Abstract
Rabbit mesenchymal stem cells (MSCs) are important seed cells in regenerative medicine research, particularly in translational research. In the current study, we showed that rabbit subchondral bone is a reliable source of MSCs. First, we harvested subchondral bone (SCB) from the rabbit knee-joint and initiated the MSC culture by cultivating enzyme-treated SCB. Adherent fibroblast-like cells that outgrew from SCB fulfill the common immuno-phenotypic criteria for defining MSCs, but with low contamination of CD45+ hematopoietic cells. Interestingly, differentiated SCB-MSCs expressed osteogenic and chondrogenic markers at significantly higher levels than those in bone marrow cell suspension-derived MSCs (BMS-MSCs) (P<0.05). No differences in the expression of adipogenic markers between SCB-MSC and BMS-MSC (P>0.05) were observed. Moreover, the results of the colony forming unit-fibroblast assay and sphere formation assay demonstrated that the SCB-MSCs had increased self-renewal potential. SCB-MSCs expressed higher levels of the stemness markers Nanog, OCT4, and Sox-2 compared to in BMS-MSCs (P<0.05). Furthermore, the results of both the CCK-8-based assay and CFSE dilution assay showed that SCB-MSCs exhibited enhanced proliferative capacity. In addition, SCB-MSCs exhibited higher phosphorylation of extracellular signal-related kinase/mitogen-activated protein kinase signaling, which is closely related to MSC proliferation. In conclusion, we identified SCB-MSCs as a novel stem cell population that met the requirements of MSCs; the unique properties of SCB-MSC are important for the potential treatment of tissue damage resulting from disease and trauma.
Keywords: bioresource, experimental animals, mesenchymal stem cell, rabbit, subchondral bone
Introduction
Mesenchymal stem cells (MSCs), also known as multipotent stromal cells, were first identified in the bone marrow [14]. In postnatal organisms, loosely woven and highly vascularized bone marrow form a unique niche for stem cells [14, 31]. Like hematopoietic stem cells, the multi-potency and self-renewal of MSCs are tightly controlled by the bone marrow microenvironment [8, 31, 36]. According to the requirements of the hosts, MSCs migrate out from connective tissue of bone marrow and regenerate mesenchymal tissues [25]. Increasing data have shown that MSCs play a role as promising seed cells for cellular replacement therapy for diabetes, rheumatoid arthritis, and bone repair [8, 17, 18, 31, 38]. The rabbit is a commonly used experimental animal for orthopedic application and tissue engineering because of its easy accessibility and convenient maneuverability. However, MSC-based therapies in rabbit models are limited because of contamination by hematopoietic cells. Notably, the structure of the MSC niche was typically destroyed, while rabbit MSCs were routinely cultured in bone marrow cell suspension [2, 16, 36]. We previously isolated and characterized MSCs and examined the potential application of these cells [24, 45, 48]. In our previous studies, we found that collagenase digestion efficiently loosened the tissue microstructure and facilitated MSC outgrowth from tissues without reducing cell viability. In addition, enzymic treatment induced the release of hematopoietic cells and made it easier to deplete them [19, 47].
Because mesenchymal stem cells were first described in the 1970s, many types of biological tissue have been developed as stem cell resources [15, 30]. Particularly, adipose tissue is an optimal source of proliferating, nonimmunogenic, and easily available stem cells [1, 22, 26, 35]. However, many studies have shown that the sources of origin and microenvironment greatly impact the differentiation ability of MSCs [26]. Researchers revealed that adipose tissue-derived MSCs show decreased osteogenic and chondrogenic differentiation capacity compared to bone marrow-derived MSCs [9, 42]. Therefore, attention should be given to subchondral bone (SCB), which is accessible in orthopedics surgery and has a similar microenvironment, to facilitate bone and cartilage injury regeneration.
Therefore, we hypothesized that culturing the SCB and allowing MSCs to migrate out from their stem cell niche may be an efficient strategy for obtaining viable and homogeneous rabbit MSC populations. We digested SCB and conducted MSC (SCB-MSC) culture using these cells. Our results showed that SCB-MSCs display enhanced osteo-chondrogenic differentiation, self-renewal, and proliferation potential compared to bone marrow suspension-derived MSCs (BMS-MSCs).
Materials and Methods
Isolation and culture of SCB-MSCs
MSCs were isolated from male New Zealand White rabbits (3–4 weeks of age, from the Laboratory Animal Center of the Academy of Military Medical Sciences of China, Beijing, China). All experiments in this study were performed in accordance with the Academy of Military Medical Sciences Guide for Laboratory Animals. To isolate SCB-MSCs, the knee joints of the rabbit were carefully excised with scissors, and the subchondral bones were collected using forceps. Subchondral bone fragments were cultured in α-MEM containing 10% (vol/vol) fetal bovine sum (FBS) (Solarbio, Hyclone, Logan, UT, USA) in the presence of 1 mg ml−1(wt/vol) of collagenase II (Gibco, Grand Island, NY, USA) at 37°C for 20 min. The digestion medium and released cells were discarded, and the enzyme-treated SCBs were seeded into a plastic culture dish (250 ml) in the presence of α-MEM supplemented with 10% (vol/vol) FBS. The culture medium was changed on the third day of culture, and the tissue debris was maintained to allow more MSCs to outgrow. To isolate BMS-MSCs, the bone marrow was flushed out from the marrow cavity in the tibiae and femurs and mononuclear cells were isolated from the bone marrow suspensions by routine density gradient centrifugation. The cells were then seeded on a plastic dish (BD Biosciences, Franklin Lakes, NJ, USA , 100 × 15 mm), and the MSCs were allowed to adhere for 72 h before the total volume of the culture medium was changed.
