kg OMT (Meilunbio, Dalian, China) 30 min before LPS administration. Meanwhile, the mice in the control group and the LPS group were pretreated with an equal volume of sterile saline.
Bronchoalveolar lavage fluid (BALF) cell count
Six hours after LPS administration, the mice were anesthetized. The trachea was cannulated and lavaged three times with 1.5 ml normal saline (0.5 ml normal saline each time). BALF was centrifuged at 2,000 rpm for 10 min, and the pellet was resuspended in 0.5 ml PBS. Inflammatory cells (white blood cells, macrophages, neutrophils, and lymphocytes) in 10 µl cell suspensions were fixed in methanol and then stained with Wright-Giemsa stain (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The inflammatory cells were counted under a microscope (Olympus, Tokyo, Japan).
H&E staining
At 6 h post-LPS exposure, the lung tissues were excised, fixed with 4% paraformaldehyde, dehydrated, and embedded in paraffin. The tissues were cut into 5 µm thick sections. After deparaffinization and rehydration, the sections were stained with hematoxylin and eosin. The pathological changes were photographed under a microscope (Olympus).
ELISA
The levels of C-reactive protein (CRP) in lung tissues were determined by ELISA kits purchased from USCN Business Co., Ltd. (Wuhan, China), according to the manufacturer’s instructions. The tumor necrosis factor (TNF)-α level in the supernatant of tissue homogenate was measured with a Mouse TNF-α ELISA Kit (Boster, Wuhan, China). The optical density at 450 nm was read on a BioTek microplate reader (BioTek, Winooski, VT, USA).
Lung wet/dry (W/D) weight ratio
Pulmonary edema was evaluated by measuring the lung wet/dry (W/D) weight ratio. The lung tissues were removed and weighed. Subsequently, they were placed in an incubator for 72 h to obtain their dry weight. The lung W/D weight ratio was calculated.
Isolation and identification of type II rat alveolar epithelial cells
Lungs from male Sprague-Dawley rats were washed twice with PBS and were filled with elastase (4.2 U/ml) via the trachea. After digestion at 37°C for 15 min, lung tissues were minced, and elastase was neutralized with 0.25% DNase I and FBS in a shaker for 10 min. The minced tissues were filtered through filters, and the resultant cell suspension was centrifuged at 1,000 rpm for 10 min at 4°C. After discarding the supernatant, the pellet was resuspended in DMEM and purified by the differential adhesion method to remove fibroblasts. Immunofluorescence was performed to identify type II alveolar epithelial cells by using an antibody against surfactant protein-C (SP-C). The cells were mounted on slides, fixed in 4% paraformaldehyde for 15 min, immersed in 0.1% Triton X-100 for 30 min, and treated with goat serum for 15 min at room temperature. Subsequently, the slides were incubated with anti-SP-C antibody (Santa Cruz Biotechnology, Dallas, Texas, USA ; 1:50 dilution) at 4°C overnight followed by 60 min of incubation with Cy3-conjugated goat anti-rabbit antibody (Beyotime Institute of Biotechnology, Haimen, China; 1:400 dilution) at room temperature. The nuclei were counterstained with DAPI, and the slides were photographed under a fluorescence microscope (Olympus).
Cell culture and treatment
Type II rat alveolar epithelial cells were cultured in DMEM containing 10% FBS in a 5% CO2 incubator. The cells were divided into 8 groups (the control group, 250 µg/ml OMT group, 500 µg/ml OMT group, 1,000 µg/ml OMT group, LPS group, LPS + 250 µg/ml OMT group, LPS + 500 µg/ml OMT group, and LPS + 1,000 µg/ml OMT group). The cells in the corresponding groups were pretreated with 250, 500, and 1,000 µg/ml OMT (Meilunbio), respectively. The cells cultured with vehicle were used as the controls. After pretreatment for 30 min, the cells in the corresponding groups were incubated with 15 µg/ml LPS (Sigma-Aldrich Corp.) at 37°C until they were subjected to analyses.
MTT assay
Cell viability was measured by MTT assay at 6, 12, and 24 h. After treatment with OMT and/or LPS, the cells were treated with 5 mg/ml MTT (Sigma-Aldrich Corp.) at 37°C for 4 h. The supernatant was discarded. The optical density was determined at 490 nm after dissolving the crystal violet with 150 µl DMSO (Sigma-Aldrich Corp.).
Real-time PCR
Total RNAs were obtained from lung tissues or type II alveolar epithelial cells by using RL lysis buffer and then subjected to reverse transcription. Real-time PCR was performed on Bioneer Quantitative Thermal Block (Bioneer, Daejeon, Republic of Korea) with the following amplification conditions: 95°C for 10 min followed by 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 30 s. All the obtained results were normalized to β-actin, and the relative expression levels of α-ENaC, β-ENaC, and γ-ENaC were calculated using the 2-ΔΔCt formula [25]. The primer sequences were as follows: 5′-ATCACGGAACAGACGCTTT-3′, forward, and 5′-CACTTGGGGATTGTTGTCGC-3′, reverse, for Mus-α-ENaC; 5′-CAGAAAGGGAGACCCAAAGAGA-3′, forward, and 5′-CACTGCCTGGCTTAGCGTCT-3′, reverse, for Rat-α-ENaC; 5′-CACACACCCCTGGTCCTTA-3′, forward, and 5′-CCGCAAGGTACACACAGTT-3′, reverse, for Mus-β-ENaC; 5′-AGTGGGGCGTCTTCATCC-3′, forward, and 5′-TGGTGGTGTTGGTGTGGCT-3′, reverse, for Rat-β-ENaC; 5′-TACTGCCTGAACACCAACA-3′, forward, and 5′-TGATGGAGACAGAGACGGTG-3′, reverse, for Mus-γ-ENaC; 5′-TCACAAACATCTACAACGCTGC-3′, forward, and 5′-GGGGTGTTGCTGGTAGTTGC-3′, reverse, for Rat-γ-ENaC; 5′-CTGTGCCCATCTACGAGGGCTAT-3′, forward, and 5′-TTTGATGTCACGCACGATTTCC-3′, reverse, for Mus-β-actin; 5′-GGAGATTACTGCCCTGGCTCCTAGC-3′, forward, and 5′-GGCCGGACTCATCGTACTCCTGCTT-3′, reverse, for Rat-β-actin.
