Abstract
Nerve activity can induce long-lasting, transcription-dependent changes in skeletal muscle fibers and thus affect muscle growth and fiber-type specificity. Calcineurin signaling has been implicated in the transcriptional regulation of slow muscle fiber genes in culture, but the functional role of calcineurin in vivo has not been unambiguously demonstrated. Here, we report that the up-regulation of slow myosin heavy chain (MyHC) and a MyHC-slow promoter induced by slow motor neurons in regenerating rat soleus muscle is prevented by the calcineurin inhibitors cyclosporin A (CsA), FK506, and the calcineurin inhibitory protein domain from cain/cabin-1. In contrast, calcineurin inhibitors do not block the increase in fiber size induced by nerve activity in regenerating muscle. The activation of MyHC-slow induced by direct electrostimulation of denervated regenerating muscle with a continuous low frequency impulse pattern is blocked by CsA, showing that calcineurin function in muscle fibers and not in motor neurons is responsible for nerve-dependent specification of slow muscle fibers. Calcineurin is also involved in the maintenance of the slow muscle fiber gene program because in the adult soleus muscle, cain causes a switch from MyHC-slow to fast-type MyHC-2X and MyHC-2B gene expression, and the activity of the MyHC-slow promoter is inhibited by CsA and FK506.
Skeletal muscles consist of different fiber types that express specific isoforms of myosin and other contractile protein genes (1). The diversification of skeletal muscle fiber types depends on both myoblast lineage and innervation (2, 3). The role of nerve activity on muscle fiber-type specification has been clearly demonstrated by denervation, cross-reinnervation, and electrostimulation studies (4). However, the signaling pathways that mediate nerve activity-dependent muscle gene regulation are largely unknown. Calcineurin (5) and Ras–mitogen-activated protein kinase signaling (6) has been recently implicated in the induction of the slow muscle fiber phenotype by nerve activity.
Calcineurin, a Ca2+/calmodulin-dependent protein serine/threonine phosphatase, is a mediator of Ca2+ signaling in different cell systems (7). The function of calcineurin and its major downstream effectors, the nuclear factors of activated T cells, has been studied most extensively in T cells (8). The increase in intracellular Ca2+ induced upon binding of antigen to T cell receptor leads to activation of calcineurin that dephosphorylates the cytosolic forms of nuclear factors of activated T cell transcription factors, resulting in their translocation to the nucleus. Nuclear factors of activated T cell factors bind cooperatively with other transcription factors to the promoters of the interleukin-2 gene and other genes critical for the immune response. Calcineurin is a major target for the immunosuppressive drugs, cyclosporin A (CsA) and FK506, which bind cytoplasmic cyclophilin and FK506-binding protein, respectively, forming complexes that inhibit calcineurin activity.
Recent studies indicate that calcineurin signaling is also involved in skeletal muscle growth and differentiation (9). Calcineurin was found to promote muscle cell differentiation in culture (10–13) and to stimulate slow muscle gene promoters and slow fiber differentiation both in culture and in vivo (5, 11, 14, 15). In addition, muscle hypertrophy in response to functional overload in vivo (16) and to insulin-like growth factor-1 in culture (17) was prevented by calcineurin inhibitors. However, the role of calcineurin in skeletal muscle growth and fiber-type specification is still controversial. For example, other reports show that overexpression of active calcineurin induced both fast and slow muscle-specific promoters in cultured myotubes (18), that a slow myosin light chain promoter injected into rat slow muscle was not activated by coinjection of activated calcineurin (18), and that CsA treatment in vivo did not induce changes in fiber type and myosin heavy chain proportions (19) nor prevented muscle hypertrophy in transgenic mice overexpressing insulin-like growth factor-1 (20). In addition, evidence for a functional role of calcineurin in skeletal muscle in vivo is based only on pharmacologic inhibition with CsA. However, this drug has intracellular targets that are independent of calcineurin (21, 22), and interpretation of CsA effects is further complicated by the fact that calcineurin is ubiquitously expressed and is especially abundant in neurons (7). Therefore, changes in muscle phenotype induced by CsA treatment do not necessarily reflect a cell-autonomous block of calcineurin activity in muscle fibers but might be due to altered calcineurin function in motor neurons.
To address this issue, we have examined the role of calcineurin in a regenerating muscle system in which muscle growth and slow fiber differentiation are dependent on nerve activity. The calcineurin inhibitors CsA and FK506, as well as the peptide inhibitor cain/cabin-1 (23, 24), were used in this study. Our results indicate that calcineurin activity in muscle fibers is required for the induction and the maintenance of the slow muscle gene program. In contrast, muscle fiber growth in regenerating muscle is not prevented by calcineurin inhibitors.