Flow cytometry analysis
SCB-MSCs and BMS-MSCs were harvested at passages 3–6 by trypsin digestion and stained individually with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies against rabbit CD44, CD45, CD14, CD79a, CD81, or CD90 (BD Biosciences and Abcam, Cambridge, UK ) for 30 min in the dark at 4°C. After two washes with PBS, the cells were collected with a FACScan (BD Biosciences) and the data were analyzed using WinMDI 2.9 software.
Multi-differentiation of MSCs
Multi-differentiation analysis of MSCs was performed as described previously with minor modifications [19, 47]. Briefly, for osteogenic differentiation, MSCs at passage 3 were seeded into 24-well culture plates (1 ml/well) at a density of 5 × 103 cells/cm2, grown in osteogenic induction medium for 14 days, and subjected to alkaline phosphatase (ALP) staining. The osteogenic induction medium consisted of culture medium, 0.1 µM dexamethasone, 10 mM β-glycerophosphate, and 50 µM ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA). The osteogenic differentiation of MSCs was assayed by in situ ALP staining with a commercial kit (Sigma-Aldrich).
For adipogenic differentiation, MSCs at passage 3 were seeded into 24-well culture plates at a density of 1 × 104 cells/cm2, incubated in adipogenic induction medium for 14 days, and subjected to Oil-Red-O staining. The adipogenic induction medium consisted of culture medium, 1 µM dexamethasone, 0.2 mM indomethacin, 0.5 mM 3-butyl-L-methylxanthine (IBMX), and 0.01 mg/ml insulin (Sigma-Aldrich). The accumulation of lipid vacuoles in MSCs was evaluated by in situ Oil-Red-O staining.
For chondrogenic differentiation, 4 × 105 MSCs were centrifuged in polypropylene tubes to form a pelleted micromass and maintained in chondrogenic induction medium consisting of α-MEM supplemented with 10−7 M dexamethasone, 1% (vol/vol) insulin-transferrin-sodium selenite, 50 µM ascorbate-2 phosphate, 1 mM sodium pyruvate, 50 µg/ml (wt/vol) proline, and 20 ng/ml (wt/vol) TGF-β3. On day 21, the pellets were fixed and sectioned as previously described [47]. The development of chondrocytes and accumulation of the cartilage matrix were evaluated by toluidine blue staining.
Colony-forming unit-fibroblast (CFU-F) assay
The clonogenic potential of MSCs was tested in a colony-forming unit-fibroblast (CFU-F) assay as described previously with minor revisions [11]. Briefly, MSCs at passage 1 were seeded into a 6-well plate (Corning, Inc., Corning, NY, USA , 16.8 ml/well) at a density of 1 × 103/well and maintained in culture medium. To detect the formation of CFU-F, the cultured cells in three replicates were stained with 3% crystal violet in methanol for 10 min at days 5, 10, and 15. All visible colonies larger than 5 mm in diameter were counted.
Sphere formation assay
The clonogenic potential of the MSCs was further tested in a sphere formation assay [20, 31]. MSCs at passage 1 were seeded at 2 × 105/cm2 on an ultra-low attachment dish (Corning) in α-MEM supplemented with 10% (vol/vol) FBS. Primary cell spheres were counted after 3 days in culture, trypsinized, and re-plated. Secondary spheres were counted on day 6.
CCK8 assay
MSC proliferation assays were performed using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) [41]. Briefly, MSCs at passage 3 were seeded into 96-well plates (Thermo Scientific, Waltham, MA, USA) at a density of 1 × 103 cells/cm2, cultured in α-MEM medium with 10% FBS (6 wells in each group), added to CCK-8 solution at a ratio of 100 µL/µL, and incubated at 37°C for 1 h. Absorbance was then measured at a wavelength of 450 nm using a microplate reader (BMG LABTECH, Offenburg, Germany). In the current study, CCK-8 experiments were performed on days 1, 5, 7, 10, and 13.
CFSE dilution assay
Moreover, the proliferation of SCB-MSCs and BMS-MSCs was also examined in a CFSE dilution assay. Briefly, MSCs were suspended at a concentration of 107 cells/ml in PBS containing 2% FBS. MSCs were incubated in the presence of 10 µM CFSE for 20 min in the dark, followed by blockage of CFSE incorporation by FBS. The cells were then washed twice before they were re-plated. MSCs were harvested on days 2 and 4. The dye dilution was assayed with a FACSCalibur instrument and data were analyzed using WinMdi2.8 software.
Cell cycle assay
MSCs were seeded at 5 × 103 cells/cm2 and cultured in α-MEM medium with 10% FBS. At 80–90% confluence, the MSCs were collected for cell cycle analysis. Briefly, the MSCs were washed and fixed overnight in 70% ethanol at −20°C in 1.5-ml microcentrifuge tubes (Biologix, Shandong, China). The fixed cells were then washed and incubated in 100 µg/ml propidium iodide (Sigma-Aldrich) and 20 ng/ml RNase (Sigma-Aldrich) in PBS for 30 min. Cell cycle analysis was then conducted by flow cytometry. Independent experiments were replicated at least three times. The cell subpopulations in the G0/G1 and S phases were calculated by gating analysis based on differences in DNA content.
Real-time polymerase chain reaction
Aliquots of MSCs (2 × 105) at passages 3–6 were seeded in 6-well culture plates and maintained in osteogenic/adipogenic/chondrogenic induction medium for 7 days before they were harvested. Total RNA was extracted from MSCs with TRIzol reagent (Invitrogen) and reverse-transcribed using the mRNA Selective PCR Kit (TaKaRa, Shiga, Japan). Rabbit HPRT, Runx-2, osteopontin (OPN), CEBP/α, PPARγ, Sox-9, collagen I, Nanog, OCT4, and Sox2 cDNA were amplified by real-time PCR using the SYBR Green PCR kit (Sigma). The primer sequences used for real-time PCR are shown in Table 1.
