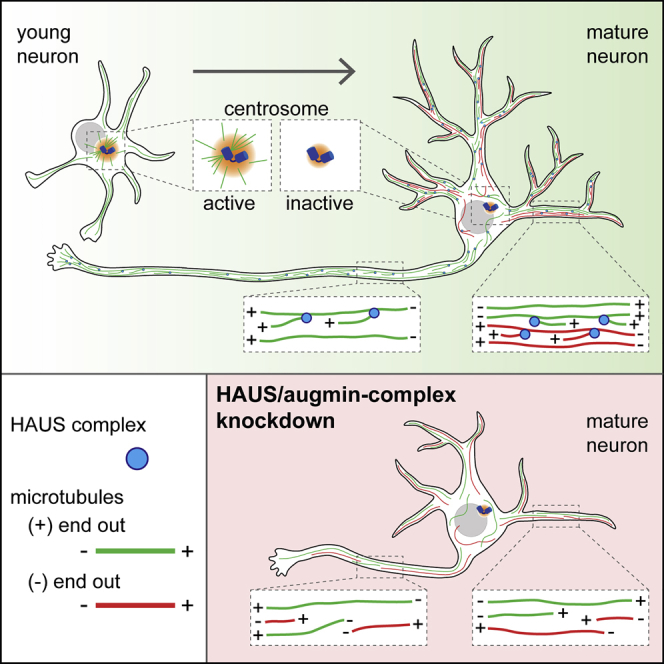
Summary
Neuron morphology and function are highly dependent on proper organization of the cytoskeleton. In neurons, the centrosome is inactivated early in development, and acentrosomal microtubules are generated by mechanisms that are poorly understood. Here, we show that neuronal migration, development, and polarization depend on the multi-subunit protein HAUS/augmin complex, previously described to be required for mitotic spindle assembly in dividing cells. The HAUS complex is essential for neuronal microtubule organization by ensuring uniform microtubule polarity in axons and regulation of microtubule density in dendrites. Using live-cell imaging and high-resolution microscopy, we found that distinct HAUS clusters are distributed throughout neurons and colocalize with γ-TuRC, suggesting local microtubule nucleation events. We propose that the HAUS complex locally regulates microtubule nucleation events to control proper neuronal development.
Keywords: centrosome, neuron, microtubule, actin, nucleation, migration, development
Graphical Abstract
Highlights
-
•
The HAUS/augmin complex regulates migration and polarization in vivo
-
•
Axonal and dendritic development are regulated by HAUS/augmin complex
-
•
HAUS/augmin regulates microtubule density in dendrites and polarity in axons
-
•
Discrete clusters of HAUS/augmin regulate local microtubule nucleation in neurons
Cunha-Ferreira et al. report that the HAUS/augmin complex regulates neuronal migration, polarization, and development. In neurons, the HAUS complex is distributed as discrete clusters regulating local microtubule nucleation. These findings shed light into how microtubules are generated in developing neurons after centrosome inactivation in early development.
Introduction
Microtubule (MT) assembly, organization, and remodeling are major determinants of neuronal morphology and function (Conde and Cáceres, 2009). In mammalian neurons, MTs are typically plus-end out in axons and of mixed polarity in dendrites, allowing directional transport of organelles and signaling molecules to specific compartments (Kapitein and Hoogenraad, 2015). Typically, MT nucleation is mediated by the centrosome and depends on γ-tubulin, which assembles into multi-subunit complexes, the γ-tubulin small complex (γ-TuSC) and the large γ-tubulin ring complex (γ-TuRC). Neurons, like other differentiated cells, undergo centrosome inactivation throughout development, and MT nucleation is reassigned at acentrosomal sites (Stiess et al., 2010, Sulimenko et al., 2017). Although great progress has been made regarding minus-end MT stabilization (Akhmanova and Hoogenraad, 2015), how acentrosomal MTs are generated in neurons is still an open question. Initial studies suggested that MTs originated from severing of pre-existing MTs nucleated from the centrosome (Baas et al., 2005). However, this mechanism fails to explain how MTs are generated in older neurons with inactive centrosomes. Dendritic MT nucleation has been suggested to occur at Golgi outposts in Drosophila (Ori-McKenney et al., 2012, Yalgin et al., 2015, Zhou et al., 2014). However, Golgi outposts are absent from dendrites that require MT nucleation for development (Nguyen et al., 2014).
MTs can be also generated on other MTs in a process that depends on the γ-TuRC complex (Goshima et al., 2008, Lawo et al., 2009, Petry et al., 2011). In mammals, this process depends on a complex of eight subunits termed the HAUS/augmin complex (Lawo et al., 2009, Uehara et al., 2009). Initially identified in Drosophila (Goshima et al., 2007), HAUS/augmin subunits have been found to regulate mitotic spindle assembly in Drosophila and human cells or cortical MT organization in plants (Goshima et al., 2008, Lawo et al., 2009, Liu et al., 2014). Real-time visualization of HAUS-dependent MT nucleation in X. laevis meiotic extracts (Petry et al., 2011, Petry et al., 2013) and Arabidopsis cortical epidermal pavement cells (Liu et al., 2014) unraveled a key feature of this complex: generation of MTs with conserved polarity (Kamasaki et al., 2013, Petry et al., 2013). Recently the HAUS complex was suggested to regulate uniform MT polarity and density in axons (Sánchez-Huertas et al., 2016). However, it remains unknown where the HAUS complex localizes in neurons, whether HAUS can nucleate MTs, and to what extent HAUS is required for development.
In this study, we assess the role of the HAUS complex during neuronal development and polarization. We show that HAUS is required for neuronal migration, axonal and dendritic development, and MT organization. Furthermore, we characterize HAUS localization as discrete clusters that seem to engage in MT nucleation events. We propose a model whereby clusters of the HAUS complex mediate MT nucleation in neurons to ensure proper development.