Western blot
The cells were trypsinized, harvested, and lysed, and they were then subjected to protein extraction. The obtained total proteins were electrophoresed on SDS-PAGE gels and subsequently transferred onto PVDF membranes (Millipore, Bedford, MA, USA). Skim milk powder was diluted in TTBS solution. After that, the membranes were blocked with diluted milk and incubated with primary antibodies against α-ENaC (Proteintech Group, Wuhan, China; 1:500 dilution), β-ENaC (Proteintech Group; 1:500 dilution), γ-ENaC (Proteintech Group; 1:1,000 dilution), ERK (Proteintech Group; 1:500 dilution), p-ERK (BIOSS, Beijing, China; 1:400 dilution), p38 (BIOSS; 1:400 dilution), p-p38 (BIOSS; 1:400 dilution), JNK (Proteintech Group; 1:1,000 dilution) and p-JNK (Abcam, Cambridge Science Park, Cambridge, UK ; 1:1,000 dilution) at 4°C overnight. HRP-conjugated secondary antibody (Beyotime Institute of Biotechnology; 1:5,000 dilution) was added after washing with TTBS. Bands were subsequently visualized using ECL reagent (Beyotime Institute of Biotechnology). The optical densities of bands were analyzed by Gel-Pro Analyzer (Media Cybernetics, Inc., Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. A P value<0.05 was considered statistically significant.
Results
OMT attenuates histopathological changes in lung tissues
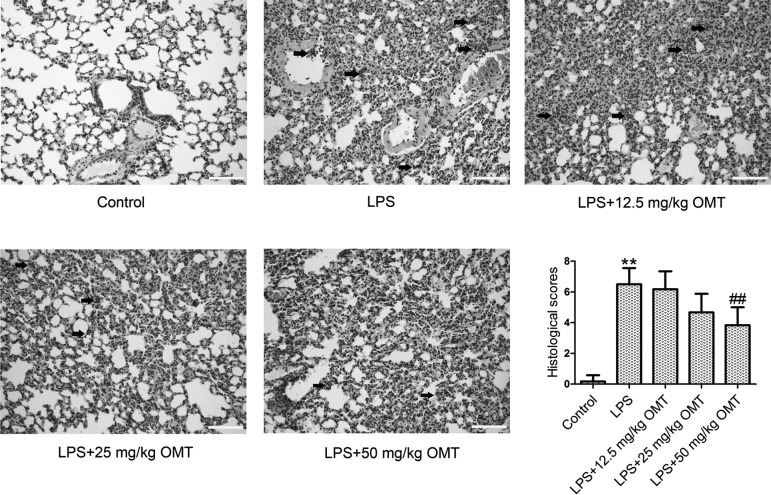
We evaluated histopathological changes in mice by H&E staining and assessed lung injury with a 9-score system. As shown in Fig. 1, the mice in the control group showed a normal structure of lung tissues and no histopathological changes. There were obvious histopathological changes in the lung tissues of the mice in the LPS group, including obvious inflammatory cell infiltration, thickened alveolar walls, severe hemorrhage in the alveolus, and alveolus collapse. After treatment with different doses of OMT, the histopathological changes of the lung were significantly ameliorated in a dose-dependent manner when compared with the LPS group.
Fig. 1.
Pulmonary morphology. The mice (18 mice/group) were pretreated with 12.5, 25, and 50 mg/kg oxymatrine (OMT) via intraperitoneal injection and then subjected to lipopolysaccharide (LPS; 50 µg) stimulation. Following 6 h of LPS exposure, lung tissues of the anaesthetized mice were excised, and the tissue sections were examined by H&E staining. Arrows indicate inflammatory cell clusters (original magnification ×200, scale bars 100 µm). Lung injury score was evaluated by a 9-score system. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. **P<0.01 compared with the control group. ##P<0.01 compared with the LPS group.
