Abstract
Immune defences often trade off with other life-history components. Within species, optimal allocation to immunity may differ between the sexes or between alternative life-history strategies. White-throated sparrows (Zonotrichia albicollis) are unusual in having two discrete plumage morphs, white-striped and tan-striped. Within each sex, white-striped individuals are more aggressive and provide less parental care than tan-striped individuals. We extended immunocompetence handicap models, which predict sex differences in immunity and parasitism, to hypothesize that infection susceptibility should be greater in white-striped than tan-striped birds. We inoculated birds of both morphs with malarial parasites. Contrary to our prediction, among birds that became infected, parasite loads were higher in tan-striped than white-striped individuals and did not differ between the sexes. Circulating androgen levels did not differ between morphs but were higher in males than females. Our findings are not consistent with androgen-mediated immunosuppression. Instead, morph differences in immunity could reflect social interactions or life-history-related differences in risk of injury, and/or genetic factors. Although plumage and behavioural morphs of white-throated sparrow may differ in disease resistance, these differences do not parallel sex differences that have been reported in animals, and do not appear to be mediated by differences in androgen levels.
Keywords: alternative reproductive strategies, avian malaria, host–parasite interactions, immunocompetence handicap hypothesis, plasmodium, Zonotrichia albicollis
1. Introduction
Trade-offs between immune defence and other life-history demands, such as growth and reproduction, are widespread. Even within a species, life-history theory predicts that individuals of different phenotypic classes should differ in the balance between immunity and other functions [1], and may thus differ in disease resistance. For example, in many animals males are more likely to be parasitized, or experience more intense infections, than females [2–4]. At a proximate level, these differences have been proposed to reflect the immunosuppressive effects of androgens [4], although support for this hypothesis remains mixed [5]. At an ultimate level, males and females generally differ in the optimal balance between immunity and mating effort [2–4].
Species with alternative reproductive strategies and other behavioural polymorphisms are particularly interesting from the perspective of ecoimmunology. Within a sex, individuals of different morphs can differ dramatically in appearance, physiology and behaviour [6–9]. Morphs can differ in hormone levels, energetic constraints, risk of injury and other traits, potentially leading to differences in immunity [10,11] and disease resistance. Morph differences in immunity and disease resistance have been posited to parallel sex differences in these traits [10]. However, reproductive polymorphisms are often expressed in one sex only. Thus, it remains an open question whether morph differences in immunity and disease resistance parallel sex differences.
White-throated sparrows (Zonotrichia albicollis) are unusual in that both sexes have two discrete colour morphs: individuals have either white-striped or tan-striped crown plumage. This dimorphism is governed by a supergene associated with an inversion on chromosome 2, with both morphs maintained at similar population frequencies by obligate disassortative mating [12]. Within each sex, white-striped individuals are more aggressive, sing more, provide less parental care, and may have higher gonadal steroid levels during early breeding than tan-striped individuals [13–15]. Given the broad suite of phenotypic differences between morphs, we reasoned that morphs might also differ in disease resistance. Specifically, we extended immunocompetence handicap models [4], originally proposed to explain sex differences in disease resistance, to predict greater disease susceptibility in white-striped than tan-striped individuals. To test this prediction, we inoculated birds with malarial (Plasmodium) parasites, then monitored infection outcome and intensity.
2. Material and methods
(a). Study animals and housing
We captured 42 white-throated sparrows during autumn migration (October 2015) at Long Point, Ontario, Canada (42.58° N, 80.40° W). Birds were transported to the Advanced Facility for Avian Research (London, Ontario, Canada) and housed indoors, in individual cages with ad libitum access to food and water. Birds were housed at 20–22°C and maintained on a short-day photoperiod (10 L : 14 D ) until February 2016.
(b). Determining prior infection status, plumage morph and sex
We collected 25 µl of whole blood from each bird by brachial venipuncture shortly after capture. We used a drop of this sample to prepare a thin-film blood smear, which we treated with Wright-Giemsa stain and examined under a light microscope (100×objective) to identify any individuals that were already naturally infected with haemosporidian parasites. We examined 10 000 erythrocytes per smear, noting any haemosporidia observed.