Results
The HAUS Complex Is Required for Neuronal Migration, Axon Formation, and Polarization In Vivo
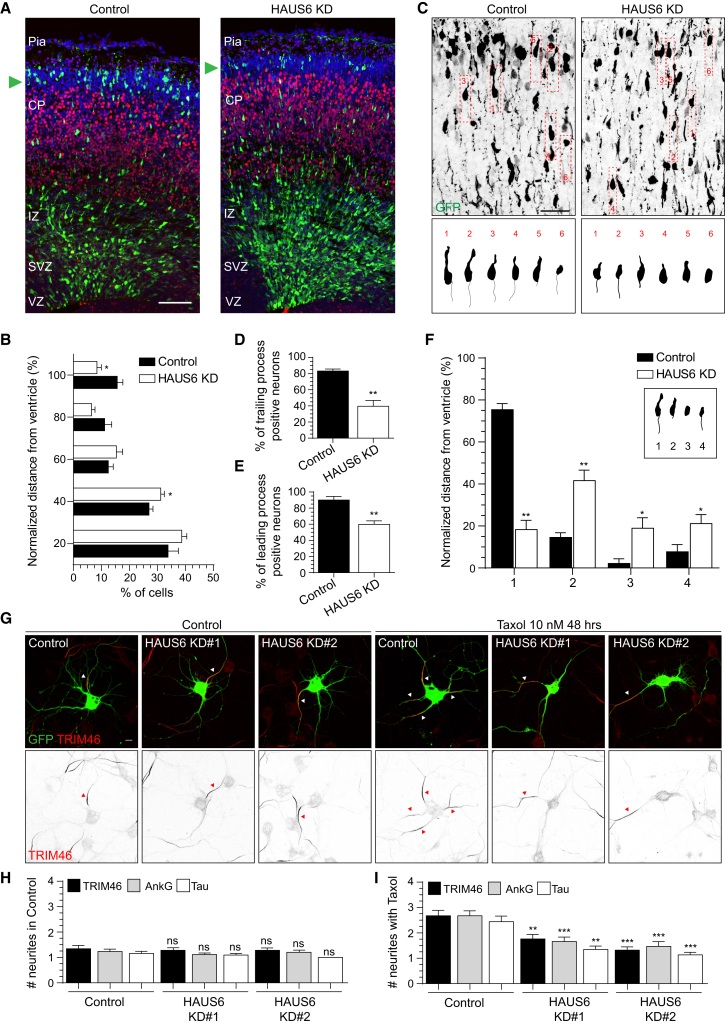
In the developing neocortex, neurons migrate from the ventricular zone across different layers toward the cortical plate (Barnes and Polleux, 2009). To test whether the HAUS complex is involved in neuronal migration, we performed in utero electroporation of E14.5 mouse embryos using short hairpin RNAs (shRNAs) targeting murine HAUS6 and a GFP plasmid to allow the identification of electroporated neurons (Figures 1A and 1B). We confirmed the efficiency of shRNA-mediated HAUS6 knockdown in murine IMCD3 cells, which showed impaired mitotic progression and increased spindle abnormalities (Figures S1A–S1C) as previously reported (Lawo et al., 2009). Following electroporation, embryos developed 3 additional days in utero before analysis. While control neurons reached the upper cortical layers, depletion of HAUS6 impaired migration and neurons remained in the sub-ventricular and intermediate zones (Figures 1A and 1B). During migration, neurons polarize and develop a trailing process that later becomes the axon and a leading edge that will develop as the apical dendrite. In our experiments, most GFP-positive control neurons showed bipolar morphology, whereas neurons depleted of HAUS6 lacked the trailing (∼53% compared with control) and leading (∼33% compared with control) (Figures 1C–1F) processes. Together these data show that the HAUS complex is required for axon formation, neuronal polarization, and migration in vivo.
Figure 1.
The HAUS Complex Regulates Neuronal Migration and Polarization In Vivo
(A) Low magnification stitched maximum-intensity projection and quantification of migrating neurons in E17.5 mouse cortex positively electroporated in utero at E14.5 with GFP and pSuper control or HAUS6 shRNAs. CP, cortical plate; IZ, intermediate zone; Pia, Pial surface; SVZ, sub-ventricular zone; VZ, ventricular zone. Green, GFP; red, Ctip2; blue, DAPI. GFP-positive neurons at the pial surface are indicated with a green arrowhead.
(B) Normalized migration distribution along the radial axis from the ventricle to the pial surface of GFP-positive neurons (n = 15 or 16, N = 6).
(C) High-magnification maximum-intensity projections of E17.5 mouse cortical neurons positively electroporated in utero at E14.5 with GFP and pSuper control or HAUS6 shRNAs.
(D and E) Percentage of trailing (D) or leading process-positive (E) neurons in pSuper control and HAUS6 shRNAs electroporated brains (n = 51–92, N = 3).
(F) Quantification of neuronal morphology in pSuper control and HAUS6 shRNAs electroporated brains (n = 51–92, N = 3).
(G) DIV6 hippocampal neurons co-transfected with pSuper control, HAUS6 knockdown (KD) #1, 2 shRNAs and GFP at DIV2 and treated at DIV4 with control vehicle (DMSO) or Taxol. Green, GFP; red, TRIM46. TRIM46-positive processes are highlighted with a white arrowhead in merged images and red arrowhead in gray-scale inverted images.
(H and I) Average number of processes positive for TRIM46, AnkG, and Tau in control (H) and Taxol treatment (I) (n = 18–34, N = 3 for TRIM46 and AnkG; N = 2 for Tau).
Graphs represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 100 μm in (A), 40 μm in (C), and 10 μm in (G). See also Figure S1 and Table S1.
The HAUS Complex Is Required for Axon Specification In Vitro
The first step in neuron polarization is axon formation. It is well known that increased MT stability along the neuritic shaft precedes axon formation and that MT dynamics drives polarization. Furthermore, the MT-stabilizing drug Taxol was shown to induce the formation of supernumerary axons (Witte et al., 2008). To test whether the HAUS complex is required for multiple axon formation, we combined Taxol treatment with HAUS6 depletion (Figures 1G–1I and S1D). We designed two separate shRNAs against HAUS6 and confirmed their efficiency by immunocytochemistry (Figures S1E–S1I). In control, Taxol treatment increased the mean number of processes positive for three axonal markers, TRIM46, AnkG, and Tau. By contrast, knockdown of HAUS6 prevented the formation of supernumerary axons in the presence of Taxol (Figures 1G–1I and S1D). These data show that HAUS is required for the formation of axons upon stabilization of MTs with Taxol in vitro. In addition, supernumerary axons positive for TRIM46, an early instructor in axon polarization (van Beuningen et al., 2015), fail to assemble after HAUS depletion, indicating that HAUS is required at an early stage of axon specification.