OMT attenuates inflammation in ALI induced by LPS in mice
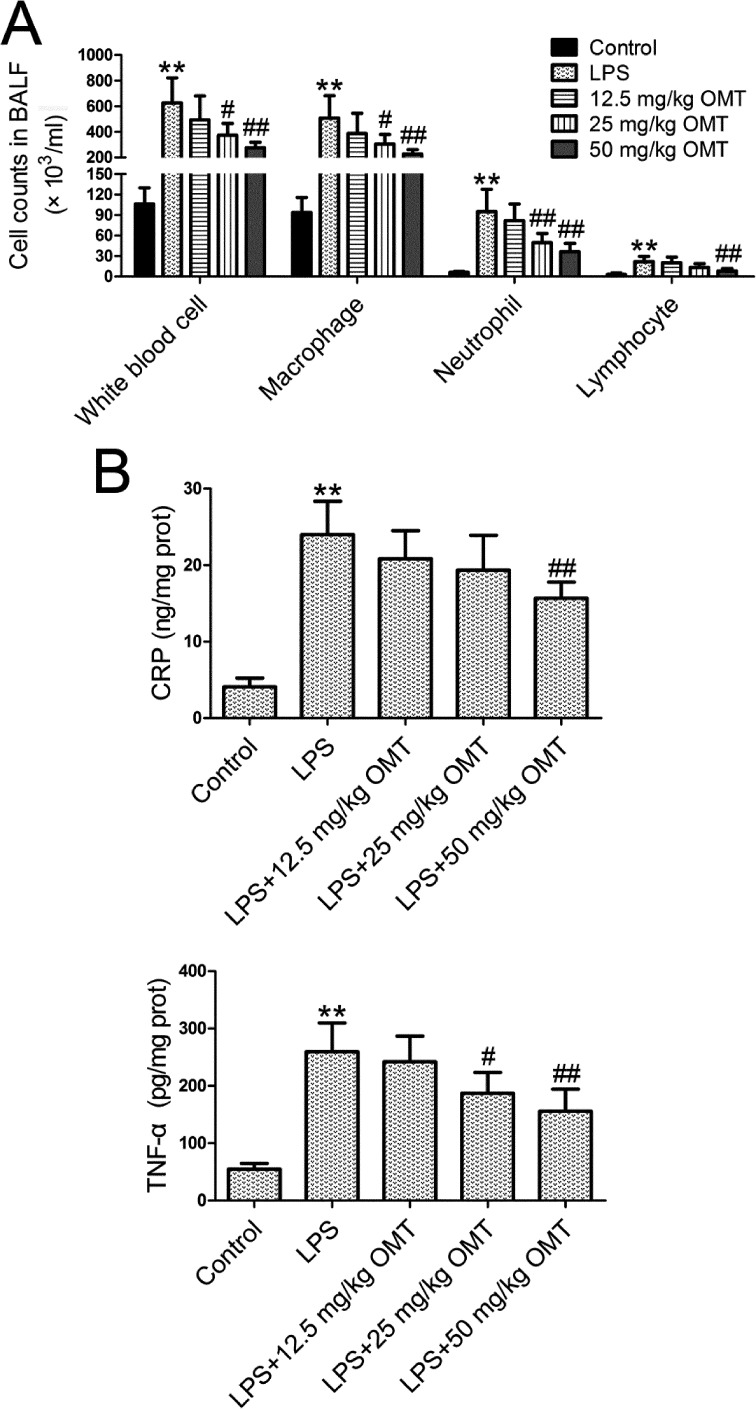
The severity of inflammation was measured by inflammatory cell (white blood cells, macrophages, neutrophils, and lymphocytes) counts in BALF and TNF-α and CRP levels in lung tissues. LPS stimulation significantly increased inflammatory cell counts in BALF (Fig. 2A) and the levels of TNF-α and CRP (Fig. 2B) compared with the control group. Compared with the LPS group, OMT markedly reduced inflammatory cell counts and lung TNF-α and CRP levels in a dose-dependent manner.
Fig. 2.
Inflammation in bronchoalveolar lavage fluid (BALF) and lung tissues. At 6 h post-LPS exposure, BALF and lung tissues were obtained. A. Inflammatory cell counts in BALF were measured by Wright-Giemsa staining. B. The lung tissue homogenate was centrifuged at 12,000 rpm for 10 min, and the supernatant was harvested. Tumor necrosis factor (TNF)-α and C-reactive protein (CRP) levels were determined using ELISA kits. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. **P<0.01 compared with the control group. #P<0.05 compared with the LPS group. ##P<0.01 compared with the LPS group.
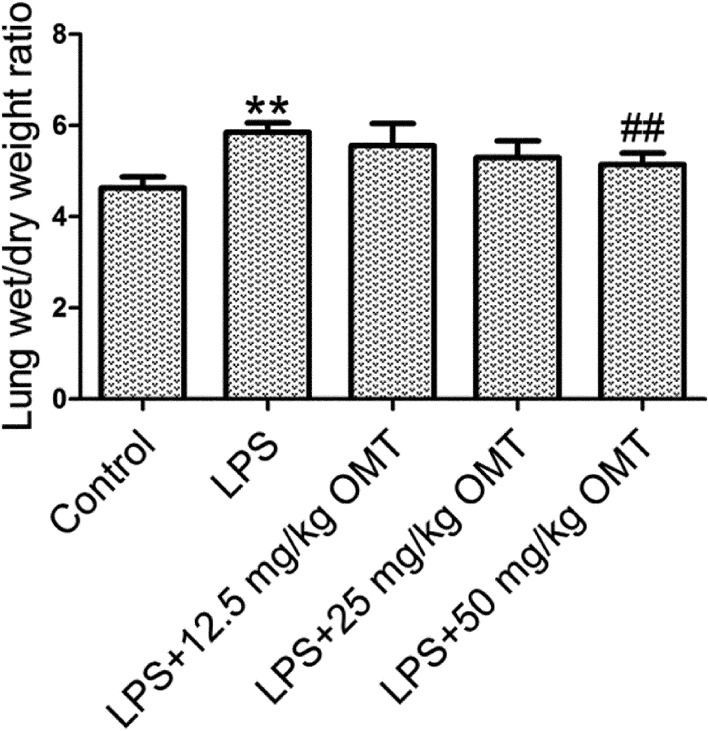
OMT attenuates pulmonary edema in mice
Pulmonary edema can be assessed by lung W/D weight ratio. The results showed that the lung W/D weight ratio was dramatically increased in the LPS group compared with the control group (Fig. 3). OMT lowered the lung W/D weight ratio in a dose-dependent manner compared with the LPS group.
Fig. 3.
Pulmonary edema. Lung wet/dry (W/D) weight ratio was measured to evaluate the severity of pulmonary edema. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni posthoc test. **P<0.01 compared with the control group. ##P<0.01 compared with the LPS group.