Methods
Muscle Regeneration, Denervation, and Electrostimulation.
Muscle regeneration was induced in 200- to 250-g male Wistar rats by intramuscular injection of bupivacaine as described (25). Denervation was produced by cutting the sciatic nerve high in the thigh. For electrostimulation experiments, regenerating rat soleus muscles were denervated to abolish nerve-evoked muscle activity and stimulated through electrodes implanted onto the muscles at 20 Hz (200 pulses every 30 s), as previously described (26). Unstimulated regenerating denervated muscles were used as controls.
Transfection of Regenerating and Adult Muscles.
Regenerating innervated or denervated muscles were injected with plasmid DNA (50 μg) at day 3 after bupivacaine treatment as described (25). We have previously shown that gene transfer efficiency is high after DNA injection at day 3, when the regenerating muscle is mostly composed of small myotubes, but is very poor after DNA injection at day 1, when only mononucleated myoblasts are present (25). Muscles were removed at day 10 after injury (day 7 after transfection) and frozen in isopentane cooled in liquid nitrogen. Adult muscles were transfected by intramuscular injection of plasmid DNA (20 μg) followed by electroporation to increase gene transfer efficiency. The electroporation procedure was similar to that described by Mir et al. (27). Muscles were removed at day 7 after transfection and frozen in isopentane cooled in liquid nitrogen.
Treatment with CsA and FK506.
Rats were injected i.p. with 5 mg/kg CsA (Novartis Pharma, Bern, Switzerland) dissolved in Cremophor 10% in saline or with 1 mg/kg FK506 (Fujisawa, München, Germany) in saline once daily starting at day 3 after muscle injury.
Plasmids and Promoter Activity.
Plasmids coding for the rat MyHC-slow promoter (−1145 bp) linked to luciferase (28) and the cain inhibitory domain fused to a myc epitope under control of a cytomegalovirus promoter (23) have been described. MyHC-slow promoter plasmid (50 μg) was coinjected with Rous sarcoma virus–chloramphenicol acetyltransferase (5 μg) to monitor for transfection efficiency and, in some experiments, with cain plasmid or empty vector (20 μg). Luciferase activity was normalized for extract protein content. Results of each transfection experiment represent the mean of at least five different muscles.
Immunocytochemistry and in Situ Hybridization.
Cryosections of regenerating muscles were processed for immunofluorescence with the monoclonal antibody BA-D5, specific for MyHC-slow (29). Myc-cain was revealed by anti-myc antibody (Roche Molecular Biochemicals) after fixation of cryosections with 4% paraformaldehyde. Serial sections were processed for in situ hybridization with 35S-labeled riboprobes complementary to the 3′-untranslated regions of MyHC-slow, MyHC-2A, MyHC-2X, and MyHC-2B transcripts as described (30).
Fiber Type and Fiber Size Measurements.
The percentage of slow fibers and fiber size were measured as the mean of at least five muscles per group and three distinct areas of each muscle cross section. Fiber cross-sectional areas were measured by using SCION IMAGE software (Scion, Frederick, MD). In muscles transfected with cain (n = 9), fiber size was measured in all fibers expressing myc-cain (n = 450). An equal number of untransfected fibers from the same muscles was used as control.
Electrophoresis.
Cryosections of regenerating muscles were dissolved in Laemmli buffer, and MyHCs were separated by SDS/PAGE as described (31). The relative amount of MyHC-slow was quantitated by densitometric analysis and expressed as percentage of total MyHCs.
Data Analysis.
All data are expressed as the mean ± SEM (error bars). Comparisons were made by using a t test, with P < 0.05 being considered statistically significant.
Results
CsA and FK506 Prevent the Up-Regulation of MyHC-Slow Induced by Slow Motor Neurons in Regenerating Soleus Muscle.