From the remaining blood we extracted DNA for analysis of haemosporidian infection, plumage morph and sex. We used two-stage nested PCR [16] to amplify a 527 bp fragment of Plasmodium or Haemoproteus cytochrome b, and purified and sequenced second-round PCR products as described elsewhere [17]. We used the Dra I genotyping assay [18] to confirm morphs, and the P2/P8 genotyping assay [19] to confirm sex. In all, we identified 12 tan-striped females, 9 tan-striped males, 7 white-striped females and 14 white-striped males. Twelve subjects were already infected with haemosporidia upon capture. Natural infection status did not differ between morphs or sexes, nor did we observe a morph×sex interaction (electronic supplementary material, table S1).
(c). Inoculation procedure
On 8 February 2016, we collected 200 µl of blood from each of two wild-caught conspecific parasite donors infected with Plasmodium lineage P-SOSP10 (GenBank accession no. KT19636). This sequence is 99% identical to that of P. homopolare (accession no. KF537294), a geographically widespread host-generalist that infects many migratory and resident species within North and South America, especially hosts of family Emberizidae [20]. We also collected 200 µl of uninfected blood from each of two sham donors that were confirmed by PCR and microscopy to be free of haemosporidian infection. We pooled blood from the two parasite donors, and from the two sham donors, and combined each mixture with saline and sodium citrate buffer [17]. We injected 200 µl of blood–buffer mixture (80 µl of freshly collected blood, 20 µl of 3.7% sodium citrate, 100 µl of 0.9% saline) into the pectoralis muscle of four amplifiers (i.e. individuals confirmed by PCR and microscopy to be free of infection). Specifically, we inoculated two parasite amplifiers with blood from parasite donors containing Plasmodium lineage P-SOSP10, and inoculated two sham amplifiers with unparasitized blood from sham donors, following previously published techniques [17].
On 15 February 2016, we switched amplifiers and experimental subjects to a long-day photoperiod (16 L : 8 D) to induce breeding condition. On 21 February—13 days after inoculating amplifiers, and 6 days after transfer to long days—we prepared and examined thin-film blood smears from amplifiers as described above. We detected Plasmodium parasites in both parasite amplifiers but not in either sham amplifier. The following day, we euthanized amplifiers by overdose of isoflurane vapours and immediately collected up to 1.2 ml of blood by cardiac puncture. We combined blood from the two parasite amplifiers, and from the two sham amplifiers, and mixed each with buffer as described above. We injected this mixture into the pectoralis muscle of experimental subjects: birds in the parasite treatment (n = 30) received parasitized blood from parasite amplifiers, and birds in the sham treatment (n = 12) received unparasitized blood from sham amplifiers. We assigned birds to treatment groups using block-randomization while balancing groups with respect to morph and sex. Fourteen days after inoculating experimental subjects (three weeks after the transition to long days), we prepared and examined thin-film blood smears as described above. This timing corresponds to peak parasitaemia in song sparrows (Melospiza melodia) inoculated with strain P-SOSP10 [17]. Smears were scored blind to treatment, sex and morph.
To assess sex and morph differences in plasma androgen levels, and to confirm that photostimulation induced reproductive condition, we collected blood from birds in the parasite and sham treatments seven days before inoculation (i.e. one day before photostimulation) and fourteen days after inoculation. We collected 100 µl of blood by brachial venipuncture, between 09.30 and 11.00, then isolated plasma and quantified androgens as detailed in the electronic supplementary material.
(d). Data analysis
Parasite loads at capture ranged from 0–5 parasitized erythrocytes per 10 000 (n = 42, mean ± s.e. = 0.90 ± 0.23). Fourteen days after inoculation, parasite loads in the sham treatment ranged from 0 to 2 parasitized erythrocytes per 10 000 (n = 12, 0.79 ± 0.23). Based on these values, we established an arbitrary threshold for infection outcome: subjects in the parasite treatment were categorized as infected if they had 6 or more parasitized erythrocytes per 10 000 at this 14-day timepoint.