Table 1. Primer sequences.
| genes | primersequences | Annealing temperature | |
|---|---|---|---|
| HPRT | forward | 5′-GACCAGTCAACAGGGGACAT-3′ | 60 °C |
| reverse | 5′-ACACTTCGAGGGGTCCTTTT-3′ | ||
| Runx2 | forward | 5′-ATTTCTCACCTCCTCAGCCC-3′ | |
| reverse | 5′-TCCCAAGTTTCCCTCATCCC-3′ | ||
| OPN | forward | 5′-TTTTGTCTCTTGGGCATGGC-3′ | |
| reverse | 5′-GCATTCTGCGGTGTTAGGAG-3′ | ||
| CEBP/α | forward | 5′-GGGACGCTAGGTGACAGAAT-3′ | |
| reverse | 5′-GAAAGGACGCTGGCTGAAAA-3′ | ||
| PPARγ | forward | 5′-TTGCTGTGGGGATGTCTCAT-3′ | |
| reverse | 5′-TTTCCTGTCAAGATCGCCCT-3′ | ||
| Sox9 | forward | 5′-ATGAAGATGACCGACGAGCA-3′ | |
| reverse | 5′-ACTTGTCCTCTTCGCTCTCC-3′ | ||
| Collage I | forward | 5′-CCAAGGGAGAGCAAGGAGAA-3′ | |
| reverse | 5′-CCTTTGGGGCCTTCTTTTCC-3′ | ||
| Nanog | forward | 5′-AAAACTCCCGACTCTGCAGA-3′ | |
| reverse | 5′-AGGCTGGAGAGTTCTTGCAT-3′ | ||
| 4-Oct | forward | 5′-CGGAAGAGAAAGCGAACGAG-3′ | |
| reverse | 5′-TGGCCTCAAAATCCTCTCGT-3′ | ||
| Sox2 | forward | 5′-AAGGGAAATGGGGAGAGGTG-3′ | |
| reverse | 5′-TGGATGGGATTGGTGGTCTC-3′ |
Western blotting
MSCs at passage 3 and 6 were plated in 6-well plates at a density of 1 × 105 cells/cm2 and starved in serum-free α-MEM medium for at least 6 h. Protein lysis buffer (Bio-Rad, Hercules, CA, USA) was added, and thawed lysates were vortexed and centrifuged. The proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked by incubation with 5% wt/vol nonfat dry milk. Membranes were then incubated with anti-ERK, anti-phospho-ERK, and β-actin (Sigma) Abs at the appropriate dilutions overnight at 4°C. After incubation, the membranes were washed in Tris-buffered saline containing Tween-20 (TBST). Secondary antibody conjugated to horseradish peroxidase was added to the membranes in 5% nonfat dry milk in TBST. The negative control was used as described previously. The western blotting assay was performed at least 3 times independently, representative results are shown.
Statistical analysis
The data were expressed as the mean values with the standard deviation. Statistical significance was analyzed by Student’s t test and two-tailed P-values were calculated, and P<0.05 was considered statistically significant. The error bars in all figures represent the standard deviation.
Results
SCB-MSC exhibit morphological features and surface antigens similar to those of BMS-MSCs
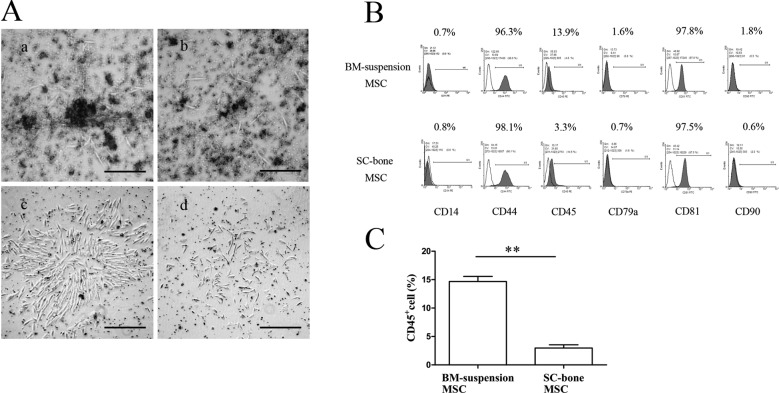
Forty-eight hours after the primary culture, fibroblast-like cells migrated out from the digested SCB fragments and adhered to the dish (Fig. 1Aa), whereas a few elongated adhesion cells were observed in the dish in which the bone marrow cell suspension cells were seeded (Fig. 1Ab). An adherent layer of vortex-shaped cells developed within 6 days (Fig. 1Ac), whereas a culture confluence of only 30–40% was achieved when the nuclear cells were cultivated (Fig. 1Ad). Further, the results of immuno-phenotyping showed that both SCB-MSCs and BMS-MSCs were homogenously positive for the mesenchymal markers CD44 and CD81 but negative for the hematopoietic markers CD14 and CD45 and co-stimulating molecule CD79α (Fig. 1B). Unlike human MSCs, it remains controversial whether rabbit MSCs are positive for CD90 [2, 28, 36]. Our results showed that SCB-MSCs and BMS-MSCs were negative for CD90 (Fig. 1B). In addition, the percentage of CD45+ cells in the SCB-MSCs (3.31 ± 0.78%) was significantly lower than that in BMS-MSCs (13.93 ± 1.63%) (**P<0.01), demonstrating that a homogeneous cell population was expanded from the digested subchondral bone (Fig. 1C).