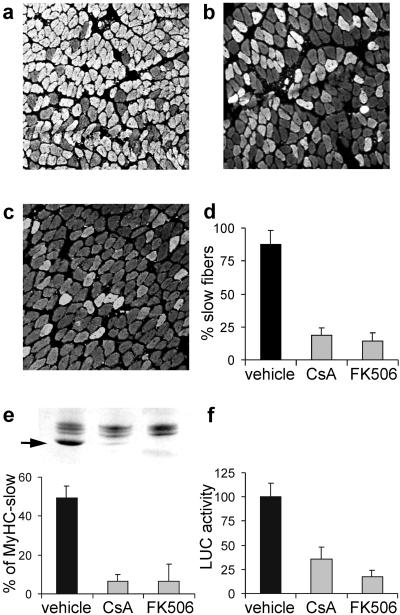
MyHC gene expression in the regenerating rat soleus muscle depends on the nerve. By days 3 and 4 after injury, regenerating fibers express MyHC-embryonic and MyHC-neonatal, and by day 5 MyHC-slow is up-regulated by slow motor neuron activity and becomes the predominant isoform during subsequent stages (6, 32). In contrast, denervated regenerating fibers express by default the fast-type MyHC-2X and MyHC-2B isoforms. The role of calcineurin in the activation of MyHC-slow gene was examined by using the calcineurin inhibitors CsA and FK506. As shown in Fig. 1, the two drugs strongly inhibit the expression of the endogenous MyHC-slow gene. By day 10 after injury, antibodies specific for MyHC-slow stain most fibers in regenerating innervated muscle but only a small proportion of fibers in CsA- and FK506-treated animals (Fig. 1 a–d). Accordingly, the relative amount of MyHC-slow detected by SDS/PAGE is markedly reduced from about 50% of total MyHCs in control to less than 10% in treated animals (Fig. 1e). To determine whether this effect is because of transcriptional regulation of the MyHC-slow gene, we examined the effect of calcineurin inhibitors on the activation of a MyHC-slow promoter, which is responsive to slow nerve activity (6). As shown in Fig. 1f, CsA and FK506 inhibit the up-regulation of the MyHC-slow promoter induced by slow motor neurons.
Figure 1.
CsA and FK506 block the expression of MyHC-slow in regenerating soleus muscle. (a–d) Immunofluorescence analysis of sections stained with an antibody specific for MyHC-slow. Note that regenerating soleus muscles from rats treated with CsA (b) or FK506 (c) show a decreased proportion of reactive fibers compared with rats treated with vehicle (a). The percentage of fibers expressing MyHC-slow in the three experimental groups is shown in d. (e) SDS/PAGE profile of MyHCs (Upper). Note that the high mobility MyHC-slow band (arrow) is abundant in control muscle (left lane) but is barely detectable after treatment with CsA (center lane) or FK506 (right lane). This decrease is confirmed by quantitation of densitometric values of the percentage of MyHC-slow relative to all MyHCs (Lower). (f) MyHC-slow promoter activity is down-regulated by CsA and FK506. A plasmid containing the MyHC-slow promoter linked to a luciferase reporter gene was injected in regenerating muscles, and luciferase activity was measured 7 days later in tissue homogenates. Luciferase (LUC) activity after treatment with calcineurin inhibitors is expressed as the percentage of that measured in rats treated with vehicle.
CsA Acts Directly on Muscle Fibers and Not by Means of Changes in Nerve Activity.
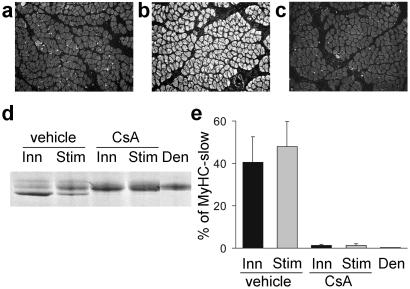
We asked whether the effect of CsA is because of a direct effect on muscle fibers or because of an indirect effect mediated through the nerve. For example, CsA could alter neurotransmitter release by nerve terminals, and this could affect secondarily MyHC-slow gene expression in muscle fibers. To identify the site of CsA action, we examined the effect of CsA in regenerating denervated muscles that were directly electrostimulated with a tonic 20-Hz pattern of impulses that resembles the firing pattern of slow motor neurons. As expected, this pattern of stimulation is able to induce MyHC-slow in denervated regenerating soleus muscle (Fig. 2 a and b). In contrast, MyHC-slow is not induced by electrostimulation with a phasic 100-Hz pattern, typical of fast motor neurons (not shown). CsA treatment markedly reduces the proportion of fibers staining for MyHC-slow (Fig. 2c) and the relative amount of MyHC-slow detected by SDS/PAGE (Fig. 2 d and e) in muscles electrostimulated with the 20-Hz pattern. These findings indicate that the inhibitory effect of CsA on slow myosin expression is not mediated through the nerve but is the result of a direct effect on the muscle fibers.
Figure 2.
CsA prevents the up-regulation of MyHC-slow induced by electrostimulation in denervated regenerating soleus muscle. Muscles were denervated and electrostimulated with a tonic 20-Hz pattern of impulses that resembles the firing pattern of slow motor neurons. (a–c) Immunofluorescence analysis with anti-MyHC-slow antibody of regenerating soleus muscles after denervation (a), denervation and electrostimulation (b), or denervation and electrostimulation plus treatment with CsA (c). Note that the up-regulation of MyHC-slow induced by electrostimulation is prevented by CsA. (d and e) Electrophoretic analysis of MyHCs shows that the high mobility MyHC-slow band is abundant in muscles innervated (Inn) or denervated and electrostimulated (Stim) but is undetectable in denervated muscles (Den). CsA treatment blocks the up-regulation of MyHC-slow in both innervated and electrostimulated muscles.