We compared support for candidate models predicting infection outcome (infected or resistant) among parasite-treated birds (electronic supplementary material, table S2). Models predicting infection outcome were fit with binomial errors, using glm in base R v. 3.3.2 [21]. Among birds that became infected, we also compared candidate models predicting infection intensity (parasitized erythrocytes per 10 000 examined; electronic supplementary material, table S3). Models predicting infection intensity were fit with negative binomial errors, using glm.nb in the R package MASS [22]. Within each set, candidate models differed in the presence versus absence of morph, sex and their interaction and prior infection status (i.e. any haemosporidian infection, confirmed through microscopy and/or PCR). We plotted model residuals to confirm assumptions.
To compare androgen levels between morphs, sexes and timepoints, we constructed a generalized linear mixed model with a log-normal (Gaussian) distribution using glmmPQL in the R package MASS [22]. Predictors included morph, sex and timepoint (pre- versus post-photostimulation) plus all first-order interactions, and a random effect of bird ID.
3. Results
White- and tan-striped morphs did not differ in infection outcome. Among birds inoculated with Plasmodium, 4 of 15 white-striped and 5 of 15 tan-striped individuals became infected, and among models predicting infection outcome, the null model was at least three times better supported than models including morph, sex, morph×sex interaction or prior infection status (electronic supplementary material, table S2). Among birds that became infected, however, parasite loads were higher in tan-striped than white-striped individuals (figure 1). Among models predicting infection intensity, the morph model was at least three times better supported than alternatives including effects of sex, morph×sex interaction, or prior infection status (electronic supplementary material, table S3), although we note that 95% confidence limits for the morph parameter estimate overlap with zero (table 1). We found no evidence of sex differences, or morph×sex interactions, in infection outcome or intensity (electronic supplementary material, tables S2 and S3).
Figure 1.
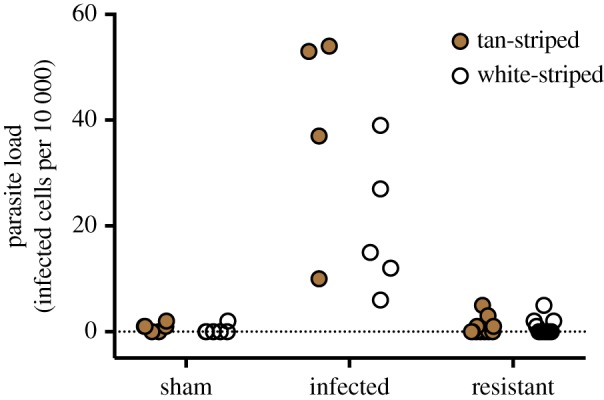
Parasite loads in white-throated sparrows, after inoculation with Plasmodium or unparasitized blood. Among individuals that became infected, parasite loads were higher on average in tan-striped than white-striped birds. n = 12 sham (6 tan-striped, 6 white-striped), 9 infected (4 tan-striped, 5 white-striped), 21 resistant (11 tan-striped, 10 white-striped). (Online version in colour.)
Table 1.
Parameter estimates from the morph model predicting parasite load in white-throated sparrows that became infected after inoculation with Plasmodium.
| parameter | ß ± s.e. | 95% CI |
|---|---|---|
| intercept | 3.65 ± 0.29 | 3.12, 4.27 |
| morph (white) | −0.67 ± 0.40 | −1.46, 0.11 |
Plasma androgens were higher in males than females, increased after the switch to long-day photoperiod, and tended to increase more in males than females, collectively suggesting birds were approaching breeding condition during the experiment. We found no evidence of morph differences, morph×sex interactions or morph×timepoint interactions (electronic supplementary material, tables S4 and S5).
4. Discussion
We compared parasite resistance between behaviourally distinct morphs of white-throated sparrow, hypothesizing that the aggressive white-striped morph would be more susceptible to infection. However, morphs did not differ in infection outcome, and infection intensity was on average higher in the tan-striped, not the white-striped, morph. Moreover, despite sex differences in androgen levels, males and females did not differ in infection outcome or intensity. We conclude that white-throated sparrow morphs may differ in disease resistance, potentially reflecting life-history-related differences in immune investment. However, these morph differences do not parallel sex differences frequently observed in animals [3] and do not appear to be mediated by morph differences in androgens.