Fig. 1.
Morphologic and immuno-phenotypic features of SCB-MSCs and BMS-MSCs. A: The morphologic characteristics of MSCs in two groups. The bar represents 200 µm. B: The immuno-phenotypic features of two groups. Both groups were homogenously positive for mesenchymal markers but negative for hematopoietic markers. C: The comparison of CD45+ cells between the SCB-MSC group and BMS-MSC group (3.31 ± 0.78% vs 13.93 ± 1.63%) (**P<0.01). SCB-MSCs, subchondral bone-derived MSCs; BMS-MSCs, bone marrow suspension-derived MSCs.
SCB-MSCs display enhanced osteogenic and chondrogenic differentiation potential
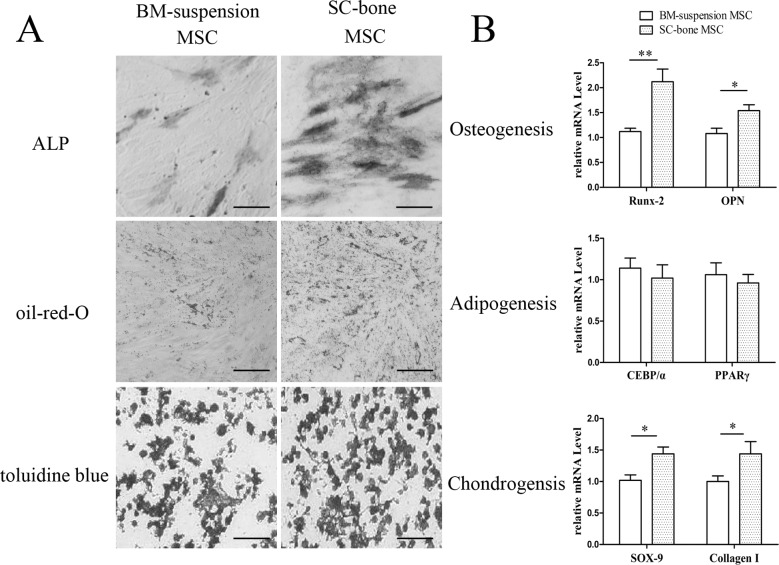
Although the SCB-MSCs and BMS-MSCs shared similar morphologic and immuno-phenotypic features, SCB-MSCs display enhanced differentiation capacity compared to BMS-MSCs. Analysis of osteogenic differentiation showed higher ALP activity in SCB-MSCs than in BMS-MSCs after 14 days of induction (Fig. 2A). Additionally, the analysis of chondrogenic differentiation showed that more SCB-MSCs developed into toluidine blue-positive chondrocytes, indicating that the cells secreted sulfated proteoglycan at a higher level to form a cartilage extracellular matrix (Fig. 2A). However, no significant differences were observed in the accumulation of intracellular Oil-Red-O-stained lipids, indicating that SCB-MSCs and BMS-MSCs shared a similar adipogenic differentiation capacity (Fig. 2A). Complementing the results of histochemical analysis, SCB-MSCs after induction exhibited high levels of mRNA expression of osteogenic markers (Runx-2 and OPN) and chondrogenic markers (Sox-9 and Collage I) (*P<0.05; **P<0.01, Fig. 2B). The mRNA expression of adipogenic transcription factor CEBP/α and PPARγ in SCB-MSCs was similar to that in BMS-MSCs (Fig. 2B).
Fig. 2.
Results of multi-differentiation induction and RT-PCR assay. A: ALP and Oil-Red-O staining showed higher osteogenic and chondrogenic potential in the SCB-MSC group after induction. There were no significant differences in adipogenic potential between the two groups. The bar represents 200 µm. B: Comparison of mRNA expression levels of osteogenic (Runx-2 and OPN), chondrogenic (Sox-9 and collagen I) and adipogenic (CEBP/α and PPARγ) markers between the two groups. SCB-MSCs, subchondral bone-derived MSCs; BMS-MSCs, bone marrow suspension-derived MSCs.
SCB-MSCs display higher self-renewal potential
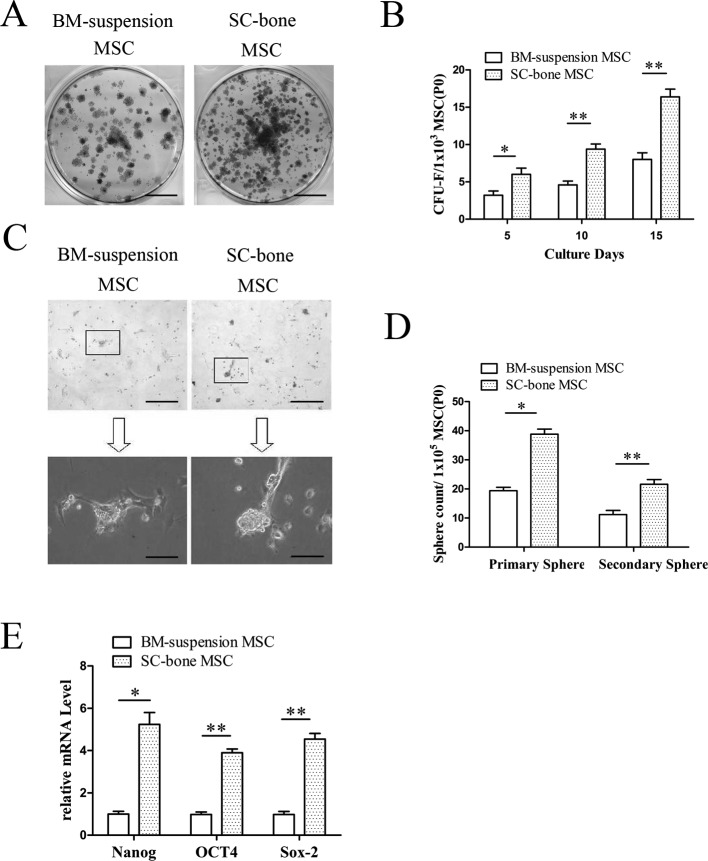
Functional MSCs were initially identified by their capacity to form clonogenic cell clusters in vitro, a common feature different to other stromal cell populations. In the current study, self-renewal potential was measured in a CFU-F assay and sphere formation assay. As indicated in Figs. 3A and B, the CFU-F frequency remained relatively higher in SCB-MSCs than in BMS-MSCs (SCB-MSCs versus BMS-MSCs: 6.33 ± 0.94 versus 3 ± 0.82, 11 ± 1.63 versus 5.67 ± 0.94, 17 ± 0.82 versus 10.67 ± 1.25 for days 5, 10, and 15, respectively. *P<0.05; **P<0.01).