Cain/Cabin-1 Prevents the Up-Regulation of MyHC-Slow Induced by Slow Motor Neurons in Regenerating Soleus Muscle.
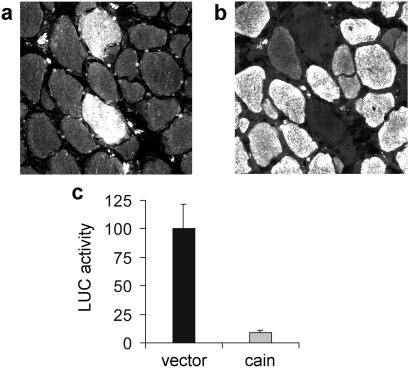
Because CsA and FK506 have other intracellular targets besides calcineurin, we also examined the effect of the peptide calcineurin inhibitor, cain/cabin-1 (23, 24), on MyHC gene expression. Regenerating soleus muscles were transfected at day 3 after injury with an expression vector encoding the cain inhibitory domain fused to a myc epitope and were examined at day 10 after injury. As shown in Fig. 3 a and b, transfected fibers are unreactive for MyHC-slow, whereas most of the surrounding untransfected fibers are stained by anti-MyHC-slow antibodies. In addition, cain/cabin-1 was found to inhibit the up-regulation of the MyHC-slow promoter induced by slow motor neurons (Fig. 3c).
Figure 3.
The calcineurin inhibitor cain/cabin-1 prevents the up-regulation of MyHC-slow and the activation of MyHC-slow promoter in regenerating soleus muscle. Serial cross sections of regenerating soleus muscle transfected with myc-tagged cain and stained with anti-myc (a) or anti-MyHC-slow (b) antibodies. Note that the two fibers expressing myc-tagged cain do not express MyHC-slow, unlike most surrounding untransfected fibers. (c) Cotransfection of MyHC-slow promoter and cain shows that MyHC-slow promoter activity is down-regulated by cain.
Muscle Fiber Growth in Regenerating Muscle Is Not Affected by Calcineurin Inhibitors.
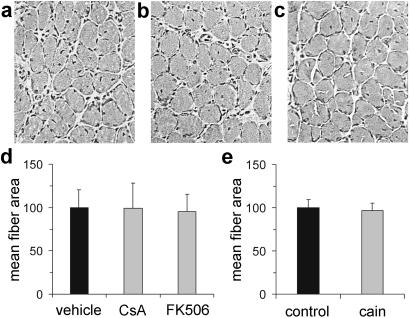
We next examined whether the effect of calcineurin inhibitors on MyHC-slow gene expression reflects a general block of muscle growth. The histology of soleus muscles from CsA- and FK506-treated animals is indistinguishable from that of vehicle-treated animals (Fig. 4 a–c), and muscle fiber size is identical in the three groups (Fig. 4d). In muscles from CsA- and FK506-treated animals, fiber size is identical in fibers expressing and nonexpressing MyHC-slow (not shown). Accordingly, the fibers transfected with the cain/cabin-1 peptide are similar in morphology and size to the surrounding untransfected fibers (Fig. 3 a and b and Fig. 4e). Therefore, we conclude that calcineurin inhibitors affect selectively the activation of the slow fiber gene program without affecting muscle hypertrophy.
Figure 4.
Muscle fiber growth in regenerating muscle is not affected by calcineurin inhibitors. (a–c) Hematoxylin and eosin staining of cross sections of soleus muscle from control (a), CsA-treated (b), and FK506-treated (c) animals shows normal growth of regenerating muscle fibers at day 10 after injury. (d) Fiber size is unchanged in CsA- and FK506-treated compared with vehicle-treated animals. (e) Fiber size is unchanged in fibers transfected with myc-tagged cain compared with untransfected surrounding fibers (control).
Differential Sensitivity of MyHC-2X → MyHC-2A and MyHC-2A → MyHC-Slow Switching to Calcineurin Inhibitors.