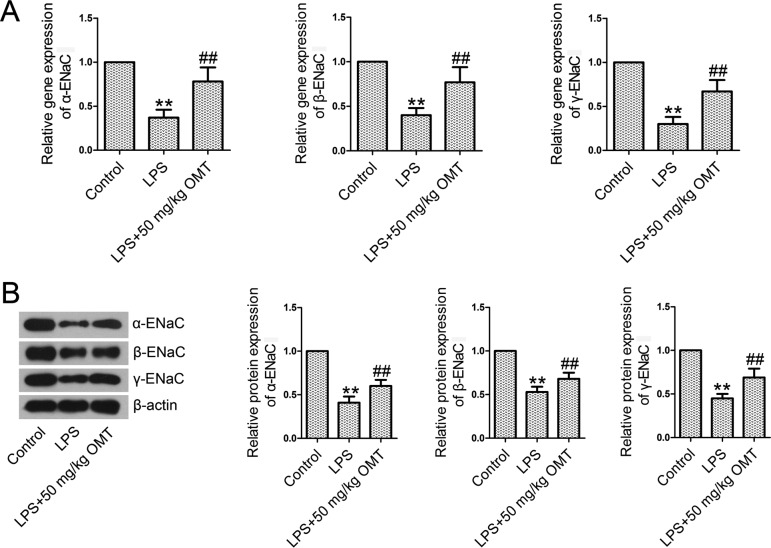
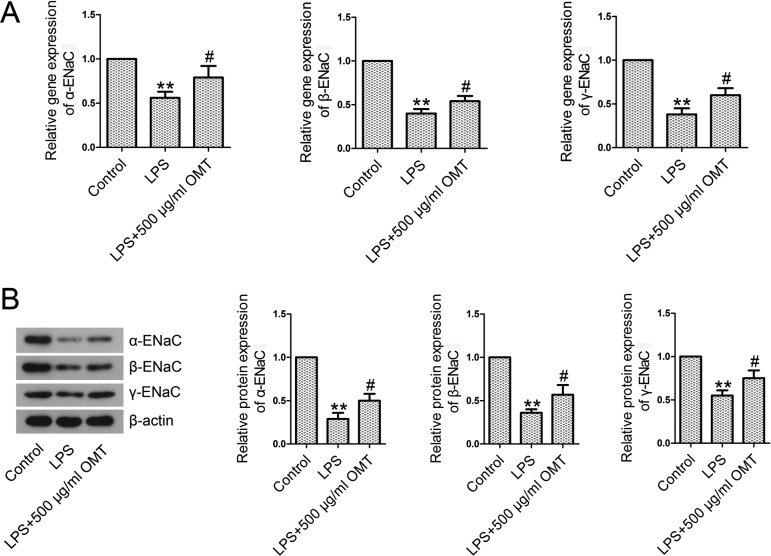
OMT activates the epithelial Na+ channel (ENaC) in mice
Real-time PCR and Western blotting results showed that the levels of expression of α-ENaC, β-ENaC, and γ-ENaC at mRNA (Fig. 4A) and protein (Fig. 4B) levels were significantly lower in the LPS group than those in the control group. However, OMT administration obviously upregulated the reduced levels of α-ENaC, β-ENaC, and γ-ENaC, indicating the activation of ENaC.
Fig. 4.
Epithelial sodium channel (ENaC) subunits in lung tissues. At 6 h post-LPS exposure, lung tissues were excised. Total RNAs and proteins extracted from the lung tissues were subjected to real-time PCR and Western blotting. A. The mRNA levels of α-ENaC, β-ENaC, and γ-ENaC were examined by real-time PCR analysis. B. The protein levels of α-ENaC, β-ENaC, and γ-ENaC were examined by Western blotting analysis. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. **P<0.01 compared with the control group. ##P<0.01 compared with the LPS group.
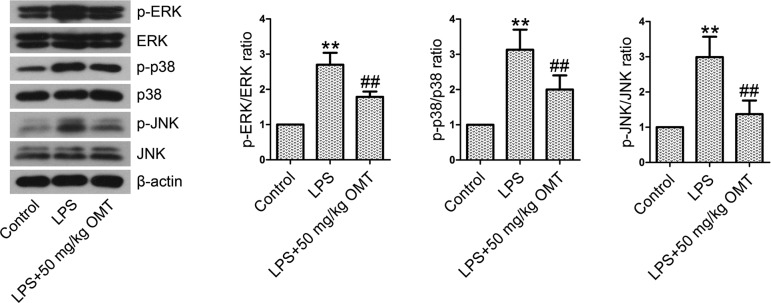
OMT inhibits the activation of the mitogen-activated protein kinase (MAPK) pathway in mice
Extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 are members of the MAPK pathway. To examine the involvement of the MAPK pathway, we measured the levels of MAPK pathway members. We found that the expression levels of phosphorylated forms of ERK, p38, and JNK were notably increased in the LPS group compared with the control group (Fig. 5). However, OMT treatment resulted in remarkable decreases in p-ERK, p-p38, and p-JNK expression compared with the LPS group.
Fig. 5.
The activation of the MAPK signaling pathway in lung tissues. The expression levels of p-ERK, ERK, p-p38, p38, p-JNK, and JNK were quantified by Western blotting analysis. The ratios of p-ERK/ERK, p-p38/p38, and p-JNK/JNK were calculated. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. **P<0.01 compared with the control group. ##P<0.01 compared with the LPS group.
