Abstract
In insects, the subdivision of the head into a lateral region, harbouring the compound eyes (CEs), and a dorsal (medial) region, where the ocelli localize, is conserved. This organization might have been already present in the insects' euarthropodan ancestors. In Drosophila, the Wnt-1 homologue wingless (wg) plays a major role in the genetic subdivision of the head. To analyse specifically the role of wg signalling in the development of the dorsal head, we attenuated this pathway specifically in this region by genetic means. We find that loss of wg signalling transforms the dorsal/medial head into lateral head structures, including the development of ectopic CEs. Our genetic analysis further suggests that wg signalling organizes the dorsal head medial–lateral axis by controlling, at least in part, the expression domains of the transcription factors Otd and Ey/Pax6.
Keywords: Wnt, Drosophila, insect head
1. Introduction
Within insects, the structure of the adult head is essentially conserved, despite the multiple morphological and functional specializations of its mouthparts and sensory organs [1–3]. In particular, the subdivision of the head into a lateral region, where the compound eyes (CEs) are located, and a dorsal/medial region, the ‘head vertex’, harbouring the ocelli (ocellar complex, OC) was present already in Cambrian euarthropods [4]. Still, the genetic mechanisms responsible for this subdivision are not well understood. In Drosophila, the Wnt gene wingless (wg) plays major roles in head development, including limiting the length of the signalling centre that induces retina development [5–10]. However, wg expression is dynamic and most studies have made use of wg mutant alleles, which make it difficult to determine what role its signalling plays in the dorsal head, where ocelli develop. Here, we have addressed this role by targeting the wg signalling pathway specifically in this region.
2. Results
(a). Attenuation of the Wnt-canonical pathway in the prospective medial head capsule results in its transformation into lateral head structures
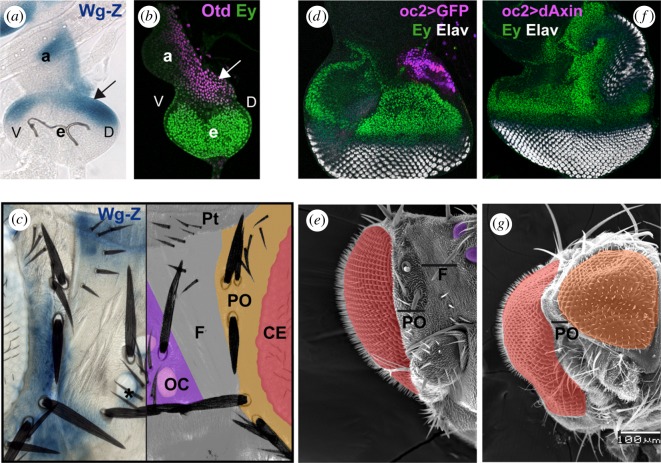
In early third-stage larvae (L3), the eye domain within the head primordium (eye-antennal imaginal disc) is subdivided into two major territories: the prospective CE expressing the Pax6 gene eyeless (ey), and the prospective dorsal head capsule, that expresses the cephalic gene orthodenticle (otd) (figure 1). wg, monitored by a wg-Z transcriptional reporter, shows a domain of strong expression within the Otd-expression territory (figure 1a,b). wg expression evolves during development so that, in the adult head, it maps to the periocular cuticle, in between the eye and the dorsal head or ‘vertex’, and to a domain anterior to the OC (figure 1c and [11]). To investigate the role played by wg signalling in the dorsal head, we targeted the Wnt-canonical signalling pathway, which is mediated by β-catenin (Armadillo, Arm; [12]), in three ways: (i) by overexpressing dAxin, an Arm-destruction complex component [13,14], (ii) by sequestering Arm to the adherens junctions through the overexpression of the intracellular moiety of E-cadherin [15], and (iii) by overexpressing a dominant-negative form of TCF (TCFDN), the nuclear cofactor of Arm [16]. These genetic manipulations were induced specifically in the developing dorsal head region using the strain oc2-GAL4 driving dAxin, DECad-5i or TCFDN, respectively (see electronic supplementary material, Methods) (figure 1d). In all three conditions, we observed an expansion of the ey-expressing domain adjacent to a new field of ELAV-positive photoreceptors in discs (figure 1f; electronic supplementary material, figure S1). In the adults, the dorsal head cuticle (including the OC) was often transformed into CEs surrounded with periocular cuticle (figure 1e–g), reflecting the disc phenotype. We interpret it as a medial-to-lateral transformation of the head. The oc2 > dAxin (WntKD) genotype expressed the medial-to-lateral phenotype with the highest penetrance (28% of adults with one or two ectopic eyes, N = 118), and was used for further analyses.