The HAUS Complex Regulates Axonal and Dendritic Development
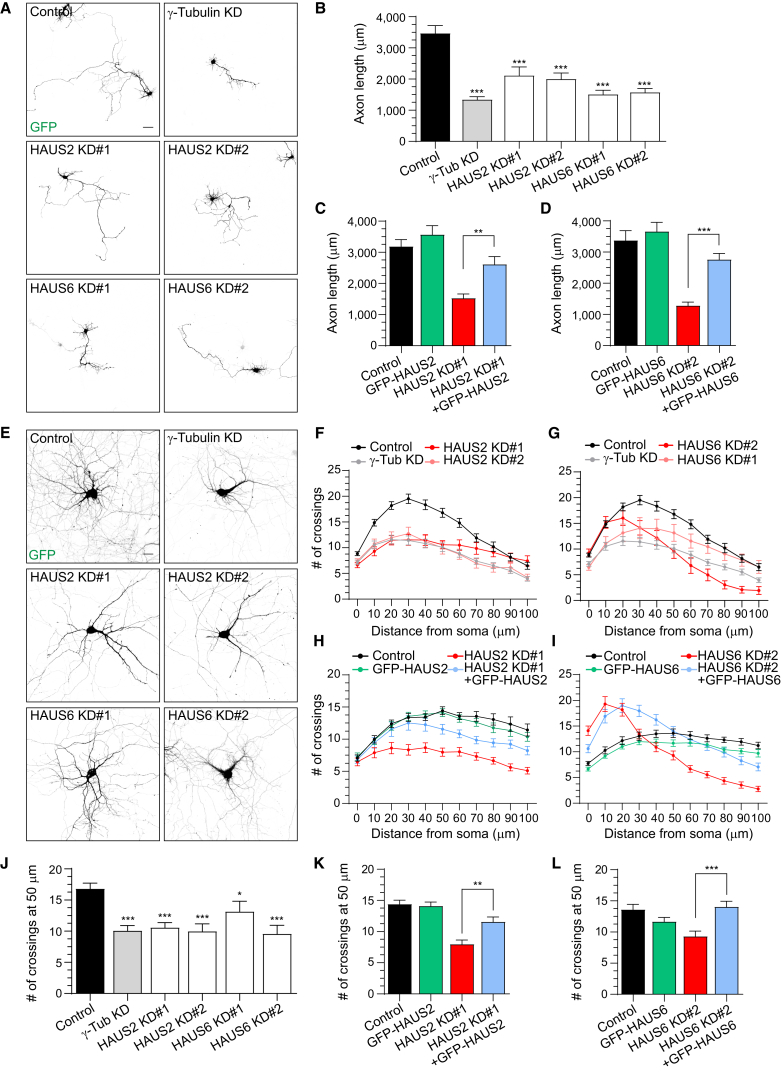
To assess whether HAUS is required for axonal development, we designed additional shRNAs for HAUS2. Axonal arborization was analyzed at day in vitro (DIV) 5 after depletion of HAUS2, HAUS6, and the positive control γ-tubulin (Yau et al., 2014). HAUS2 and HAUS6 knockdown severely impaired axonal growth, similar to γ-tubulin depletion (Figures 2A and 2B). Contrary to HAUS depletion, overexpression of GFP-HAUS2 or GFP-HAUS6 had no effect on total axon arborization (Figures 2C, 2D, and S2A). Co-expression of GFP-HAUS2 and GFP-HAUS6 rescued their respective axonal phenotypes (Figures 2C, 2D, and S2A). We also determined the effect of HAUS2 and HAUS6 depletion on dendritic development using Sholl analysis. Depletion of HAUS2 and HAUS6 phenocopied γ-tubulin depletion with a reduction in total dendritic branching and complexity compared with controls (Figures 2E–2G and 2J). We were able to partially rescue knockdown phenotypes with GFP-HAUS2 and GFP-HAUS6 co-transfection, and overexpression of GFP-HAUS2 or GFP-HAUS6 alone did not alter dendritic complexity (Figures 2H, 2I, 2K, 2L, and S2B). Collectively, these data show that the HAUS complex is required for both axonal and dendritic development in hippocampal neurons.
Figure 2.
The HAUS Complex Regulates Axonal and Dendritic Development In Vitro
(A) DIV5 hippocampal neurons co-transfected with GFP and pSuper control, HAUS2 KD#1, 2, HAUS6 KD#1, 2 or γ-tubulin shRNAs.
(B) Axon arborization related to (A) (n = 18–20, N = 2).
(C and D) Axon arborization of DIV5 neurons co-transfected with HA-β-galactosidase and pSuper control, HAUS2 KD#1 (C) or HAUS6 KD#2 (D) with GFP, GFP-HAUS2 or GFP HAUS6 shRNA resistant (n = 18–24 in C, n = 19–22 in D, N = 2).
(E) DIV12 hippocampal neurons co-transfected with GFP and pSuper control, HAUS2 KD#1, 2, HAUS6 KD#1, 2 or γ-tubulin shRNAs.
(F and G) Sholl analysis related to (E) (n = 20–46, N = 2; the control in F and G is the same because these are data from one common set of experiments).
(H and I) Sholl analysis of DIV12 hippocampal neurons co-transfected with HA-β-galactosidase and pSuper control, HAUS2 KD#1 (H) or HAUS6 KD#2 (I) with GFP, GFP-HAUS2 or GFP HAUS6 shRNA resistant (n = 20, N = 2 in H; n = 30, N = 3 in I).
(J) Average number of crossings at 50 μm distance from the cell body related to (F) and (G).
(K and L) Average number of crossings at 50 μm distance from the cell body related to (H) and (I).
Graphs represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 50 μm in (A) and 20 μm in (E). See also Figure S2 and Table S1.