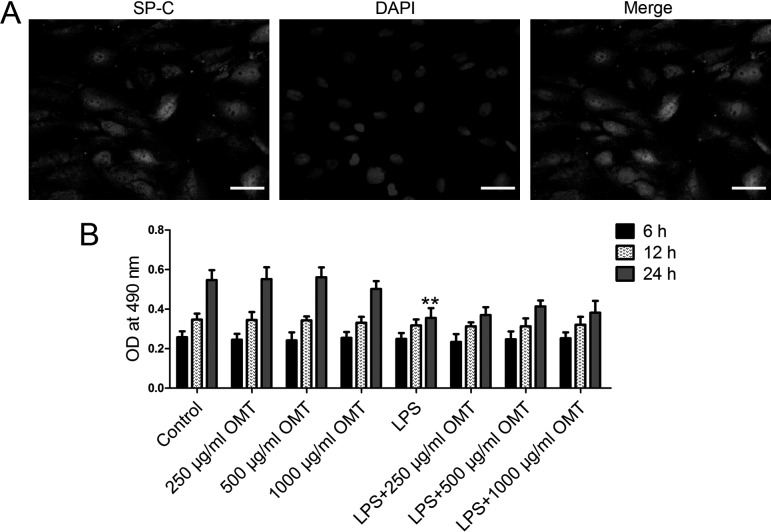
Identification of type II alveolar epithelial cells and cytotoxicity of OMT
In our in vitro study, we firstly identified the isolated cells using immunofluorescence staining for SP-C. As shown in Fig. 6A, the results showed that type II rat alveolar epithelial cells were successfully isolated. Then, we examined the cytotoxicity of OMT in type II rat alveolar epithelial cells by MTT assay. The results showed that OMT (250, 500, and 1,000 µg/ml) had no cytotoxicity in type II rat alveolar epithelial cells (Fig. 6B). The addition of LPS significantly reduced the cell viability of type II alveolar epithelial cells. OMT preconditioning, especially at the dose of 500 µg/ml, slightly abolished the effect of LPS at 24 h.
Fig. 6.
Identification of type II alveolar epithelial cells and cytotoxicity of OMT. A. The isolated cells were identified by immunofluorescence using anti-SP-C antibody. B. Rat type II alveolar epithelial cells were pretreated with different doses of OMT (250, 500, and 1,000 µg/ml OMT), and then LPS (15 µg/ml) was added 30 min post-OMT addition. After 6, 12, and 24 h, cytotoxicity of OMT was examined by MTT assay. The optical density was determined at 490 nm. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. **P<0.01 compared with the control group.
OMT activates ENaC in alveolar epithelial cells
We further confirmed whether ENaC was activated in type II alveolar epithelial cells using real-time PCR and Western blotting. In the LPS group, the mRNA (Fig. 7A) and protein (Fig. 7B) levels of α-ENaC, β-ENaC, and γ-ENaC were decreased compared with the control group. However, OMT injection restored the levels of α-ENaC, β-ENaC, and γ-ENaC.
Fig. 7.
ENaC subunits in rat type II alveolar epithelial cells. The cells were treated with 500 µg/ml OMT; 30 min later, the cells were exposed to LPS (15 µg/ml) for 6 h. A. Real-time PCR analysis of α-ENaC, β-ENaC, and γ-ENaC. B. Western blotting analysis of α-ENaC, β-ENaC, and γ-ENaC. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. **P<0.01 compared with the control group. #P<0.05 compared with the LPS group.
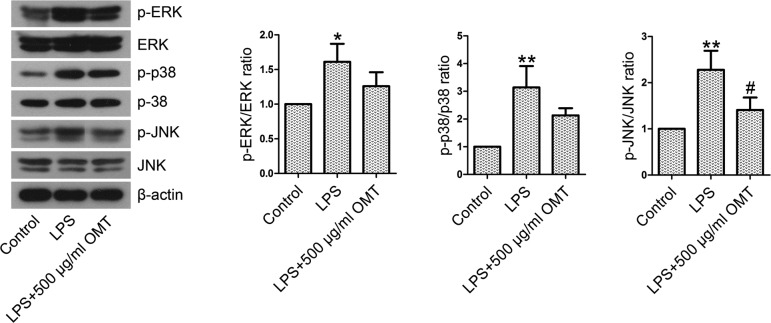
OMT inhibits the activation of the JNK pathway in type II alveolar epithelial cells
The expression levels of MAPK pathway members and their phosphorylated forms in type II alveolar epithelial cells were examined by Western blotting. As shown in Fig. 8, the protein levels of p-ERK, p-p38, and p-JNK were shown to be elevated by LPS exposure. OMT preconditioning significantly reduced the ratios of p-JNK/JNK in LPS-stimulated cells. The differences were not statistically significant, although p-ERK/ERK and p-p38/p38 ratios were decreased by OMT preconditioning.
Fig. 8.
The activation of the MAPK signaling pathway in rat type II alveolar epithelial cells. Thirty minutes following OMT (500 µg/ml) treatment, the cells were exposed to LPS (15 µg/ml) for 30 min. The expression levels of p-ERK, ERK, p-p38, p38, p-JNK, and JNK were quantified by Western blotting analysis. The ratios of p-ERK/ERK, p-p38/p38, and p-JNK/JNK were calculated. Data are expressed as the mean ± SD. Results were analyzed by one-way ANOVA followed by Bonferroni post hoc test. *P<0.05 compared with the control group. **P<0.01 compared with the control group. #P<0.05 compared with the LPS group.
Discussion
LPS is the pathogenic constituent of P. aeruginosa and can induce strong immune responses in bodies [2]. LPS has been widely used to establish the experimental model of ALI [32, 42]. Our present study investigated the protective effect of oxymatrine (OMT) preconditioning on acute lung injury (ALI) and further elucidated the possible mechanisms.