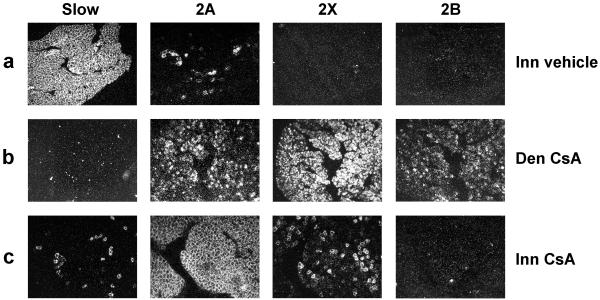
We have previously shown that regenerating soleus muscle fibers display an increased expression of MyHC-2A and a decreased expression of MyHC-2X and MyHC-2B during the early phase of reinnervation (32). This MyHC-2X (-2B) → MyHC-2A switch is immediately followed by a MyHC-2A → MyHC-slow switch, so that by day 10 after injury regenerating soleus muscles contain mostly MyHC-slow transcripts and a minor proportion of MyHC-2A confined to a small population of fibers (Fig. 5a). These sequential myosin switches are reminiscent of the MyHC transitions observed during the conversion of fast into slow muscles induced by continuous low-frequency electrostimulation (4). We asked whether both myosin switches are controlled by calcineurin. In the absence of the nerve, CsA treatment has no effect on MyHC gene expression, with denervated muscles being characterized by a predominance of MyHC-2X and MyHC-2B and complete lack of MyHC-slow transcripts (Fig. 5b) (see also ref. 32). In the presence of the nerve, the MyHC-2A → MyHC-slow switch is markedly repressed by CsA treatment, but the MyHC-2X → MyHC-2A switch is essentially unaffected (Fig. 5c). As a result of the differential effect of CsA on the two myosin transitions, regenerating soleus muscles contain mostly MyHC-2A transcripts in rats treated with CsA. The effect of CsA may be due to the fact that calcineurin is involved only in MyHC-2A → MyHC-slow switching or may simply reflect an incomplete inhibition of calcineurin activity with the dose of CsA used in our experiments. To address this question, we examined the effect of cain/cabin-1 on MyHC transcript expression in regenerating innervated soleus. MyHC-slow transcripts are never detected in fibers transfected with cain/cabin-1, and the relative amount of MyHC-2A, MyHC-2X, and MyHC-2B transcripts was found to vary in relation to the level of myc-cain expression. Fibers strongly reactive for myc-cain tend to express high levels of MyHC-2B and MyHC-2X transcripts, whereas fibers weakly reactive for myc-cain tend to express low levels of these transcripts (not shown). Thus, it seems that calcineurin controls both MyHC transitions, but the sensitivity to calcineurin inhibitors is higher for MyHC-2A → MyHC-slow switching.
Figure 5.
CsA blocks the MyHC-2A → MyHC-slow switch but not the MyHC-2X (-2B) → MyHC-2A switch in regenerating soleus muscle. Serial cross sections of innervated regenerating soleus muscle were processed for in situ hybridization with probes specific for MyHC-slow, MyHC-2A, MyHC-2X, and MyHC-2B transcripts and viewed by dark-field microscopy. Note that innervated muscles (Top) show a predominance of MyHC-slow transcripts with a minor population of fibers expressing MyHC-2A transcripts. In contrast, denervated muscles both in rats treated with vehicle (not shown) or CsA (Middle) show a predominance of MyHC-2X and MyHC-2B with minor amounts of MyHC-2A but complete absence of MyHC-slow. CsA treatment (Bottom) blocks almost completely the switch to MyHC-slow in innervated muscles, whereas the transition from MyHC-2X (-2B) to MyHC-2A is almost unaffected, thus leading to a predominant expression of MyHC-2A transcripts with a minor population of fibers expressing MyHC-2X transcripts.
Calcineurin Controls the Maintenance of MyHC-Slow Gene Expression and the Repression of Fast MyHC-2X and MyHC-2B Genes in Adult Soleus Muscle.