Figure 1.
Attenuating the wg/Wnt signalling pathway results in the medial to lateral transformation of the Drosophila head. (a,b) Early third larva stage discs. (a) X-gal staining of the wg transcriptional reporter wg-Z. (b) Disc co-stained with anti-Otd (magenta) and anti-Ey (green) antibodies, showing complementary expression domains. The arrow in (a) and (b) points to the prospective dorsal head region, where ocelli develop. (c) X-Gal-stained wg-Z adult head (left) and schematic representation of dorsal head regions (right). wg expression is detected in the periocular cuticle (PO) and the anterior region of the dorsal head (ptillinum, PT). CE, compound eye; OC, ocellar complex; F, frons. The asterisk marks a late-appearing wg expression domain around the ocelli (see also [11]). (d,f) Late discs from oc2-GAL4; UAS-GFP (oc2 > GFP; d) or oc2-GAL4; UAS-dAxin2.28 (oc2 > dAxin; f), stained for the retinal marker Elav (white) and Ey (green). GFP expression in (d) is shown in magenta and marks the oc2-GAL4 expression domain. In oc2 > Axin discs, a duplicated eye field arises from the ocellar domain. (e,g) SEM images of oc2 > GFP (e) and oc2 > Axin adult half-heads shown at the same magnification. CEs are pseudocoloured in red. Ocelli in (e) are pseudocoloured in purple and the ectopic eye in (g) in orange. PO and F as in (c).
(b). Wnt signalling and otd exclude Pax6/ey expression from the dorsal head
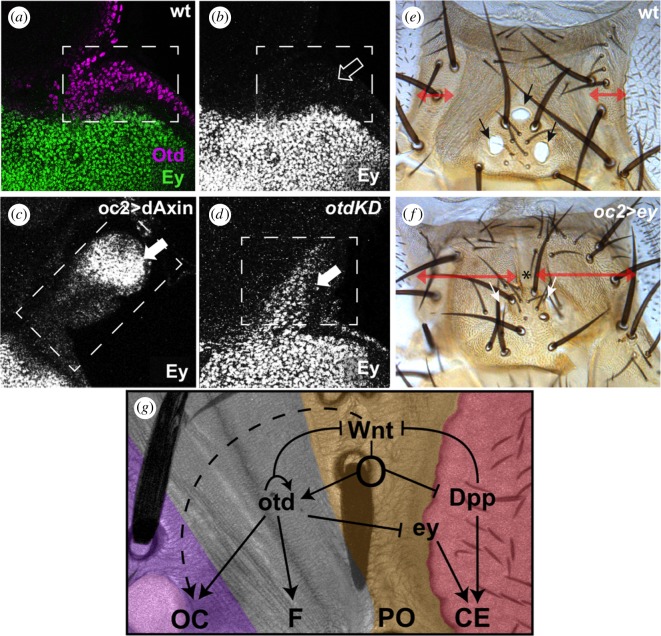
To understand the mechanisms by which Wnt signalling controls the choice between medial and lateral head fates, we focused on key medial/lateral genetic differences and analysed how these could contribute to the action of Wnt. otd expression is almost complementary to that of ey in L3 (figure 2a,b) filling the prospective dorsal head. In WntKD discs, a new domain of Ey expression appeared where the ectopic dorsal eyes are to develop (figure 2a–c). As otd lies downstream of wg [9,11,17], we tested if otd could repress ey expression. Indeed, in discs where otd expression was attenuated (oc2 > otd-RNAi, or otdKD) ey expression extended medially into the ocellar region (figure 2d). Ectopic expression of ey is known to cause the respecification of fly appendages into CEs [18] so, in principle, ey deprepression could be causing the development of dorsal eyes in WntKD individuals. However, the ectopic expression of ey that we detected in WntKD and otdKD is unlikely to be the sole responsible of the transformation. This is because despite ey de-repression, there is no medial-to-lateral transformation in otd mutants [19] or otdKD [20]. As ey derepression was weaker in otdKD than in WntKD (figure 2d,c, respectively), we tested if higher ey levels were capable of respecifying the dorsal head as CE by forcing ey expression in the ocellar region (oc2 > ey). This caused the obliteration of the ocelli and the expansion of the periocular cuticle, characterized by long bristles (figure 2e,f), but not the respecification of the medial head into CE. Therefore, otd expression attenuates ey expression in the prospective dorsal head, but the sole derepression of ey is insufficient to explain the medial-to-lateral transformation in WntKD flies. In addition, we noted that in late L3 discs, the expression domains of otd and ey overlap in a strip of cells (electronic supplementary material, figure S2) that would correspond in the adult to the periocular cuticle. As mentioned above, the