The HAUS Complex Is Required for MT Organization in Axons and Dendrites
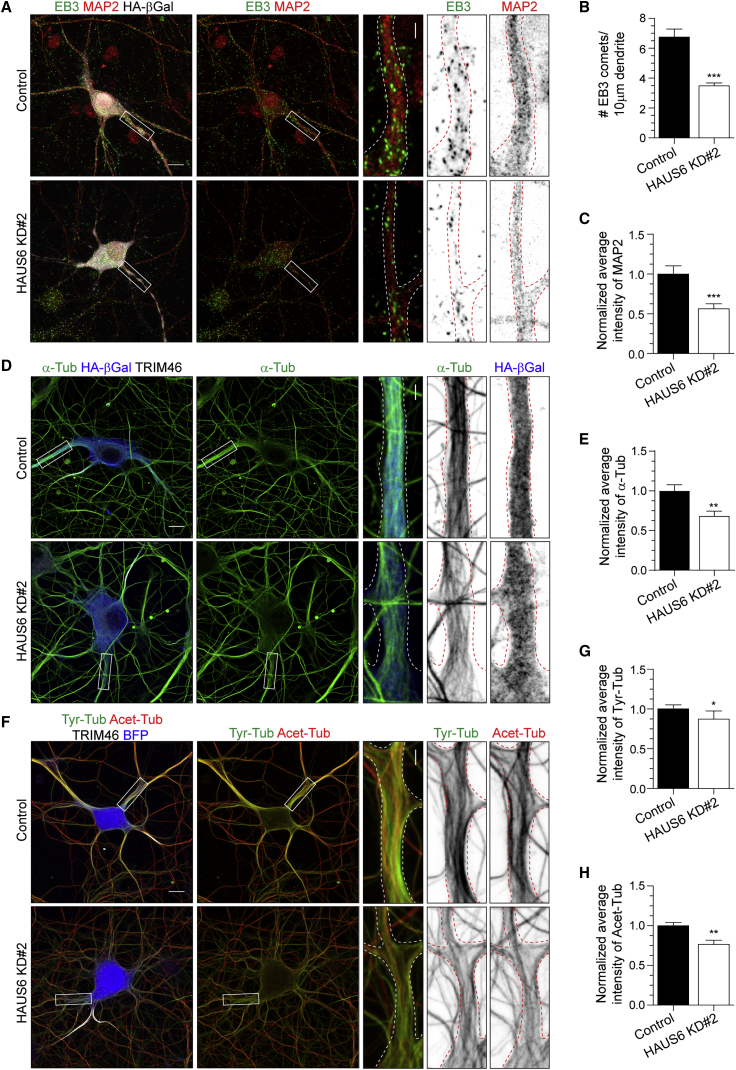
To test whether the HAUS complex is required for MT organization in neurons, we investigated the consequences of depleting HAUS6 on the cytoskeleton. The number of MT plus-ends (EB3) was analyzed along proximal dendrites after HAUS6 depletion at DIV12. In HAUS6-depleted neurons, the number of EB3 comets was reduced by ∼48% (Figures 3A and 3B). This decrease was also found in the soma (data not shown). In line with these results, MAP2 (dendritic MT-associated protein) intensity was reduced by ∼44% in proximal dendrites of HAUS6-depleted neurons (Figures 3A and 3C). To investigate if HAUS is required for maintenance of neuronal MTs, levels of polymerized tubulin were analyzed after a pre-extraction protocol that allows the extraction of soluble tubulin. Depletion of HAUS6 led to a ∼31% reduction of polymerized α-tubulin in dendrites of DIV11 neurons (Figures 3D and 3E). Total levels of α-tubulin were not changed after depletion of HAUS6, indicating that this effect is specific for polymerized α-tubulin (Figures S2C and S2D). More specifically, tyrosinated tubulin (a marker of newly assembled MTs) and acetylated tubulin (a marker of stable MTs) levels showed reductions of ∼13% and 23%, respectively, in HAUS6-depleted neurons (Figures 3F–3H). As for α-tubulin, western blot analysis showed no changes in the total pool of these proteins (Figures S2E–S2H). Taken together, these results reveal that HAUS is required for the maintenance of MT density in dendrites.
Figure 3.
The HAUS Complex Regulates MT Organization in Hippocampal Neurons
(A) DIV12 hippocampal neurons co-transfected with HA-β-galactosidase and pSuper control or HAUS6 KD#2 shRNAs. Insets depict dendrite areas. Green, EB3; red, MAP2; and gray, HA-β-galactosidase.
(B) Average number of EB3 comets per 10 μm proximal dendrite in DIV12 neurons related to (A) (n = 20, N = 2).
(C) Normalized average intensity of MAP2 at 10 μm proximal dendrite in DIV12 neurons related to (A) (n = 20, N = 2).
(D) DIV11 hippocampal neurons co-transfected with HA-β-galactosidase and pSuper control or HAUS6 KD#2 shRNAs. Insets depict dendrite areas. Green, α-tubulin; gray, TRIM46; and blue, HA-β-galactosidase.
(E) Normalized average intensity of α-tubulin at 10 μm proximal dendrite in DIV11 neurons related to (D) (n = 17, N = 2).
(F) DIV12 hippocampal neurons co-transfected with BFP and pSuper control or HAUS6 KD#2 shRNAs. Insets depict dendrite areas. Green, tyrosinated tubulin; red, acetylated tubulin; gray, TRIM46; blue, BFP.
(G and H) Normalized average intensity of tyrosinated (G) and acetylated (H) tubulin at 10 μm proximal dendrites of DIV11–12 neurons related to (F) (n = 17–19, N = 2).
Graphs represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 10 μm in panels and 2 μm in insets in (A), (D), and (F). See also Figure S2 and Table S1.
To test if HAUS regulates MT polarity in axons, we analyzed the orientation of MTs live using a GFP-MT+TIP marker in DIV11 neurons depleted of HAUS6. HAUS6 depletion increased the percentage of retrograde comets in axons ∼2.5-fold, showing that HAUS regulates MT polarity in axons (Figures S2I–S2K; Video S1), as previously reported (Sánchez-Huertas et al., 2016). In mature dendrites, dynamic MTs are maintained at a ratio of 20% minus-end out and 80% plus-end out (Yau et al., 2016). In contrast to axons, HAUS6 depletion did not change MT polarity in dendrites (Figure S2L). Collectively, these data indicate that the HAUS complex regulates the MT cytoskeleton in neurons by maintaining MT polarity in axons and density in dendrites.
This movie corresponds to Figures S2I and S2J. DIV11 neurons co-transfected with GFP-MT+TIP and pSuper control or HAUS6 KD#2 shRNAs. Increased numbers of retrograde comets can be seen after depletion of HAUS6. Total time is 10 minutes. 1 frame per second. Displayed at 60 frames per second. Scale bar 5 μm (AVI 8.20 MB).