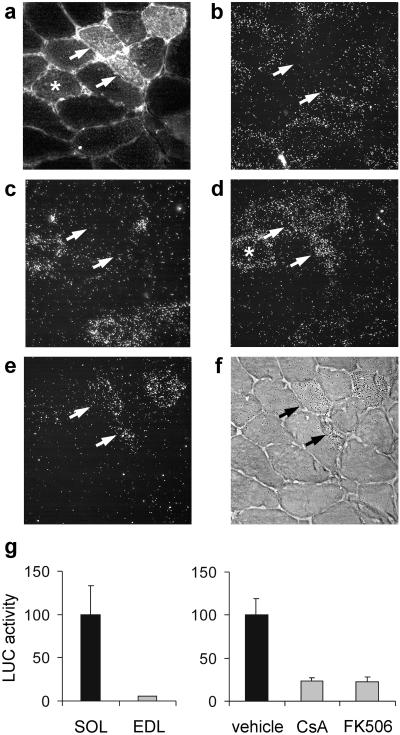
To determine the effect of calcineurin inhibitors on MyHC gene expression in adult nonregenerating soleus muscle, plasmids containing cain/cabin-1 were electroporated into rat muscles, and MyHC transcripts were detected by in situ hybridization 7 days later. In normal adult soleus, most fibers contain MyHC-slow and a minor proportion contains MyHC-2A transcripts, whereas fibers containing MyHC-2X transcripts are rare and fibers expressing MyHC-2B transcripts are absent. As shown in Fig. 6 a–f, fibers strongly reactive for myc-cain express MyHC-2X and MyHC-2B but not MyHC-slow and MyHC-2A transcripts, whereas fibers weakly reactive for myc-cain tend to express MyHC-2X but not MyHC-2B. Thus, calcineurin activity seems to control both the maintenance of MyHC-slow gene expression and the repression of the fast MyHC-2X and MyHC-2B genes. To determine whether this myosin switch results from transcriptional regulation of the MyHC-slow gene, we examined the effect of calcineurin inhibitors on the activation of the MyHC-slow promoter. Plasmids containing luciferase driven by the MyHC-slow promoter were electroporated into adult rat muscles. MyHC-slow promoter activity is more than 10 times higher in the slow-twitch soleus compared with the fast-twitch extensor digitorum longus muscle, and CsA or FK506 treatment markedly inhibits MyHC-slow promoter activity in adult soleus muscle (Fig. 6g).
Figure 6.
MyHC-slow gene expression is down-regulated by calcineurin inhibitors in the adult soleus muscle. (a–f) Serial cross sections of adult soleus transfected with myc-cain were processed for immunofluorescence with anti-myc antibody (a) or for in situ hybridization with probes specific for MyHC-slow (b), MyHC-2A (c), MyHC-2X (d), and MyHC-2B transcripts (e and f) and viewed by dark-field microscopy (b–e). MyHC-2B transcript distribution is also illustrated by light-field microscopy (f) to allow direct comparison with myc-cain distribution. Note that fibers strongly reactive for myc-cain (arrows) contain MyHC-2X and MyHC-2B but not MyHC-2A and MyHC-slow transcripts, whereas fibers weakly reactive for myc-cain (asterisk) express MyHC-2X but not MyHC-2B. (g) MyHC-slow promoter activity is higher in the adult slow soleus (SOL) compared with the fast extensor digitorum longus (EDL) muscle (Left) and is markedly inhibited by CsA and FK506 in soleus muscle (Right).
Discussion
The induction of MyHC-slow and the repression of the fast MyHC-2X and MyHC-2B genes in regenerating rat soleus muscle depend on the activity of slow motor neurons (6, 32). We report here that both pharmacologic (CsA and FK506) and genetic (cain/cabin-1) inhibition of calcineurin activity blocks the up-regulation of MyHC-slow, supporting the notion that calcineurin is a key component of the transduction pathways relaying nerve electrical activity and transcriptional regulation of slow muscle fiber-type specificity (5). This seems to be true both for the induction of the MyHC-slow gene in regenerating muscle and the maintenance of the slow myosin phenotype in the adult. Calcineurin seems to be involved also in the repression of the fast MyHC-2X and MyHC-2B genes, as shown by the up-regulation of these genes in regenerating and adult muscle fibers overexpressing cain/cabin-1. However, the repression of fast MyHC genes by calcineurin seems to be less sensitive to calcineurin inhibition, as shown by the finding that the same dose of CsA that blocks the up-regulation of the MyHC-slow gene is unable to block the repression of MyHC-2X and MyHC-2B genes. Accordingly, only fibers overexpressing high levels of cain/cabin-1 show up-regulation of the MyHC-2B gene.
A major result of the present study is the demonstration that the activation of the slow muscle gene program depends on calcineurin function in muscle fibers and not in motor neurons, an issue that was not addressed in previous studies. Calcineurin is highly expressed in nerve cells and is known to affect synaptic transmission and synaptic plasticity (8). Synaptic vesicle recycling induced by stimulation in nerve terminals depends on the dephosphorylation of different presynaptic proteins and is blocked by calcineurin inhibitors (33, 34). In addition, activity-induced potentiation of developing neuromuscular synapses depends on calcineurin (35). The changes in muscle fiber-type profile induced by CsA and FK506 might thus be due to calcineurin activity in motor neurons rather than in muscle fibers. To distinguish between direct and indirect effects of calcineurin inhibitors, we took advantage of the observation that the up-regulation of MyHC-slow by slow motor neurons can be reproduced by electrostimulation of denervated soleus with a tonic 20-Hz pattern resembling the firing pattern of slow motor neurons. The finding that CsA inhibits the up-regulation of MyHC-slow produced by direct electrostimulation points to a primary role of calcineurin in muscle fibers. The muscle cell-autonomous role of calcineurin is supported by gene transfer experiments with the calcineurin inhibitor cain/cabin-1, which blocks both the induction of endogenous MyHC-slow gene and the activation of the MyHC-slow promoter in transfected muscle fibers.