We detected morph but not sex differences in parasite loads. By contrast, we detected sex but not morph differences in androgen levels, suggesting that androgen-mediated immunosuppression [4] does not explain morph differences in parasite loads. Several non-exclusive processes may explain these findings. First, tan-striped birds are behaviourally subordinate to white-striped birds [23] and may have been immunosuppressed due to social stress from aggressive interactions. Although our animals were housed individually, they remained in acoustic and visual contact. Second, morphs may differ in infection tolerance, defined as the ability to minimize damage associated with a given pathogen burden [24]. If morphs differ in the relative benefits of tolerance versus resistance (minimizing pathogen burden), one morph might rely primarily on tolerance and the other on resistance. Third, life-history differences in injury risk [10] could favour greater immune investment by the more aggressive white-striped morph. Finally, the chromosomal inversion associated with this plumage and behavioural dimorphism may harbour immune loci that affect disease resistance.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Long Point Bird Observatory, the Advanced Facility for Avian Research, A. Boyer, M. Brodbeck, S. DesRoches, J. Slade and M. Watson for assistance.
Ethics
Animal use was approved by the University of Western Ontario's Animal Care Committee (protocol no. 2015-047 to E.A.M.-S.) and was in agreement with Canadian legislation regarding animal research. Animals were collected under Environment Canada Scientific Collecting Permit CA0244.
Data accessibility
Supporting data are on the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.hp26sv7 [25].
Authors' contributions
T.R.K., S.A.M.-S. and E.A.M.-S contributed to study design. T.R.K contributed to animal work. R.J.B contributed to genetic analysis and microscopy. T.R.K. and S.A.M.-S. contributed to androgen assays. Data analysis was contributed by R.J.B., T.R.K., S.A.M.-S. and E.A.M.-S. Manuscript preparation and revision by R.J.B., T.R.K., S.A.M.-S. and E.A.M.-S. All authors approved the final version of the manuscript and agree to be accountable for this work's accuracy and integrity.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NSERC Canada Discovery Grants to E.A.M.-S. (293123-RGPIN) and S.A.M.-S. (217381-RGPIN).
References
- 1.Lee KA. 2006. Linking immune defenses and life history at the levels of the individual and the species. Int. Compar. Biol. 46, 1000–1015. ( 10.1093/icb/icl049) [DOI] [PubMed] [Google Scholar]
- 2.Poulin R. 1996. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 147, 287–295. ( 10.1086/285851) [DOI] [Google Scholar]
- 3.Zuk M, McKean KA. 1996. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 26, 1009–1023. ( 10.1016/S0020-7519(96)80001-4) [DOI] [PubMed] [Google Scholar]
- 4.Folstad I, Karter AJ. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622. ( 10.1086/285346) [DOI] [Google Scholar]
- 5.Roberts ML, Buchanan KL, Evans MR. 2004. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 68, 227–239. ( 10.1016/j.anbehav.2004.05.001) [DOI] [Google Scholar]
- 6.Gross MR, Charnov EL. 1980. Alternative male life histories in bluegill sunfish. Proc. Natl Acad. Sci. USA 77, 6937–6940. ( 10.1073/pnas.77.11.6937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lank DB, Smith CM, Hanotte O, Burke T, Cooke F. 1995. Genetic polymorphism for alternative mating behaviour in lekking male ruff Philomachus pugnax. Nature 378, 59–62. ( 10.1038/378059a0) [DOI] [Google Scholar]
- 8.Jukema J, Piersma T. 2006. Permanent female mimics in a lekking shorebird. Biol. Lett. 2, 161–164. ( 10.1098/rsbl.2005.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinervo B, Lively CM. 1996. The rock–paper–scissors game and the evolution of alternative male reproductive strategies. Nature 380, 240–243. ( 10.1038/380240a0) [DOI] [Google Scholar]