Fig. 3.
CFU-F assay, sphere formation assay, and stemness markers. A, B: CFU-F frequency remained relatively higher in the SCB-MSC group than in the BMS-MSC group. The bar represents 1 cm in 3A. SCB-MSCs, subchondral bone-derived MSCs; BMS-MSCs, bone marrow suspension-derived MSCs. C, D: The results of primary and secondary sphere culture revealed a significant difference between the two groups. The bars represent 100 µm in 3C upper and 200 µm in 3C low, respectively. E: Comparison of mRNA expression of several stemness markers (Nanog, OCT4, and Sox-2) between two groups.
Sphere formation assays have long been used to evaluate progenitor/multipotent cell populations in epithelial systems. Recent studies suggested that MSCs can also produce spheres [20, 31]. Three days after culture on ultra-low adherent tissue culture plates, sphere formation was evident in the SCB-MSC group and BMS-MSC group (Fig. 3C). These spheres were disassociated and re-plated on non-adherent plates. Fewer spheres developed after another 3 days of culture. Interestingly, there was a noticeable difference in primary and secondary sphere number in SCB-MSC culture compared with to in BMS culture (SCB-MSCs versus BMS-MSCs: 38 ± 9.53 versus 22 ± 0.82, 24.67 ± 3.21 versus 10 ± 1.63 for primary spheres and secondary spheres, respectively. Fig. 3D, *P<0.05; **P<0.01).
The results of the CUF-F and sphere formation assays strongly suggest that SCB-MSCs have an increased stem cell population that can self-renew. To further explore the cause of enhanced self-renewal, we next measured the mRNA expression of several stemness markers (Nanog, OCT4, and Sox-2) in SCB-MSCs [4, 7, 27, 34]. The data indicated that SCB-MSCs displayed significantly higher transcription levels of Nanog, OCT4, and Sox-2 than in BMS-MSCs (Fig. 3E, *P<0.05; **P<0.01).
SCB-MSCs display enhanced proliferative capacity
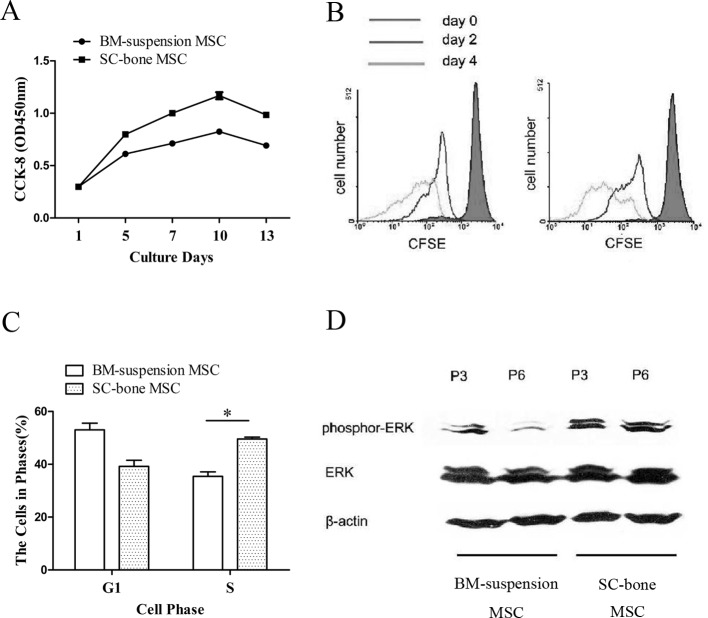
To investigate the proliferation ability of SCB-MSCs, a CCK-8 assay and CFSE dilution assay were performed. The results of the CCK-8-based cell proliferation assay (Fig. 4A) showed that SCB-MSCs exerted stronger proliferative effects than BMS-MSCs (*P<0.05). Consistently, the CFSE data showed that a higher proportion of SCB-MSCs underwent cell division on days 2 and 4 (Fig. 4B), indicating that these cells had an enhanced proliferation capacity.
Fig. 4.
SCB-MSCs display enhanced proliferative capacity. A: CCK-8-based cell proliferation assay indicated that the SCB-MSC group harbors stronger proliferative potential than the BMS-MSC group (*P<0.05). B: CFSE data on days 2 and 4 showed that a greater proportion of SCB-MSCs underwent cell division, indicating enhanced proliferation potential. C: The results of cell cycle analysis showed a higher percentage of SCB-MSCs (50 ± 1.41%) were in the S phase compared to BMS-MSCs (36.5 ± 3.55%). D: The data showed enhanced Erk1/2 phosphorylation in passages 3 and 6 SCB-MSCs. SCB-MSCs, subchondral bone-derived MSCs; BMS-MSC, bone marrow suspension-derived MSCs.