Xu et al. demonstrated that OMT attenuates oleic acid-induced ALI by inhibiting pulmonary edema and inflammatory cell infiltration in alveolar spaces [34]. Yuan et al. found that sodium ferulate combined with OMT decreases inflammatory cells in BALF and alleviates pulmonary edema in a LPS-induced ALI mouse model [36]. Additionally, OMT has been applied in the treatment of acute pancreatitis and acute intestinal inflammation due to its anti-inflammatory effect [13, 41]. In our study, Wright-Giemsa staining results consistently showed that OMT dose-dependently decreased LPS-induced increases of inflammatory cell (white blood cells, macrophages, neutrophils, and lymphocyte) numbers in BALF. C-reactive protein (CRP) is an important indicator of inflammation and correlates with the severity of diseases, which is markedly increased after bacterial infection [21]. TNF-α is a vital pro-inflammatory cytokine that can activate other cytokines and chemokines to aggravate lung tissue injury. Zhang et al. found that OMT pretreatment inhibits the expression of TNF-α and IL-1β in LPS-stimulated macrophages [40]. Moreover, we found that OMT significantly reduced the upregulated levels of CRP and TNF-α in lung tissues induced by LPS exposure. The data suggest that OMT administration may protect against LPS-induced ALI via its anti-inflammatory effect.
ALI is characterized by inflammation, pulmonary edema, and alveolar-capillary barrier damage. The lung W/D weight ratio is an index of pulmonary edema [39]. In our study, we found that OMT significantly alleviated histopathological changes and inhibited pulmonary edema induced by LPS.
The epithelial sodium channel (ENaC) consists of three subunits (α, β, and γ) [26]. Alpha-ENaC forms the channel pore, while β-ENaC and γ-ENaC are associated with the channel activity [24, 26]. ENaC plays a major role in sodium and water homeostasis [4]. Knockdown of α-ENaC by siRNA transfection inhibits lung fluid absorption in SD rats [23]. Hummler et al. found that α-ENaC-deficient neonatal mice fail to clear lung fluid, develop respiratory distress, and die within 40 h of birth [19]. Roux et al. reported that IL-1β reduces α-ENaC expression and activity, inhibits lung epithelial sodium absorption, and ultimately contributes to alveolar edema via the p38 MAPK signaling pathway [31]. We found that LPS administration decreased the expression levels of α-ENaC, β-ENaC, and γ-ENaC in lung tissues and alveolar epithelial cells, whereas OMT treatment increased α-ENaC, β-ENaC, and γ-ENaC levels. The data suggest that OMT administration may alleviate pulmonary edema via ENaC.
MAPKs, including extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun N-terminal kinase (JNK), are members of the serine/threonine kinase family, which participate in signal transduction in eukaryotic organisms [22]. MAPKs play important roles in inflammation, oncogenesis, cell proliferation, apoptosis, differentiation, and stress responses [17, 20]. The MAPK signaling pathway is inhibited by OMT administration in LPS-induced mastitis in mice [35]. Moreover, OMT protects rats against myocardial injury in a rat model of sepsis via suppression of the p38 MAPK signaling pathway [37]. We found that OMT administration significantly inhibited LPS-induced activation of the MAPK signaling pathway in a mouse model of ALI. As expected, we consistently observed suppression of the JNK signaling pathway in OMT-treated alveolar epithelial cells in vitro. LPS-induced activation of the ERK and p38 pathways was slightly inhibited by OMT; however, the difference was not statistically significant. The data suggest that OMT administration may protect against LPS-induced ALI in vivo and in vitro by inhibiting the JNK signaling pathway. Toll-like receptor 4 (TLR4) can recognize and interact with LPS and subsequently activate the downstream MAPK pathways, eventually leading to an inflammatory response [12, 15]. Previous studies reported that LPS-induced ALI can be attenuated by other drugs through the TLR4-mediated MAPK pathway [16, 33]. OMT may also ameliorate ALI induced by LPS by inhibiting the TLR4-mediated signaling pathways; however, the precise mechanism requires further investigation and verification.
In summary, the results demonstrated that pretreatment with OMT attenuated LPS-induced ALI in both C57BL/6 mice and rat alveolar epithelial cells. The inhibitory effects of OMT on ALI were associated with the ENaC and JNK signaling pathway.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Boncoeur E., Tardif V., Tessier M.C., Morneau F., Lavoie J., Gendreau-Berthiaume E., Grygorczyk R., Dagenais A., Berthiaume Y.2010. Modulation of epithelial sodium channel activity by lipopolysaccharide in alveolar type II cells: involvement of purinergic signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 298: L417–L426. doi: 10.1152/ajplung.00170.2009 [DOI] [PubMed] [Google Scholar]
- 2.Buyck J.M., Verriere V., Benmahdi R., Higgins G., Guery B., Matran R., Harvey B.J., Faure K., Urbach V.2013. P. aeruginosa LPS stimulates calcium signaling and chloride secretion via CFTR in human bronchial epithelial cells. J. Cyst. Fibros. 12: 60–67. doi: 10.1016/j.jcf.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Wang L., Kang Q., Zhang X., Yu G., Wan X., Wang J., Zhu K.2017. Heat Shock Protein A12B Protects Vascular Endothelial Cells Against Sepsis-Induced Acute Lung Injury in Mice. Cell. Physiol. Biochem. 42: 156–168. doi: 10.1159/000477308 [DOI] [PubMed] [Google Scholar]