overexpression of ey in the otd-expressing developing head vertex (oc > ey) resulted in an expansion of periocular-like cuticle (figure 2e,f). The converse expression of otd in the ey-domain (in optix > otd individuals, see electronic supplementary material, Methods) resulted in a reduced eye and extra cuticle with bristles (electronic supplementary material, figure S2d)—again this could be interpreted as an eye-to-periocular cuticle transformation. Therefore, the overlap of otd and ey in the disc seems to specify periocular head fate.
Figure 2.
Repression of ey by wg and otd in the medial head. (a–d) Confocal images of control (‘wt’, a,b), oc2 > dAxin (c) and oc2 > otd-RNAi (‘otdKD’, d) discs stained for Ey (a–d) and Otd (a). The boxes mark the prospective ocellar region. While Ey is not expressed in the ocellar region of control discs (open arrow), Ey is derepressed in this area in both oc2 > dAxin and otdKD discs (white arrows). Derepression is stronger in oc2 > dAxin discs. Overexpression of Ey in the developing ocellar region (f; oc2 > ey) results in the obliteration of the ocelli and the replacement of the ridged cuticle of the head vertex by periorbital-type cuticle, characterized by bearing bristles (compare with control in ‘e’. Double-headed arrows mark the extent of the periorbital cuticle in ‘e’ and ‘f’). A wild-type dorsal head is shown for comparison (e). Arrows in (e) and (f) mark the ocelli. The lateral ocelli in (f) are very reduced, while the anterior ocellus is missing (*). (g) Medial identity of the dorsal head is imparted by Wnt signalling through Otd-dependent and Otd-independent mechanisms. The Wnt target Otd represses ey, which is a CE selector transcription factor. In addition, attenuation of Wnt signal leads to the derepression of dpp. ey and dpp are both necessary for further CE development. Although Otd represses ey, both genes are coexpressed in the periocular cuticle, where they may instruct this fate. See the main text for references.
3. Discussion
The overall anatomy of the head is conserved within insects, even if its segmental composition and the genetic specification of these segments have been subject to much debate (see [3]). Recent work points to a consensus regarding the genetic underpinnings of cephalic structures. This includes the consecutive domains of Six3/optix and Otd expression at the anterior-most cephalic/brain region, and the contribution of wg/Wnt-1 to the specification of anterior head/ocular structures and the origin of the dorsal head, including eyes and head vertex, in the most anterior embryonic segment, the ocular/pre-antennal segment [21]. Most of these studies focused on the embryonic development of the larval head. Depending on the degree of metaboly in the species under study, conclusions on larval head development can be projected into the adult head. In Drosophila, an extreme holometabolous insect with a highly modified involuted larval head, these studies ought to be performed on the eye–antennal disc, the larval primordium of most structures of the adult head. However, the developmental convergence into a similar adult head in insects suggests a conservation of the genetic mechanisms specifying and patterning it, despite the intermediate diversification of the larval head in different insect groups. Therefore, it is likely that the functions performed by wg/Wnt-1 during the patterning of the Drosophila head are also widely conserved.