Enhanced cell proliferation is also reflected by an increased number of cells in the S phase and decreased number of cells arrested in the G0/G1 phase. A higher percentage of SCB-MSCs (50 ± 1.41%) were in S phase compared to BMS-MSCs (36.5 ± 3.55%) (Fig. 4C), indicating that an increased number of cells proceeded into G2/S phase (*P<0.05).
Because ERK-MAPK signaling is involved in controlling cell proliferation [6, 13], we further examined the phosphorylation of ERK-MAPK in the cells. The data in Fig. 4D shows enhanced Erk1/2 phosphorylation in passages 3 and 6 SCB-MSCs. The results support that SCB-MSC harbors an enhanced proliferation capacity.
Discussion
Rabbit MSCs are important seed cells in regenerative medicine research, particularly in translational research. A variety of healthy tissues have been developed as stem cell resources, including bone marrow, blood, umbilical cord, placenta, fat, heart, brain, skin, muscle, liver, gonads, and teeth [37]. Many studies have shown that the differentiation ability of MSCs varies greatly from different resources. In the orthopedics field, SCB has received attention in regeneration research [10, 21, 40, 44, 46].
To identify MSCs, surface antigen markers were tested. It remains controversial weather rabbit MSC express CD90 based on previous studies. Tan et al. (2013) characterized rabbit MSCs and found that they expressed CD90 [36]. Bakhtina (2014) and Lee (2014) compared the surface markers between human and rabbit MSCs and found rabbit MSCs did not express CD90 [2, 28]. The results of flow cytometry analysis in the present study showed that rabbit MSCs were CD90-negative, which is in accordance with the previous reports. The adult bone marrow contains niches that control the multi-differentiation potential and self-renewal capacity of stem cells [3]. Several studies demonstrated that implanted bone marrow could support long-term repopulating cells in vivo [5, 39]. Therefore, maintaining the bone marrow niche in primary culture may be beneficial for MSC properties. In the present study, we initiated MSC culture using digested rabbit SCBs, which are mainly composed of adipose tissue and vessel networks.
Our results suggest that SCB-MSCs meet the generally accepted criteria, [12] including the fibroblast-like morphology, typical cell surface profile, and multi-lineage differentiation capacity. It had been widely accepted that MSCs cultured from different tissues share many common features, but the differentiation potential vary [9, 26]. In this study, the results showed that SCB-MSCs gain enhanced osteogenic and chodrogenic differentiation potential that is comparable to that of BMS-MSCs, which is important for the potential treatment of tissue damage resulting from disease and trauma.
Several factors have been reported to influence MSC self-renewal capacity, including cell passages, differentiation, and other factors [23, 33, 43]. In the present study, we demonstrated that SCB-MSCs, when undergoing differentiation into osteoblasts and chondrocytes, maintain a higher self-renewal capacity. The results of the CFU-F and sphere forming assays suggest that SCB-MSCs contain more potent cells. Nanog, OCT4, and Sox-2 are crucial stemness transcription factors, and lower expression of these proteins leads to a deficiency of self-renewal [4, 7, 27, 34]. Based on the results of the colony formation assay, SCB-MSCs expressed high levels of Nanog, OCT4, and Sox-2.
High proliferation is a fundamental property of MSCs and is important for the potential treatment of tissue damage resulting from disease and trauma. The CCK-8 assay and CFSE dilution assay are widely used to analyze the proliferation of stem cells [29, 32]. Because ERK-MAPK signaling is involved in controlling cell proliferation, phosphorylation of ERK-MAPK in MSCs was also detected in this present study. The results showed SCB-MSCs grew at a higher rate than their marrow counterparts. These results demonstrate that the proliferation of MSCs was improved in SCB culture.
There were also many limitations in our study. First, the most widely used MSC resource in regenerative medicine domain is fat tissue, umbilical cord, and placenta. We only compared bone marrow-derived MSCs and SCB-derived MSCs in the present study. Second, all tests were performed in vitro in this study, and an animal joint injury model would be useful in further studies to explore the differences between different source origin-derived MSCs in vivo. Third, the mechanism of differentiation and proliferation potential changes should be evaluate in further studies.
Conclusion
In conclusion, our results support that maintaining the bone marrow niche in MSC culture minimizes the negative impact on cell yield and purity while retaining enhanced multi-potency, self-renewal, and proliferation potential of MSCs. However, the precise mechanism regulating the fate of SCB-MSCs requires further investigation. The results also suggest that SCB is a novel resource for rabbit MSCs and may provide helpful information for understanding MSC niches.
Conflict of Interest
The authors declare no competing financial interests.
Acknowledgments
This study was supported by the National Natural Science Foundation (81572159, and 81371945) and the Beijing Natural Sciences Grants (No. 7182123).