- 4.Chifflet S., Hernandez J.A.2016. The epithelial sodium channel and the processes of wound healing. BioMed Res. Int. 2016: 5675047. doi: 10.1155/2016/5675047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornell T.T., Fleszar A., McHugh W., Blatt N.B., Le Vine A.M., Shanley T.P.2012. Mitogen-activated protein kinase phosphatase 2, MKP-2, regulates early inflammation in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 303: L251–L258. doi: 10.1152/ajplung.00063.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagenais A., Denis C., Vives M.F., Girouard S., Massé C., Nguyen T., Yamagata T., Grygorczyk C., Kothary R., Berthiaume Y.2001. Modulation of alpha-ENaC and alpha1-Na+-K+-ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 281: L217–L230. doi: 10.1152/ajplung.2001.281.1.L217 [DOI] [PubMed] [Google Scholar]
- 7.Dagenais A., Gosselin D., Guilbault C., Radzioch D., Berthiaume Y.2005. Modulation of epithelial sodium channel (ENaC) expression in mouse lung infected with Pseudomonas aeruginosa. Respir. Res. 6: 2. doi: 10.1186/1465-9921-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng W., Li C.Y., Tong J., Zhang W., Wang D.X.2012. Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury. Respir. Res. 13: 29. doi: 10.1186/1465-9921-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong X.Q., Du Q., Yu W.H., Zhang Z.Y., Zhu Q., Che Z.H., Chen F., Wang H., Chen J.2013. Anti-inflammatory Effects of Oxymatrine Through Inhibition of Nuclear Factor-kappa B and Mitogen-activated Protein Kinase Activation in Lipopolysaccharide-induced BV2 Microglia Cells. Iran. J. Pharm. Res. 12: 165–174. [PMC free article] [PubMed] [Google Scholar]
- 10.Elias N., Rafii B., Rahman M., Otulakowski G., Cutz E., O’Brodovich H.2007. The role of alpha-, beta-, and gamma-ENaC subunits in distal lung epithelial fluid absorption induced by pulmonary edema fluid. Am. J. Physiol. Lung Cell. Mol. Physiol. 293: L537–L545. doi: 10.1152/ajplung.00373.2006 [DOI] [PubMed] [Google Scholar]
- 11.Fu L., Xu Y., Tu L., Huang H., Zhang Y., Chen Y., Tao L., Shen X.2016. Oxymatrine inhibits aldosterone-induced rat cardiac fibroblast proliferation and differentiation by attenuating smad-2,-3 and-4 expression: an in vitro study. BMC Complement. Altern. Med. 16: 241. doi: 10.1186/s12906-016-1231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J., Su S., Guo J., Zhu Y., Zhao M., Duan J.A.2018. Anti-inflammatory and anti-apoptotic effects of the combination of Ligusticum chuanxiong and Radix Paeoniae against focal cerebral ischaemia via TLR4/MyD88/MAPK/NF-κB signalling pathway in MCAO rats. J. Pharm. Pharmacol. 70: 268–277. doi: 10.1111/jphp.12841 [DOI] [PubMed] [Google Scholar]
- 13.Guzman J.R., Koo J.S., Goldsmith J.R., Mühlbauer M., Narula A., Jobin C.2013. Oxymatrine prevents NF-κB nuclear translocation and ameliorates acute intestinal inflammation. Sci. Rep. 3: 1629. doi: 10.1038/srep01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J., Zhao Y., Deng W., Wang D.X.2014. Netrin-1 promotes epithelial sodium channel-mediated alveolar fluid clearance via activation of the adenosine 2B receptor in lipopolysaccharide-induced acute lung injury. Respiration 87: 394–407. doi: 10.1159/000358066 [DOI] [PubMed] [Google Scholar]
- 15.Hu G., Hong D., Zhang T., Duan H., Wei P., Guo X., Mu X.2017. Cynatratoside-C from Cynanchum atratum displays anti-inflammatory effect via suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Chem. Biol. Interact. 279: 187–195. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y., Tao L., Tan H., Zhang M., Shimizu K., Zhang F., Zhang C.2017. An Active Drimane-Type Lactone from Polygonum jucundum Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Mice Through TLR4-MAPKs Signaling Pathway. Inflammation 40: 1204–1213. doi: 10.1007/s10753-017-0563-z [DOI] [PubMed] [Google Scholar]
- 17.Huang C., Jacobson K., Schaller M.D.2004. MAP kinases and cell migration. J. Cell Sci. 117: 4619–4628. doi: 10.1242/jcs.01481 [DOI] [PubMed] [Google Scholar]
- 18.Huang M., Hu Y.Y., Dong X.Q., Xu Q.P., Yu W.H., Zhang Z.Y.2012. The protective role of oxymatrine on neuronal cell apoptosis in the hemorrhagic rat brain. J. Ethnopharmacol. 143: 228–235. doi: 10.1016/j.jep.2012.06.028 [DOI] [PubMed] [Google Scholar]
- 19.Hummler E., Barker P., Gatzy J., Beermann F., Verdumo C., Schmidt A., Boucher R., Rossier B.C.1996. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat. Genet. 12: 325–328. doi: 10.1038/ng0396-325 [DOI] [PubMed] [Google Scholar]
- 20.Jiang M., Li J., Peng Q., Liu Y., Liu W., Luo C., Peng J., Li J., Yung K.K., Mo Z.2014. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J. Neuroinflammation 11: 167. doi: 10.1186/s12974-014-0167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juan X., Lu Y.M., Shi J.D., Deng X.Q., Long W.2011. Visfatin levels in patients with severe pneumonia. World J. Emerg. Med. 2: 132–136. doi: 10.5847/wjem.j.1920-8642.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D., Hu J., Wang T., Zhang X., Liu L., Wang H., Wu Y., Xu D., Wen F.2016. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci. Rep. 6: 37751. doi: 10.1038/srep37751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T., Folkesson H.G.2006. RNA interference for alpha-ENaC inhibits rat lung fluid absorption in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 290: L649–L660. doi: 10.1152/ajplung.00205.2005 [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Jiang B.J., Zhao R.Z., Ji H.L.2016. Epithelial sodium channels in pulmonary epithelial progenitor and stem cells. Int. J. Biol. Sci. 12: 1150–1154. doi: 10.7150/ijbs.15747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K.J., Schmittgen T.D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 26.Loh S.Y., Giribabu N., Salleh N.2016. Sub-chronic testosterone treatment increases the levels of epithelial sodium channel (ENaC)-α, β and γ in the kidney of orchidectomized adult male Sprague-Dawley rats. PeerJ 4: e2145. doi: 10.7717/peerj.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H., Zhang L., Gu L.L., Hou B.Y., Du G.H.2016. Oxymatrine induces liver injury through JNK signalling pathway mediated by TNF-α in vivo. Basic Clin. Pharmacol. Toxicol. 119: 405–411. doi: 10.1111/bcpt.12608 [DOI] [PubMed] [Google Scholar]
- 28.Migneault F., Boncoeur E., Morneau F., Pascariu M., Dagenais A., Berthiaume Y.2013. Cycloheximide and lipopolysaccharide downregulate αENaC mRNA via different mechanisms in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 305: L747–L755. doi: 10.1152/ajplung.00023.2013 [DOI] [PubMed] [Google Scholar]
- 29.Mirzapoiazova T., Kolosova I.A., Moreno L., Sammani S., Garcia J.G., Verin A.D.2007. Suppression of endotoxin-induced inflammation by taxol. Eur. Respir. J. 30: 429–435. doi: 10.1183/09031936.00154206 [DOI] [PubMed] [Google Scholar]
- 30.Randrianarison N., Clerici C., Ferreira C., Fontayne A., Pradervand S., Fowler-Jaeger N., Hummler E., Rossier B.C., Planès C.2008. Low expression of the beta-ENaC subunit impairs lung fluid clearance in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 294: L409–L416. doi: 10.1152/ajplung.00307.2007 [DOI] [PubMed] [Google Scholar]
- 31.Roux J., Kawakatsu H., Gartland B., Pespeni M., Sheppard D., Matthay M.A., Canessa C.M., Pittet J.F.2005. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J. Biol. Chem. 280: 18579–18589. doi: 10.1074/jbc.M410561200 [DOI] [PubMed] [Google Scholar]
- 32.Wu G., Wang J., Luo P., Li A., Tian S., Jiang H., Zheng Y., Zhu F., Lu Y., Xia Z.2017. Hydrostatin-SN1, a sea snake-derived bioactive peptide, reduces inflammation in a mouse model of acute lung injury. Front. Pharmacol. 8: 246. doi: 10.3389/fphar.2017.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu K.C., Huang S.S., Kuo Y.H., Ho Y.L., Yang C.S., Chang Y.S., Huang G.J.2017. Ugonin M, a Helminthostachys zeylanica Constituent, Prevents LPS-Induced Acute Lung Injury through TLR4-Mediated MAPK and NF-κB Signaling Pathways. Molecules 22: E573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu G.L., Yao L., Rao S.Y., Gong Z.N., Zhang S.Q., Yu S.Q.2005. Attenuation of acute lung injury in mice by oxymatrine is associated with inhibition of phosphorylated p38 mitogen-activated protein kinase. J. Ethnopharmacol. 98: 177–183. doi: 10.1016/j.jep.2005.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z., Yin R., Cong Y., Yang Z., Zhou E., Wei Z., Liu Z., Cao Y., Zhang N.2014. Oxymatrine lightened the inflammatory response of LPS-induced mastitis in mice through affecting NF-κB and MAPKs signaling pathways. Inflammation 37: 2047–2055. doi: 10.1007/s10753-014-9937-7 [DOI] [PubMed] [Google Scholar]
- 36.Yuan X., Wang Y., Du D., Hu Z., Xu M., Xu M., Liu Z.2012. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice. Inflammation 35: 1161–1168. doi: 10.1007/s10753-011-9424-3 [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Wang X., Bai B., Zhang R., Li Y., Wang Y.2016. Oxymatrine protects against sepsis-induced myocardial injury via inhibition of the TNF-α/p38-MAPK/caspase-3 signaling pathway. Mol. Med. Rep. 14: 551–559. doi: 10.3892/mmr.2016.5250 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Li X., Grailer J.J., Wang N., Wang M., Yao J., Zhong R., Gao G.F., Ward P.A., Tan D.X., Li X.2016. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 60: 405–414. doi: 10.1111/jpi.12322 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Xu T., Wu B., Chen H., Pan Z., Huang Y., Mei L., Dai Y., Liu X., Shan X., Liang G.2017. Targeting myeloid differentiation protein 2 by the new chalcone L2H21 protects LPS-induced acute lung injury. J. Cell. Mol. Med. 21: 746–757. doi: 10.1111/jcmm.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Yan R., Hu Y.2015. Oxymatrine inhibits lipopolysaccharide-induced inflammation by down-regulating Toll-like receptor 4/nuclear factor-kappa B in macrophages. Can. J. Physiol. Pharmacol. 93: 253–260. doi: 10.1139/cjpp-2014-0362 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z., Wang Y., Dong M., Cui J., Rong D., Dong Q.2012. Oxymatrine ameliorates L-arginine-induced acute pancreatitis in rats. Inflammation 35: 605–613. doi: 10.1007/s10753-011-9352-2 [DOI] [PubMed] [Google Scholar]
- 42.Zhu G., Xin X., Liu Y., Huang Y., Li K., Wu C.2017. Geraniin attenuates LPS-induced acute lung injury via inhibiting NF-κB and activating Nrf2 signaling pathways. Oncotarget 8: 22835–22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu T., Zhang W., Wang D.X.2012. Insulin up-regulates epithelial sodium channel in LPS-induced acute lung injury model in rats by SGK1 activation. Injury 43: 1277–1283. doi: 10.1016/j.injury.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y., Chen X., Liu Z., Peng Y.P., Qiu Y.H.2015. Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int. J. Mol. Sci. 17: E25. doi: 10.3390/ijms17010025 [DOI] [PMC free article] [PubMed] [Google Scholar]