(a). Early wg/Wnt signalling specifies medial head structures
Our results show that Wnt signalling is necessary for the specification of dorsal/medial head structures as, in its absence, the lateral fate (including eye formation) is taken by default. Indeed, forced expression of wg, or the activation of its pathway in the eye, leads to its transformation into head capsule-like tissue (although no ectopic ocelli, indicative of dorsal head identity, have been reported in these experiments [22]). Selection of medial head/vertex development by wg/Wnt signalling seems to proceed through a complex mechanism, because the loss of its target otd alone (or the concomitant gain of ey expression) does not recapitulate the effects of wg/Wnt signalling attenuation. We have noted that the WntKD disc phenotypes resemble those reported for ectopic dpp expression [23]. Therefore, medial head identity would be regulated by wg through two mechanisms: establishing an otd+/ey− domain where ocelli develop, and by preventing dpp expression and/or signalling (figure 2g; see [10,24]). Recent work by Zattara et al. [25] found that RNAi-mediated otd attenuation in Onthophagus beetles resulted in the development of CEs on the dorsal head. These authors acknowledge that most beetle families have lost ocelli and speculate that otd attenuation might have triggered the atavistic ocellar program expressed as CE [25]. As in Drosophila otd is necessary for ocellar development, this hypothesis would indicate a lack of conservation of dorsal head patterning mechanisms between coleopterans and dipterans. Alternatively, otd loss could result in ey derepression and acquisition of CE competence of the beetle dorsal head. The fact that dorsal head identity in Drosophila requires wg signalling upstream of otd indicates that the way wg signalling is wired into the gene network controlling head development differs in Onthophagus, which agrees with the lack of ocelli in most beetle families.
4. Material and methods
Targeted gene expression manipulation was carried out using the GAL4/UAS system [26] using oc2-GAL4 as OC-specific driver line [17]. Immunofluorescence was carried out as in [9]. Additional methods are available as online electronic supplementary material.
Supplementary Material
Acknowledgements
We thank F. Pignoni, F. Pichaud and T. Cook for reagents, and the CABD ALMI platform for confocal microscopy support.
Data accessibility
Available as electronic supplementary material.
Authors' contributions
F.C. conceived the study. M.S.M. and M.A.D.-C. acquired data. All authors helped with interpretation of results and writing of the manuscript. In addition, all authors have given their approval for the publication of this manuscript, and have agreed to be accountable for the accuracy and integrity of the work.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by MINECO (Spain) through grant nos. BFU2015-66040-P (to F.C.) and MDM-2016-0687.
References
- 1.Snodgrass RE. 1935. Principles of insect morphology, p. 667 New York, NY: McGraw-Hill Book Co., Inc. [Google Scholar]
- 2.Matsuda R.1965. Morphology and evolution of the insect head. Memoirs of the American Entomological Institute. Logan, UT: American Entomological Institute.
- 3.Posnien N, Schinko JB, Kittelmann S, Bucher G. 2010. Genetics, development and composition of the insect head—a beetle's view. Arthropod. Struct. Dev. 39, 399–410. ( 10.1016/j.asd.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 4.Ortega-Hernandez J. 2015. Homology of head sclerites in Burgess Shale euarthropods. Curr. Biol. 25, 1625–1631. ( 10.1016/j.cub.2015.04.034) [DOI] [PubMed] [Google Scholar]
- 5.Treisman JE, Rubin GM. 1995. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development 121, 3519–3527. [DOI] [PubMed] [Google Scholar]
- 6.Ma C, Moses K. 1995. Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development 121, 2279–2289. [DOI] [PubMed] [Google Scholar]