References
- 1.Bajek A., Gurtowska N., Olkowska J., Kazmierski L., Maj M., Drewa T.2016. Adipose-derived stem cells as a tool in cell-based therapies. Arch. Immunol. Ther. Exp. (Warsz.) 64: 443–454. doi: 10.1007/s00005-016-0394-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakhtina A., Tohfafarosh M., Lichtler A., Arinzeh T.L.2014. Characterization and differentiation potential of rabbit mesenchymal stem cells for translational regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 50: 251–260. doi: 10.1007/s11626-013-9702-5 [DOI] [PubMed] [Google Scholar]
- 3.Bardelli S., Moccetti M.2017. Remodeling the human adult stem cell niche for regenerative medicine applications. Stem Cells Int. 2017: 6406025. doi: 10.1155/2017/6406025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu-Roy U., Ambrosetti D., Favaro R., Nicolis S.K., Mansukhani A., Basilico C.2010. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 17: 1345–1353. doi: 10.1038/cdd.2010.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigildeev A.E., Zhironkina O.A., Lubkova O.N., Drize N.J.2013. Interleukin-1 beta is an irradiation-induced stromal growth factor. Cytokine 64: 131–137. doi: 10.1016/j.cyto.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Cao Y., Xia D.S., Qi S.R., Du J., Ma P., Wang S.L., Fan Z.P.2013. Epiregulin can promote proliferation of stem cells from the dental apical papilla via MEK/Erk and JNK signalling pathways. Cell Prolif. 46: 447–456. doi: 10.1111/cpr.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A.2007. Nanog safeguards pluripotency and mediates germline development. Nature 450: 1230–1234. doi: 10.1038/nature06403 [DOI] [PubMed] [Google Scholar]
- 8.da Silva Meirelles L., Caplan A.I., Nardi N.B.2008. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26: 2287–2299. doi: 10.1634/stemcells.2007-1122 [DOI] [PubMed] [Google Scholar]
- 9.Danisovic L., Varga I., Polák S., Ulicná M., Hlavacková L., Böhmer D., Vojtassák J.2009. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen. Physiol. Biophys. 28: 56–62. doi: 10.4149/gpb_2009_01_56 [DOI] [PubMed] [Google Scholar]
- 10.de Girolamo L., Bertolini G., Cervellin M., Sozzi G., Volpi P.2010. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury 41: 1172–1177. doi: 10.1016/j.injury.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 11.Ding L., Zhu H., Yang Y., Wang Z.D., Zheng X.L., Yan H.M., Dong L., Zhang H.H., Han D.M., Xue M., Liu J., Zhu L., Guo Z.K., Wang H.X.2014. Functional mesenchymal stem cells remain present in bone marrow microenvironment of patients with leukemia post-allogeneic hematopoietic stem cell transplant. Leuk. Lymphoma 55: 1635–1644. doi: 10.3109/10428194.2013.858815 [DOI] [PubMed] [Google Scholar]
- 12.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E.2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 13.Eom Y.W., Oh J.E., Lee J.I., Baik S.K., Rhee K.J., Shin H.C., Kim Y.M., Ahn C.M., Kong J.H., Kim H.S., Shim K.Y.2014. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 445: 16–22. doi: 10.1016/j.bbrc.2014.01.084 [DOI] [PubMed] [Google Scholar]
- 14.Friedenstein A.J., Chailakhyan R.K., Latsinik N.V., Panasyuk A.F., Keiliss-Borok I.V.1974. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17: 331–340. doi: 10.1097/00007890-197404000-00001 [DOI] [PubMed] [Google Scholar]
- 15.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P.1968. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6: 230–247. doi: 10.1097/00007890-196803000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Galindo S., Herreras J.M., López-Paniagua M., Rey E., de la Mata A., Plata-Cordero M., Calonge M., Nieto-Miguel T.2017. Therapeutic effect of human adipose tissue-derived mesenchymal stem cells in experimental corneal failure due to limbal stem cell niche damage. Stem Cells 35: 2160–2174. doi: 10.1002/stem.2672 [DOI] [PubMed] [Google Scholar]
- 17.Ge Y., Gomez N.C., Adam R.C., Nikolova M., Yang H., Verma A., Lu C.P., Polak L., Yuan S., Elemento O., Fuchs E.2017. Stem cell lineage infidelity drives wound repair and cancer. Cell 169: 636–650.e14. doi: 10.1016/j.cell.2017.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin M.D., Elliman S.J., Cahill E., English K., Ceredig R., Ritter T.2013. Concise review: adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells 31: 2033–2041. doi: 10.1002/stem.1452 [DOI] [PubMed] [Google Scholar]
- 19.Guo Z., Li H., Li X., Yu X., Wang H., Tang P., Mao N.2006. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells 24: 992–1000. doi: 10.1634/stemcells.2005-0224 [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez G.M., Kong E., Sabbagh Y., Brown N.E., Lee J.S., Demay M.B., Thomas D.M., Hinds P.W.2008. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1-/- mice. Proc. Natl. Acad. Sci. USA 105: 18402–18407. doi: 10.1073/pnas.0805925105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilas D.C., Churchman S.M., McGonagle D., Jones E.2017. Targeting subchondral bone mesenchymal stem cell activities for intrinsic joint repair in osteoarthritis. Future Sci OA 3: FSO228. doi: 10.4155/fsoa-2017-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im G.I.2017. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. A 105: 2640–2648. doi: 10.1002/jbm.a.36089 [DOI] [PubMed] [Google Scholar]
- 23.Ishii M., Kino J., Ichinohe N., Tanimizu N., Ninomiya T., Suzuki H., Mizuguchi T., Hirata K., Mitaka T.2017. Hepatocytic parental progenitor cells of rat small hepatocytes maintain self-renewal capability after long-term culture. Sci. Rep. 7: 46177. doi: 10.1038/srep46177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X.X., Zhang Y., Liu B., Zhang S.X., Wu Y., Yu X.D., Mao N.2005. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105: 4120–4126. doi: 10.1182/blood-2004-02-0586 [DOI] [PubMed] [Google Scholar]