- 10.Lozano GA, Lank DB, Addison B. 2013. Immune and oxidative stress trade-offs in four classes of ruffs (Philomachus pugnax) with different reproductive strategies. Can. J. Zool. 91, 212–218. ( 10.1139/cjz-2012-0324) [DOI] [Google Scholar]
- 11.Sacchi R, Rubolini D, Gentilli A, Pupin F, Razzetti E, Scali S, Galeotti P, Fasola M. 2007. Morph-specific immunity in male Podarcis muralis. Amphib. Reptil. 28, 408–412. ( 10.1163/156853807781374700) [DOI] [Google Scholar]
- 12.Tuttle EM, et al. 2016. Divergence and functional degradation of a sex chromosome-like supergene. Curr. Biol. 26, 344–350. ( 10.1016/j.cub.2015.11.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle EM. 2003. Alternative reproductive strategies in the white-throated sparrow: behavioral and genetic evidence. Behav. Ecol. 14, 425–432. ( 10.1093/beheco/14.3.425) [DOI] [Google Scholar]
- 14.Spinney LH, Bentley GE, Hau M. 2006. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis). Horm. Behav. 50, 762–771. ( 10.1016/j.yhbeh.2006.06.034) [DOI] [PubMed] [Google Scholar]
- 15.Horton BM, Moore IT, Maney DL. 2014. New insights into the hormonal and behavioural correlates of polymorphism in white-throated sparrows, Zonotrichia albicollis. Anim. Behav. 93, 207–219. ( 10.1016/j.anbehav.2014.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellgren O, Waldenström J, Bensch S. 2004. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 90, 797–802. ( 10.1645/GE-184R1) [DOI] [PubMed] [Google Scholar]
- 17.Sarquis-Adamson Y, MacDougall-Shackleton EA. 2016. Song sparrows Melospiza melodia have a home-field advantage in defending against sympatric malarial parasites. R. Soc. open sci. 3, 160216 ( 10.1098/rsos.160216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michopoulos V, Maney DL, Morehouse CB, Thomas JW. 2007. A genotyping assay to determine plumage morph in the white-throated sparrow (Zonotrichia albicollis). Auk 124, 1330–1335. ( 10.1642/0004-8038(2007)124%5B1330:AGATDP%5D2.0.CO;2) [DOI] [Google Scholar]
- 19.Griffiths R, Double MC, Orr K, Dawson RJ. 1998. A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. ( 10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 20.Walther EL, Valkiūnas G, González AD, Matta NE, Ricklefs RE, Cornel A, Sehgal RN. 2014. Description, molecular characterization, and patterns of distribution of a widespread New World avian malaria parasite (Haemosporidae: Plasmodiidae), Plasmodium (Novyella) homopolare sp. nov. Parasitol. Res. 113, 3319–3332. ( 10.1007/s00436-014-3995-5) [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 22.Venables WN, Ripley BD. 2002. Modern applied statistics with S. 4th edn. New York, NY: Springer. [Google Scholar]
- 23.Watt DJ, Ralph CJ, Atkinson CT. 1984. The role of plumage polymorphism in dominance relationships of the white-throated sparrow. Auk 101, 110–120. [Google Scholar]
- 24.Ayres JS, Schneider DS. 2008. Two ways to survive infection: what resistance and tolerance can teach us about treatments for infectious diseases. Nat. Rev. Immunol. 8, 889–895. ( 10.1038/nri2432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd RJ, Kelly TR, MacDougall-Shackleton SA, MacDougall-Shackleton EA. 2018. Data from: Alternative reproductive strategies in white-throated sparrows are associated with differences in parasite load following experimental infection Dryad Digital Repository. ( 10.5061/dryad.hp26sv7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Boyd RJ, Kelly TR, MacDougall-Shackleton SA, MacDougall-Shackleton EA. 2018. Data from: Alternative reproductive strategies in white-throated sparrows are associated with differences in parasite load following experimental infection Dryad Digital Repository. ( 10.5061/dryad.hp26sv7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supporting data are on the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.hp26sv7 [25].