The HAUS Complex Colocalizes with the γ-TuRC in Discrete Clusters in Neurons
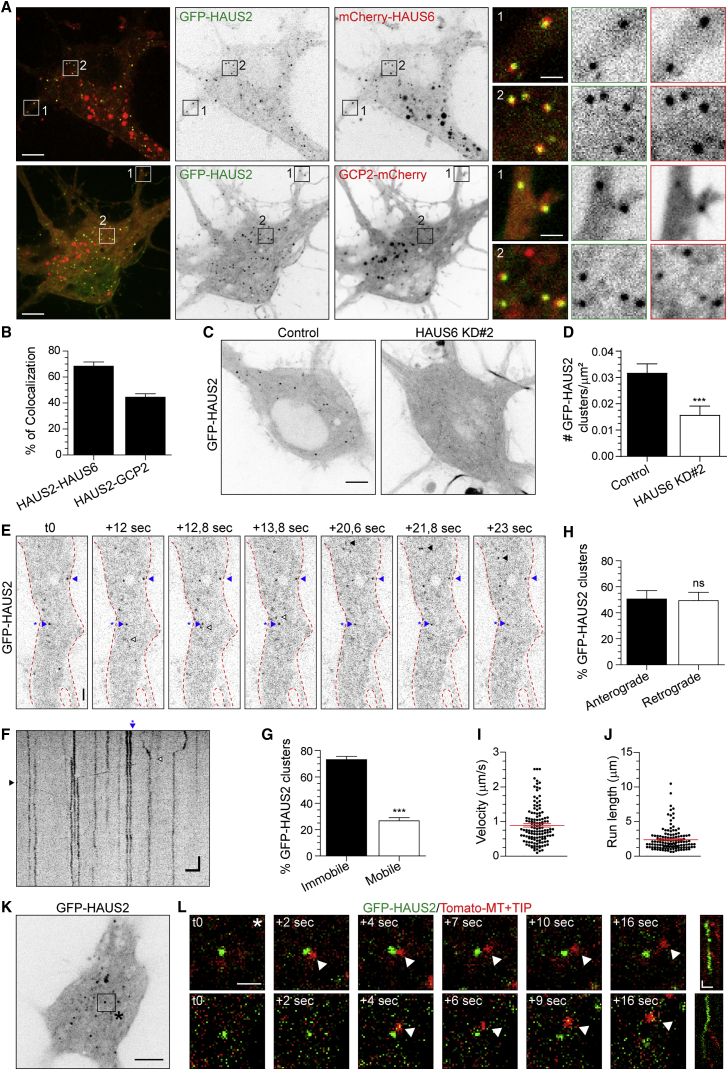
To gain insight into the function of the HAUS complex in neurons, we analyzed the localization of GFP-HAUS2. In young neurons (DIV2/4), GFP-HAUS2 localized to centrioles, as discrete clusters in the soma and along the axon (Figures S3A and S3B). GFP-HAUS2 and mCherry-HAUS6 co-expressed at DIV10–11 showed both proteins localizing at centrioles and in clusters present in soma and dendrites. HAUS2 and HAUS6 colocalized ∼68% in clusters, suggesting that the majority of clusters represent complexes containing different HAUS subunits (Figures 4A, 4B, and S3C). Furthermore, depletion of HAUS6 reduced the number of GFP-HAUS2 clusters in DIV12–15 neurons (Figures 4C and 4D). This result is in line with the observation that HAUS complex subunit localization is interdependent (Lawo et al., 2009).
Figure 4.
The HAUS Complex Colocalizes with the γ-Tubulin Complex at Acentrosomal Clusters in Hippocampal Neurons
(A) Average intensity projections of stream acquisitions of DIV10 hippocampal neurons expressing GFP-HAUS2 (green) and mCherry-HAUS6 (top) or GCP2-mCherry (bottom) (red). Insets depict indicated areas.
(B) Percentage of GFP-HAUS2 clusters colocalizing with mCherry-HAUS6 or GCP2-mCherry in DIV10–11 neurons (n = 28–51, N = 4).
(C) Average intensity projection of stream acquisition of DIV12 hippocampal neurons co-transfected with GFP-HAUS2 and pSuper control or HAUS6 KD#2 shRNAs.
(D) Density of GFP-HAUS2 clusters in DIV12–15 neurons (n = 43, N = 2).
(E and F) Stills (E) and kymograph (F) of GFP-HAUS2 in a proximal dendrite of a DIV10 neuron from 1 min stream acquisition. Time is in seconds. Anterograde and retrograde GFP-HAUS2 events are indicated with black and white arrowheads, respectively, and stationary events with blue arrowhead.
(G) Percentage of mobile and immobile GFP-HAUS2 clusters in DIV10–11 neurons (n = 24, N = 3).
(H) Percentage of anterograde and retrograde GFP-HAUS2 clusters in DIV10–11 neurons (n = 22, N = 3).
(I and J) Average velocity and run length of GFP-HAUS2 clusters in DIV10–11 neurons (n = 22, N = 3).
(K and L) Average intensity projection, stills (K) and kymographs (L) of 10 min time-lapse acquisitions of DIV17 neurons co-transfected with GFP-HAUS2 (green) and Tomato-MT+TIP (red). Top insets in (L) depict the area indicated in (K); bottom insets are from another neuron.
Graphs represent mean ± SEM. ∗∗∗p < 0.001. Scale bar, 5 μm in panels and 1 μm in insets in (A), 5 μm in (C), 2 μm in (E) and (F), 5 s in (F), 5 μm in (K), 1 μm in stills in (L), and 1 μm and 10 s in kymographs in (L). See also Figures S3 and S4 and Table S1.
Analysis of GFP-HAUS2 cluster motility in proximal dendrites of DIV10–11 neurons showed that ∼73% of clusters are immobile (Figures 4E–4G; Video S2). Remaining HAUS clusters mostly showed anterograde and retrograde mobility at ∼50% ratio (Figures 4E–4J; Video S2). To gain further insight into the mechanism underlying HAUS localization and dynamics, we searched for putative binding partners of the HAUS complex. To this end, lysates of HEK293 cells co-expressing Bio-GFP-HAUS2 or HAUS6 and the biotin-protein ligase BirA were incubated with adult brain extracts, and isolated proteins were analyzed by affinity purification-mass spectrometry (AP-MS). All subunits of the HAUS complex were co-affinity-purified in HEK293 cells (Figure S4A) as previously reported (Lawo et al., 2009). Affinity purification of HAUS2 and HAUS6 brought down various α- and β-tubulin subunits and MT-associated proteins in rat adult brains (Figures S4B–S4E), consistent with the association of the HAUS complex with the MT cytoskeleton (Sánchez-Huertas and Lüders, 2015). Interestingly, several actin-related proteins were enriched in the list of proteins pulled down with HAUS6 (Figures S4B–S4D). Analysis of protein interaction networks (Cytoscape, GeneMANIA plugin) revealed how these proteins are interconnected (Figure S4B). To better map the localization of the HAUS complex with respect to actin and MTs, we performed three-color super-resolution imaging (see Supplemental Experimental Procedures for details). In DIV4 neurons, GFP-HAUS2 clusters colocalized with the MT cytoskeleton. Surprisingly, GFP-HAUS2 clusters also localized to the actin cytoskeleton (Figures S4F and S4G). Although most clusters colocalized with both actin and MTs, some GFP-HAUS2 clusters localized exclusively to actin. These data suggest that GFP-HAUS2 clusters may associate both with the MT and the actin cytoskeleton.