The effect of calcineurin inhibitors on MyHC gene expression in vivo is not due to a block of muscle cell growth because calcineurin inhibitors do not affect muscle fiber hypertrophy in the regenerating soleus. This is true for regenerating muscles from rats treated with CsA or FK506 and regenerating muscle fibers transfected with cain/cabin-1. Abbott et al. (10) reported that CsA inhibits muscle regeneration when CsA treatment is started immediately after muscle injury in mice. Calcineurin activity is required for the initiation of skeletal muscle cell differentiation in culture (10–13); therefore, calcineurin is presumably involved also in the early stages of myotube formation in regenerating muscle. On the other hand, we started CsA and FK506 treatment, as well as transfection with cain/cabin-1, at day 3 after injury, namely at a stage when the formation of myotubes has already taken place. Our results indicate that the subsequent growth of regenerating muscle fibers is unaffected by three different calcineurin inhibitors. The notion that calcineurin signaling is not required for muscle fiber growth in vivo is consistent with the finding that CsA does not prevent muscle hypertrophy in transgenic mice overexpressing insulin-like growth factor-1 (20) and that transgenic mice that express activated calcineurin show no evidence for skeletal muscle hypertrophy (15). CsA was reported to inhibit skeletal muscle hypertrophy in response to functional overload in vivo (16); however, using a similar model of hypertrophy induced by elimination of synergistic muscles, we found that hypertrophy is unaffected in muscle fibers overexpressing cain/cabin-1 (unpublished observations).
We have previously reported that the Ras–mitogen-activated protein kinase pathway is involved in the nerve activity-dependent induction of the slow-muscle-fiber gene program in regenerating rat soleus muscle (6). The role of Ras–mitogen-activated protein kinase is supported by the finding that a dominant negative Ras mutant (RasN17) prevents the induction of MyHC-slow by slow motor neurons, whereas a Ras double mutant (RasV12S35) that selectively stimulates the mitogen-activated protein kinase/ERK pathway is able to induce MyHC-slow in the absence of innervation. Ras and calcineurin are known to cooperate in other cell systems; for example, a synergy between the two signaling pathways is required to stimulate the transcription of the interleukin-2 gene and T cell activation (8, 36). Additional studies will be necessary to establish whether there is a cross-talk between the Ras and calcineurin pathways in skeletal muscle fibers, similar to that recently described in cardiac muscle cells (37, 38). However, it seems that Ras and calcineurin pathways affect differentially fiber-type specificity in regenerating and adult skeletal muscle. In fact, calcineurin inhibitors markedly decrease the transcription of the MyHC-slow gene promoter in both regenerating and adult soleus muscle (this study), whereas dominant negative Ras inhibits MyHC-slow promoter activity in regenerating (6) but not in adult muscle (unpublished observations). Therefore, the factors responsible for the initiation of the slow-muscle-fiber gene program differ from those involved in the maintenance of the slow-fiber phenotype.
Acknowledgments
This paper is dedicated to the memory of our friend and colleague Franco Tatò. We thank R. Kitsis and K. Hasegawa for MyHC-slow promoter-luciferase plasmid and S. Snyder and M. Lai for cain/cabin-1 plasmid. We also thank Novartis (Bern, Switzerland) for CsA and Fujisawa (München, Germany) for FK506. This work was supported by European Commission Grants ERBBIO4CT960216 and QLK6-2000-00530, the Giovanni Armenise-Harvard Foundation for Advanced Scientific Research, the Italian Space Agency, and the Italian Ministry of University and Scientific and Technological Research.