- 25.Kfoury Y., Scadden D.T.2015. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 16: 239–253. doi: 10.1016/j.stem.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 26.Kocan B., Maziarz A., Tabarkiewicz J., Ochiya T., Banaś-Ząbczyk A.2017. Trophic activity and phenotype of adipose tissue-derived mesenchymal stem cells as a background of their regenerative potential. Stem Cells Int. 2017: 1653254. doi: 10.1155/2017/1653254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavial F., Acloque H., Bertocchini F., Macleod D.J., Boast S., Bachelard E., Montillet G., Thenot S., Sang H.M., Stern C.D., Samarut J., Pain B.2007. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 134: 3549–3563. doi: 10.1242/dev.006569 [DOI] [PubMed] [Google Scholar]
- 28.Lee T.C., Lee T.H., Huang Y.H., Chang N.K., Lin Y.J., Chien P.W., Yang W.H., Lin M.H.2014. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One 9: e111390. doi: 10.1371/journal.pone.0111390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma X.H., Xu X., Zou C.Y., Zhao Y., Wang Z.J., Wang H.Y., Wang Y.F., Hu Z.B.2016. [Effect of human umbilical cord-derived mesenchymal stem cells on proliferation and differentiation of leukemia cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 24: 1710–1715(in Chinese). [DOI] [PubMed] [Google Scholar]
- 30.McKee C., Chaudhry G.R.2017. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 159: 62–77. doi: 10.1016/j.colsurfb.2017.07.051 [DOI] [PubMed] [Google Scholar]
- 31.Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma’ayan A., Enikolopov G.N., Frenette P.S.2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834. doi: 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochoa-Gonzalez F., Cervantes-Villagrana A.R., Fernandez-Ruiz J.C., Nava-Ramirez H.S., Hernandez-Correa A.C., Enciso-Moreno J.A., Castañeda-Delgado J.E.2016. Metformin induces cell cycle arrest, reduced proliferation, wound healing impairment in vivo and is associated to clinical outcomes in diabetic foot ulcer patients. PLoS One 11: e0150900. doi: 10.1371/journal.pone.0150900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders A., Faiola F., Wang J.2013. Concise review: pursuing self-renewal and pluripotency with the stem cell factor Nanog. Stem Cells 31: 1227–1236. doi: 10.1002/stem.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo E., Basu-Roy U., Zavadil J., Basilico C., Mansukhani A.2011. Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage. Mol. Cell. Biol. 31: 4593–4608. doi: 10.1128/MCB.05798-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J.2012. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 21: 2724–2752. doi: 10.1089/scd.2011.0722 [DOI] [PubMed] [Google Scholar]
- 36.Tan S.L., Ahmad T.S., Selvaratnam L., Kamarul T.2013. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 222: 437–450. doi: 10.1111/joa.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatullo M., Codispoti B., Pacifici A., Palmieri F., Marrelli M., Pacifici L., Paduano F.2017. Potential use of human periapical cyst-mesenchymal stem cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 5: 103. doi: 10.3389/fcell.2017.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udalamaththa V.L., Jayasinghe C.D., Udagama P.V.2016. Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res. Ther. 7: 110. doi: 10.1186/s13287-016-0366-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varas F., Grande T., Ramírez A., Bueren J.A.2000. Implantation of bone marrow beneath the kidney capsule results in transfer not only of functional stroma but also of hematopoietic repopulating cells. Blood 96: 2307–2309. [PubMed] [Google Scholar]
- 40.Wang Y., Xu J., Zhang X., Wang C., Huang Y., Dai K., Zhang X.2017. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 8: e2715. doi: 10.1038/cddis.2017.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu F.F., Zhu H., Li X.M., Yang F., Chen J.D., Tang B., Sun H.G., Chu Y.N., Zheng R.X., Liu Y.L., Wang L.S., Zhang Y.2014. Intercellular adhesion molecule-1 inhibits osteogenic differentiation of mesenchymal stem cells and impairs bio-scaffold-mediated bone regeneration in vivo. Tissue Eng. Part A 20: 2768–2782. doi: 10.1089/ten.tea.2014.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L., Liu Y., Sun Y., Wang B., Xiong Y., Lin W., Wei Q., Wang H., He W., Wang B., Li G.2017. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 8: 275. doi: 10.1186/s13287-017-0716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S.J., Kim H.J., Lee E.S., Park C.G., Cho S.J., Jeon S.H.2017. β-Catenin accumulation is associated with increased expression of Nanog protein and predicts maintenance of MSC self-renewal. Cell Transplant. 26: 365–377. doi: 10.3727/096368916X693040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zedde P., Cudoni S., Giachetti G., Manunta M.L., Masala G., Brunetti A., Manunta A.F.2016. Subchondral bone remodeling: comparing nanofracture with microfracture. An ovine in vivo study. Joints 4: 87–93. doi: 10.11138/jts/2016.4.2.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Li C., Jiang X., Zhang S., Wu Y., Liu B., Tang P., Mao N.2004. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp. Hematol. 32: 657–664. doi: 10.1016/j.exphem.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 46.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C., Askin F.B., Frassica F.J., Chang W., Yao J., Carrino J.A., Cosgarea A., Artemov D., Chen Q., Zhao Z., Zhou X., Riley L., Sponseller P., Wan M., Lu W.W., Cao X.2013. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19: 704–712. doi: 10.1038/nm.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H., Guo Z.K., Jiang X.X., Li H., Wang X.Y., Yao H.Y., Zhang Y., Mao N.2010. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 5: 550–560. doi: 10.1038/nprot.2009.238 [DOI] [PubMed] [Google Scholar]
- 48.Zhu H., Jiang X.X., Guo Z.K., Li H., Su Y.F., Yao H.Y., Wang X.Y., Li X.S., Wu Y., Liu Y.L., Zhang Y., Mao N.2009. Tumor necrosis factor-alpha alters the modulatory effects of mesenchymal stem cells on osteoclast formation and function. Stem Cells Dev. 18: 1473–1484. doi: 10.1089/scd.2009.0021 [DOI] [PubMed] [Google Scholar]