This movie corresponds to Figure 4E. DIV10 neurons transfected with GFP-HAUS2. Movie shows that most clusters of GFP-HAUS2 are immobile in a proximal dendrite (25 μm). Total time is 60 s. 5 frames per second. Displayed at 30 frames per second. Scale bar 2 μm (AVI 7.28 MB).
Interestingly, in plants, MT nucleation events are associated with discrete, immobile HAUS clusters along cortical MTs (Liu et al., 2014). To address whether HAUS clusters nucleate MTs in neurons we tested for colocalization with the γ-TuRC complex. GFP-HAUS2 and GCP2-mCherry showed ∼45% colocalization, suggesting that approximately half of HAUS clusters are potential MT nucleation sites (Figures 4A, 4B, and S3C). Additionally, growing MT plus tips were found emerging from GFP-labeled HAUS2 clusters in several instances, suggesting that these represent authentic MT nucleation sites in neurons (Figures 4K, 4L, and S3D; Video S3). Collectively, these data suggest that the HAUS complex localizes as discrete clusters along the neuronal cell body and processes to allow local MT nucleation events.
This movie corresponds to Figure 4L. DIV15 neurons co-transfected with GFP-HAUS2 and Tomato-MT+TIP. Total time is 1 minute and 39 s. Displayed at 15 frames per second. Scale bar 1 μm (AVI 1.01MB).
Discussion
The ability of neurons to migrate, polarize, and differentiate depends on the MT cytoskeleton (Barnes and Polleux, 2009). In this paper, we demonstrate that the HAUS complex is required for neuronal migration, polarization, and development. Interestingly, some processes occur at a stage when the centrosome is actively nucleating MTs in neurons. These data suggest that both centrosomal and acentrosomal MT nucleation occurs at the same time during early development. One main feature of the HAUS complex is generation of MTs with conserved polarity (Kamasaki et al., 2013, Petry et al., 2013). Consistent with this, depletion of HAUS6 increased the percentage of retrograde growing MTs in axons, as previously reported by Sánchez-Huertas et al. (2016). Recent findings showing that HAUS additionally regulates MT density in axons (Sánchez-Huertas et al., 2016) suggest that HAUS mediates MT nucleation in axons to ensure MT polarity. Similarly to γ-tubulin, we found that the HAUS complex regulates neuronal polarity and axonal development, supporting the idea that HAUS-mediated MT nucleation regulates early neuronal development.
One stage of neuronal development that is likely to depend exclusively on acentrosomal MT nucleation is dendritic outgrowth, because at this stage the centrosome is fully inactivated (Nguyen et al., 2011, Nguyen et al., 2014, Ori-McKenney et al., 2012, Sánchez-Huertas et al., 2016, Stiess et al., 2010, Yau et al., 2014). In fact, γ-tubulin has been found to localize in dendrites and multiple MT growing ends emerge from varied sites suggesting that MTs are locally nucleated (Nguyen et al., 2014, Ori-McKenney et al., 2012, Yau et al., 2014). We found that depletion of the HAUS complex decreases MT density and impairs dendritic development. Depletion of HAUS6 led to a decrease in dynamic MTs (EB3), MAP2, α-tubulin, and tyrosinated tubulin. Contrary to a previous report by Sánchez-Huertas et al. (2016), we found that acetylated tubulin levels are also reduced in hippocampal neurons after depletion of HAUS6. This most likely reflects differences in analysis: Sánchez-Huertas et al. (2016) investigated axons of young neurons (DIV4), whereas our analysis was performed in dendrites of older neurons (DIV11–12). Axons are highly enriched in acetylated tubulin, so one may perhaps expect that depletion of HAUS in young neurons would not impair total levels of acetylated tubulin. Contrary to axons, HAUS6 depletion had no impact on MT polarity in dendrites, suggesting that antiparallel MT organization is regulated by alternative mechanisms (Kapitein and Hoogenraad, 2015). We propose that HAUS acts on dendritic MTs with already established polarity and regulates MT density within MT bundles (Tas et al., 2017). Collectively, these data suggest that the function of the HAUS complex is broader than previously anticipated. It is critical for neuronal migration, polarization, and development through local regulation of the MT cytoskeleton in axons and dendrites.
As in other systems (Lawo et al., 2009), localization of different HAUS subunits is interdependent in neurons, indicating that HAUS clusters represent sites of assembly for HAUS subunits into one complex. Furthermore, our findings that GCP2, a member of the γ-TuRC, colocalizes with HAUS and that dynamic MTs emerge from HAUS clusters strongly suggests that HAUS mediates local MT nucleation. We also observed a small population of HAUS2 clusters that did not colocalize with HAUS6 and GCP2. Those clusters likely represent immature complexes that are still undergoing assembly. Similar mechanisms are used in interphase pavement cells in Arabidopsis (Liu et al., 2014). A key signature of the HAUS complex is the generation of MTs along mother MTs: branched MT nucleation (Sánchez-Huertas and Lüders, 2015). Here we found that GFP-HAUS2 clusters colocalize with MTs, which could indicate that branched MT nucleation occurs in neurons. The high density of the MT cytoskeleton and/or the short lifespan of these MT configurations could explain why we have not been able to detect branched MTs in neurons. In fact, HAUS-nucleated MTs have been shown to be highly mobile or hardly detectable in other systems (Kamasaki et al., 2013, Lecland and Lüders, 2014, Liu et al., 2014, Petry et al., 2013).
Results from AP-MS indicate that the HAUS complex interacts with the actin and MT cytoskeleton in adult brains, suggesting that both cytoskeleton networks may cooperate with the HAUS complex to control local MT nucleation events. It is possible that MTs and actin contribute to the transport of HAUS subunits for complex assembly and local MT nucleation. In fact, ∼27% of GFP-HAUS2 clusters are mobile. Unraveling the mechanism by which the actin or MT cytoskeleton regulates HAUS complex function is an interesting challenge for future studies.
In summary, we show that the HAUS complex regulates MT organization in neurons, playing an important role in neuronal polarization, development, and migration. HAUS appears as discrete clusters throughout the soma and neurites of hippocampal neurons, suggesting local MT nucleation. This work provides a novel mechanism whereby decentralized MT nucleation at HAUS clusters ensures proper MT remodeling during neuronal development.
Experimental Procedures
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Casper Hoogenraad (c.hoogenraad@uu.nl).