Abbreviations
- CsA
cyclosporin A
- MyHC
myosin heavy chain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Schiaffino S, Reggiani C. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 2.DiMario J X, Stockdale F E. Dev Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- 3.Hughes S M, Salinas P C. Curr Opin Neurobiol. 1999;9:54–64. doi: 10.1016/s0959-4388(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 4.Pette D, Vrbova G. Rev Physiol Biochem Pharmacol. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- 5.Chin E R, Olson E N, Richardson J A, Yang Q, Humphries C, Shelton J M, Wu H, Zhu W, Bassel-Duby R, Williams R S. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murgia M, Serrano A L, Calabria E, Pallafacchina G, Lømo T, Schiaffino S. Nat Cell Biol. 2000;2:142–147. doi: 10.1038/35004013. [DOI] [PubMed] [Google Scholar]
- 7.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 8.Aramburu J, Rao A, Klee C B. Curr Top Cell Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- 9.Olson E N, Williams R S. Cell. 2000;101:689–692. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- 10.Abbott K L, Friday B B, Thaloor D, Murphy T J, Pavlath G K. Mol Biol Cell. 1998;9:2905–2916. doi: 10.1091/mbc.9.10.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delling U, Tureckova J, Lim H W, De Windt L J, Rotwein P, Molkentin J D. Mol Cell Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friday B B, Horsley V, Pavlath G K. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardiman O, Sklar R M, Brown R H., Jr Neurology. 1993;43:1432–1434. doi: 10.1212/wnl.43.7.1432. [DOI] [PubMed] [Google Scholar]
- 14.Bigard X, Sanchez H, Zoll J, Mateo P, Rousseau V, Veksler V, Ventura-Clapier R. J Biol Chem. 2000;275:19653–19660. doi: 10.1074/jbc.M000430200. [DOI] [PubMed] [Google Scholar]
- 15.Naya F J, Mercer B, Shelton J, Richardson J A, Williams R S, Olson E N. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- 16.Dunn S E, Burns J L, Michel R N. J Biol Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- 17.Musarò A, McCullagh K J, Naya F J, Olson E N, Rosenthal N. Nature (London) 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 18.Swoap S J, Hunter R B, Stevenson E J, Felton H M, Kansagra N V, Lang J M, Esser K A, Kandarian S C. Am J Physiol. 2000;279:C915–C924. doi: 10.1152/ajpcell.2000.279.4.C915. [DOI] [PubMed] [Google Scholar]
- 19.Biring M S, Fournier M, Ross D J, Lewis M I. J Appl Physiol. 1998;84:1967–1975. doi: 10.1152/jappl.1998.84.6.1967. [DOI] [PubMed] [Google Scholar]
- 20.Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton E R, Sweeney H L, Rosenthal N. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 21.Avdonin P V, Cottet-Maire F, Afanasjeva G V, Loktionova S A, Lhote P, Ruegg U T. Kidney Int. 1999;55:2407–2414. doi: 10.1046/j.1523-1755.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 22.duBell W H, Gaa S T, Lederer W J, Rogers T B. Am J Physiol. 1998;275:H2041–H2052. doi: 10.1152/ajpheart.1998.275.6.H2041. [DOI] [PubMed] [Google Scholar]
- 23.Lai M M, Burnett P E, Wolosker H, Blackshaw S, Snyder S H. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Youn H D, Loh C, Stolow M, He W, Liu J O. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 25.Vitadello M, Schiaffino M V, Picard A, Scarpa M, Schiaffino S. Hum Gene Ther. 1994;5:11–18. doi: 10.1089/hum.1994.5.1-11. [DOI] [PubMed] [Google Scholar]
- 26.Ausoni S, Gorza L, Schiaffino S, Gundersen K, Lømo T. J Neurosci. 1990;10:153–160. doi: 10.1523/JNEUROSCI.10-01-00153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mir L M, Bureau M F, Gehl J, Rangara R, Rouy D, Caillaud J M, Delaere P, Branellec D, Schwartz B, Scherman D. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa K, Lee S J, Jobe S M, Markham B E, Kitsis R N. Circulation. 1997;96:3943–3953. doi: 10.1161/01.cir.96.11.3943. [DOI] [PubMed] [Google Scholar]
- 29.Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo T. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 30.DeNardi C, Ausoni S, Moretti P, Gorza L, Velleca M, Buckingham M, Schiaffino S. J Cell Biol. 1993;123:823–835. doi: 10.1083/jcb.123.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talmadge R J, Roy R R. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 32.Jerkovic R, Argentini C, Serrano-Sanchez A, Cordonnier C, Schiaffino S. Cell Struct Funct. 1997;22:147–153. doi: 10.1247/csf.22.147. [DOI] [PubMed] [Google Scholar]
- 33.Lai M M, Luo H R, Burnett P E, Hong J J, Snyder S H. J Biol Chem. 2000;275:34017–34020. doi: 10.1074/jbc.C000429200. [DOI] [PubMed] [Google Scholar]
- 34.Slepnev V I, Ochoa G C, Butler M H, Grabs D, Camilli P D. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 35.Wan J, Poo M. Science. 1999;285:1725–1728. doi: 10.1126/science.285.5434.1725. [DOI] [PubMed] [Google Scholar]
- 36.Crabtree G R. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 37.Ichida M, Finkel T. J Biol Chem. 2000;276:3524–3530. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- 38.Murat A, Pellieux C, Brunner H R, Pedrazzini T. J Biol Chem. 2000;275:40867–40873. doi: 10.1074/jbc.M008071200. [DOI] [PubMed] [Google Scholar]