- 7.Royet J, Finkelstein R. 1995. Pattern formation in Drosophila head development: the role of the orthodenticle homeobox gene. Development 121, 3561–3572. [DOI] [PubMed] [Google Scholar]
- 8.Blanco J, Pauli T, Seimiya M, Udolph G, Gehring WJ. et al. 2010. Genetic interactions of eyes absent, twin of eyeless and orthodenticle regulate sine oculis expression during ocellar development in Drosophila. Dev. Biol. 344, 1088–1099. ( 10.1016/j.ydbio.2010.05.494) [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Cejudo MA, Casares F. 2015. Anteroposterior patterning of Drosophila ocelli requires an anti-repressor mechanism within the hh pathway mediated by the Six3 gene Optix. Development 142, 2801–2809. ( 10.1242/dev.125179) [DOI] [PubMed] [Google Scholar]
- 10.Heslip TR, Theisen H, Walker H, Marsh JL. 1997. Shaggy and dishevelled exert opposite effects on wingless and decapentaplegic expression and on positional identity in imaginal discs. Development 124, 1069–1078. [DOI] [PubMed] [Google Scholar]
- 11.Royet J, Finkelstein R. 1996. Hedgehog, wingless and orthodenticle specify adult head development in Drosophila. Development 122, 1849–1858. [DOI] [PubMed] [Google Scholar]
- 12.Dierick H, Bejsovec A. 1999. Cellular mechanisms of wingless/Wnt signal transduction. Curr. Top. Dev. Biol. 43, 153–190. ( 10.1016/S0070-2153(08)60381-6) [DOI] [PubMed] [Google Scholar]
- 13.Hamada F, et al. 1999. Negative regulation of wingless signaling by D-axin, a Drosophila homolog of axin. Science 283, 1739–1742. ( 10.1126/science.283.5408.1739) [DOI] [PubMed] [Google Scholar]
- 14.Willert K, Logan CY, Arora A, Fish M, Nusse R. 1999. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development 126, 4165–4173. [DOI] [PubMed] [Google Scholar]
- 15.Sanson B, White P, Vincent JP. 1996. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383, 627–630. ( 10.1038/383627a0) [DOI] [PubMed] [Google Scholar]
- 16.Parker DS, Jemison J, Cadigan KM. 2002. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129, 2565–2576. [DOI] [PubMed] [Google Scholar]
- 17.Blanco J, Seimiya M, Pauli T, Reichert H, Gehring WJ. 2009. Wingless and Hedgehog signaling pathways regulate orthodenticle and eyes absent during ocelli development in Drosophila. Dev. Biol. 329, 104–115. ( 10.1016/j.ydbio.2009.02.027) [DOI] [PubMed] [Google Scholar]
- 18.Halder G, Callaerts P, Gehring WJ. 1995. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, 1788–1792. ( 10.1126/science.7892602) [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein R, Smouse D, Capaci TM, Spradling AC, Perrimon N. 1990. The orthodenticle gene encodes a novel homeo domain protein involved in the development of the Drosophila nervous system and ocellar visual structures. Genes Dev. 4, 1516–1527. ( 10.1101/gad.4.9.1516) [DOI] [PubMed] [Google Scholar]
- 20.Brockmann A, Domínguez-Cejudo MA, Amore G, Casares F. 2011. Regulation of ocellar specification and size by twin of eyeless and homothorax. Dev. Dyn. 240, 75–85. ( 10.1002/dvdy.22494) [DOI] [PubMed] [Google Scholar]
- 21.Steinmetz PR, et al. 2010. Six3 demarcates the anterior-most developing brain region in bilaterian animals. Evodevo 1, 14 ( 10.1186/2041-9139-1-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royet J, Finkelstein R. 1997. Establishing primordia in the Drosophila eye–antennal imaginal disc: the roles of decapentaplegic, wingless and hedgehog. Development 124, 4793–4800. [DOI] [PubMed] [Google Scholar]
- 23.Pignoni F, Zipursky SL. 1997. Induction of Drosophila eye development by decapentaplegic. Development 124, 271–278. [DOI] [PubMed] [Google Scholar]
- 24.Hazelett DJ, Bourouis M, Walldorf U, Treisman JE. 1998. Decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development 125, 3741–3751. [DOI] [PubMed] [Google Scholar]
- 25.Zattara EE, Macagno AL, Busey HA, Moczek AP. 2017. Development of functional ectopic compound eyes in scarabaeid beetles by knockdown of orthodenticle. Proc. Natl Acad. Sci. USA 114, 12 021–12 026. ( 10.1073/pnas.1714895114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available as electronic supplementary material.