Animals
All experiments were approved by the Dutch Animal Experiments Committee (Dier Experimenten Commissie [DEC]), performed in line with institutional guidelines of University Utrecht and UMC (University Medical Center) Utrecht, and conducted in agreement with Dutch law (Wet op de Dierproeven, 1996) and European regulations (Directive 2010/63/EU). Female pregnant C57BL/6J mice were obtained from The Jackson Laboratory and female pregnant Wistar rats from Janvier. For in utero electroporation experiments, embryos of both genders were used at E14.5 stage of development. For hippocampal and cortical neuron culture experiments obtained from rat embryos, embryos of both genders at E18 stage of development were used. For further details see Supplemental Experimental Procedures.
Cell Lines
HEK293 cells (CRL1573) and mouse inner medullary collecting duct 3 (IMCD3) cells (CRL2123) were purchased from American Type Culture Collection (ATCC). Cell lines were not authenticated by the authors after purchase. For details on culturing conditions see Supplemental Experimental Procedures.
DNA and shRNA Constructs
pGW2-GFP-HAUS2 and HAUS6 were generated using a PCR-based strategy using pEGFP-C1-HAUS2 and pEGFP-C1-HAUS6 (a kind gift from Dr. Laurence Pelletier, Lunenfeld-Tanenbaum Research Institute). These constructs were used to reclone HAUS2/6 in pGW2-mCherry or Bio-GFP constructs. GCP2 was cloned in pGW2-mCherry by recloning it from pGW1-GCP2-GFP construct (a kind gift from Dr. Kah W. Yau and Dr. Kai Jiang, Department of Cell Biology, University of Utrecht). For further details on these and other constructs see Supplemental Experimental Procedures.
Antibodies and Reagents
The following antibodies were used in this study: rabbit anti-HAUS6 (Uehara et al., 2009), mouse anti-centrin clone 20H5 (04-1624; Millipore), rabbit anti-EB3 (02-1005-07; Stepanova et al., 2003), mouse anti-α-tubulin (T5168; Sigma-Aldrich), rat anti-tubulin tyrosinated clone YL1/2 (ab6160; Abcam), mouse anti-acetylated tubulin (T7451; Sigma-Aldrich). For more details on antibodies and reagents see Supplemental Experimental Procedures.
Primary Neuronal Cultures, Transfection, and Electroporation
Primary hippocampal and cortical cultures were prepared from embryonic day 18 (E18) rat brains (both genders). Hippocampal neurons were transfected with Lipofectamine 2000. Primary cortical neurons were electroporated using the Amaxa Rat Neuron Nucleofector kit (Lonza). For details see Supplemental Experimental Procedures.
In Utero Electroporation
In utero electroporation was performed as described previously (van Beuningen et al., 2015). For details see Supplemental Experimental Procedures.
AP-MS
Streptavidin beads pull-down assays were performed with HEK293 cells transfected with BirA and Bio-GFP-HAUS2, Bio-GFP-HAUS6, and Bio-GFP using polyethylenimine (PEI; Polysciences) for 48 hr according to the manufacturer’s instructions. For further details see Supplemental Experimental Procedures.
Live Imaging and Super-resolution Imaging
Live imaging of neurons was performed using spinning-disk confocal microscopy with an inverted microscope Nikon Eclipse Ti-E or a Nikon Eclipse TE2000E. Three-color single molecule localization microscopy (SMLM) was performed on a Nikon Ti microscope. For further details see Supplemental Experimental Procedures.
Statistical Analysis
All data processing and statistical analysis were performed in Excel (Microsoft) and GraphPad Prism (GraphPad Software). Diagrams were made using GraphPad Prism. Significance was defined as follows: ns, not significant; ∗p < 0.05; ∗∗p < 0.01; and ∗∗∗p < 0.001. Statistical analyses included two-tailed unpaired t tests, Mann-Whitney U tests, Kruskal-Wallis tests followed by Dunn’s multiple-comparison tests, one-way ANOVA followed by Holm-Sidak multiple-comparisons tests, Dunnett’s multiple-comparisons tests, Sidak’s multiple-comparisons tests or Tukey’s multiple-comparisons tests, two-way ANOVA followed by Dunnett’s multiple-comparisons tests or Tukey’s multiple-comparisons tests, two-tailed chi-square tests with Yates’s correction, and two-tailed Fisher’s exact tests. The assumption of normality was tested using the D’Agostino-Pearson omnibus test. The exact values of n (total number of cells analyzed), N (total number of experiments), and statistical tests for each graph presented in the paper can be found in Table S1. N and n can also be found in figure legends.
Acknowledgments
We thank Gabriela Plucinska (Utrecht University) for critical reading of the manuscript. We thank Dr. Laurence Pelletier (Lunenfeld-Tanenbaum Research Institute) for sharing HAUS reagents and Dr. Ryota Uehara (Hokkaido University) and Prof. Gohta Goshima (Nagoya University) for the HAUS6 antibody. This work was supported by the following grants: Human Frontier Science Program fellowship LT000506/2013-L to I.C.-F.; the Netherlands Organization for Scientific Research (NWO), as part of the Graduate Programme project, grant 022.003.003 to R.R.B.; European Research Council (ERC) Consolidator Grant 617050 to C.C.H.; ERC Starting Grant 336291 to L.C.K.; Marie Curie fellowship 661401 to A.C.; NWO grant NWO-ALW-VICI 865.10.010 to C.C.H.; grant NWO-ALW-VICI 865.14.004 to R.J.P.; grant NWO-ALW-VIDI 864.12.008 to L.C.K.; NWO, as part of the National Roadmap Large-Scale Research Facilities of the Netherlands, Proteins@Work, grant 184.032.201 to M.A.; and VIDI grant 723.012.102 to M.A.
Author Contributions
Conceptualization, I.C.-F., C.C.H., and L.C.K.; Validation, I.C.-F., A.C., and R.R.B.; Formal Analysis, I.C.-F., A.C., R.R.B., R.S., X.P., and J.C.W.; Investigation, I.C.-F., A.C., R.R.B., R.S., L.W., X.P., Y.A., C.M., J.C.W., and O.I.K.; Resources, I.C.-F., R.R.B., J.C.W., P.S., C.C.H., R.J.P., and M.A.; Writing – Original Draft, I.C.-F. and C.C.H.; Writing – Review & Editing, I.C.-F. and C.C.H.; Visualization, I.C.-F., A.C., and R.R.B.; Supervision, C.C.H., L.C.K., R.J.P., and M.A.; Project Administration, C.C.H. and L.C.K.; Funding Acquisition, C.C.H., L.C.K., R.J.P., M.A., I.C.-F., A.C., and R.R.B.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and three videos and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.06.093.
Contributor Information
Lukas C. Kapitein, Email: l.kapitein@uu.nl.
Casper C. Hoogenraad, Email: c.hoogenraad@uu.nl.
Supplemental Information
References
- Akhmanova A., Hoogenraad C.C. Microtubule minus-end-targeting proteins. Curr. Biol. 2015;25:R162–R171. doi: 10.1016/j.cub.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Baas P.W., Karabay A., Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–524. doi: 10.1016/j.tcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Barnes A.P., Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C., Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Goodwin S.S., Zhang N., Scholey J.M., Vale R.D., Stuurman N. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science. 2007;316:417–421. doi: 10.1126/science.1141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Mayer M., Zhang N., Stuurman N., Vale R.D. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasaki T., O’Toole E., Kita S., Osumi M., Usukura J., McIntosh J.R., Goshima G. Augmin-dependent microtubule nucleation at microtubule walls in the spindle. J. Cell Biol. 2013;202:25–33. doi: 10.1083/jcb.201304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein L.C., Hoogenraad C.C. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87:492–506. doi: 10.1016/j.neuron.2015.05.046. [DOI] [PubMed] [Google Scholar]
- Lawo S., Bashkurov M., Mullin M., Ferreria M.G., Kittler R., Habermann B., Tagliaferro A., Poser I., Hutchins J.R., Hegemann B. HAUS, the 8-subunit human augmin complex, regulates centrosome and spindle integrity. Curr. Biol. 2009;19:816–826. doi: 10.1016/j.cub.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Lecland N., Lüders J. The dynamics of microtubule minus ends in the human mitotic spindle. Nat. Cell Biol. 2014;16:770–778. doi: 10.1038/ncb2996. [DOI] [PubMed] [Google Scholar]
- Liu T., Tian J., Wang G., Yu Y., Wang C., Ma Y., Zhang X., Xia G., Liu B., Kong Z. Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Curr. Biol. 2014;24:2708–2713. doi: 10.1016/j.cub.2014.09.053. [DOI] [PubMed] [Google Scholar]
- Nguyen M.M., Stone M.C., Rolls M.M. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 2011;6:38. doi: 10.1186/1749-8104-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.M., McCracken C.J., Milner E.S., Goetschius D.J., Weiner A.T., Long M.K., Michael N.L., Munro S., Rolls M.M. Γ-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol. Biol. Cell. 2014;25:2039–2050. doi: 10.1091/mbc.E13-09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K.M., Jan L.Y., Jan Y.N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S., Pugieux C., Nédélec F.J., Vale R.D. Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc. Natl. Acad. Sci. U S A. 2011;108:14473–14478. doi: 10.1073/pnas.1110412108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S., Groen A.C., Ishihara K., Mitchison T.J., Vale R.D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Huertas C., Lüders J. The augmin connection in the geometry of microtubule networks. Curr. Biol. 2015;25:R294–R299. doi: 10.1016/j.cub.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Sánchez-Huertas C., Freixo F., Viais R., Lacasa C., Soriano E., Lüders J. Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat. Commun. 2016;7:12187. doi: 10.1038/ncomms12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T., Slemmer J., Hoogenraad C.C., Lansbergen G., Dortland B., De Zeeuw C.I., Grosveld F., van Cappellen G., Akhmanova A., Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J. Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiess M., Maghelli N., Kapitein L.C., Gomis-Rüth S., Wilsch-Bräuninger M., Hoogenraad C.C., Tolić-Nørrelykke I.M., Bradke F. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- Sulimenko V., Hájková Z., Klebanovych A., Dráber P. Regulation of microtubule nucleation mediated by γ-tubulin complexes. Protoplasma. 2017;254:1187–1199. doi: 10.1007/s00709-016-1070-z. [DOI] [PubMed] [Google Scholar]
- Tas R.P., Chazeau A., Cloin B.M.C., Lambers M.L.A., Hoogenraad C.C., Kapitein L.C. Differentiation between oppositely oriented microtubules controls polarized neuronal transport. Neuron. 2017;96:1264–1271.e5. doi: 10.1016/j.neuron.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara R., Nozawa R.S., Tomioka A., Petry S., Vale R.D., Obuse C., Goshima G. The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc. Natl. Acad. Sci. U S A. 2009;106:6998–7003. doi: 10.1073/pnas.0901587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen S.F.B., Will L., Harterink M., Chazeau A., van Battum E.Y., Frias C.P., Franker M.A.M., Katrukha E.A., Stucchi R., Vocking K. TRIM46 controls neuronal polarity and axon specification by driving the formation of parallel microtubule arrays. Neuron. 2015;88:1208–1226. doi: 10.1016/j.neuron.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalgin C., Ebrahimi S., Delandre C., Yoong L.F., Akimoto S., Tran H., Amikura R., Spokony R., Torben-Nielsen B., White K.P., Moore A.W. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nat. Neurosci. 2015;18:1437–1445. doi: 10.1038/nn.4099. [DOI] [PubMed] [Google Scholar]
- Yau K.W., van Beuningen S.F., Cunha-Ferreira I., Cloin B.M., van Battum E.Y., Will L., Schätzle P., Tas R.P., van Krugten J., Katrukha E.A. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82:1058–1073. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Yau K.W., Schätzle P., Tortosa E., Pagès S., Holtmaat A., Kapitein L.C., Hoogenraad C.C. Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. J. Neurosci. 2016;36:1071–1085. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Chang J., Wang X., Savelieff M.G., Zhao Y., Ke S., Ye B. GM130 is required for compartmental organization of dendritic Golgi outposts. Curr. Biol. 2014;24:1227–1233. doi: 10.1016/j.cub.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This movie corresponds to Figures S2I and S2J. DIV11 neurons co-transfected with GFP-MT+TIP and pSuper control or HAUS6 KD#2 shRNAs. Increased numbers of retrograde comets can be seen after depletion of HAUS6. Total time is 10 minutes. 1 frame per second. Displayed at 60 frames per second. Scale bar 5 μm (AVI 8.20 MB).
This movie corresponds to Figure 4E. DIV10 neurons transfected with GFP-HAUS2. Movie shows that most clusters of GFP-HAUS2 are immobile in a proximal dendrite (25 μm). Total time is 60 s. 5 frames per second. Displayed at 30 frames per second. Scale bar 2 μm (AVI 7.28 MB).
This movie corresponds to Figure 4L. DIV15 neurons co-transfected with GFP-HAUS2 and Tomato-MT+TIP. Total time is 1 minute and 39 s. Displayed at 15 frames per second. Scale bar 1 μm (AVI 1.01MB).