Abstract
Cardiac oxygenation is achieved via both coronary arterial and luminal venous oxygen supply routes in many fish species. However, the relative importance of these supplies for cardiac and aerobic metabolic performance is not fully understood. Here, we investigated how coronary artery ligation in rainbow trout (Oncorhynchus mykiss), implanted with heart rate loggers, affected cardiorespiratory performance in vivo. While coronary ligation significantly elevated resting heart rate, the standard metabolic rate was unchanged compared to sham-treated controls. However, coronary ligation reduced the maximum metabolic rate while heart rate remained unchanged following enforced exercise. Thus, coronary ligation reduced metabolic and heart rate scopes by 29% and 74%, respectively. Our findings highlight the importance of coronary oxygen supply for overall cardiorespiratory performance in salmonid fish, and suggest that pathological conditions that impair coronary flow (e.g. coronary arteriosclerosis) constrain the ability of fish to cope with metabolically demanding challenges such as spawning migrations and environmental warming.
Keywords: bio-logger, cardiac oxygen supply, compact myocardium, STAR-ODDI
1. Introduction
The capacity of fish to elevate oxygen uptake above resting levels to sustain additional metabolically demanding activities (e.g. exercise and digestion) is reflected in their aerobic scope (AS), defined as the difference between maximum and standard metabolic rates (i.e. MMR–SMR) [1,2]. MMR depends primarily on the capacity to increase cardiac output (heart rate×stroke volume) and tissue oxygen extraction [2,3]. The pumping capacity of the heart is directly affected by myocardial oxygen availability [2,4], which in many active teleost fish species is delivered via two alternative routes. The luminal oxygen supply comprises the oxygen remaining in the venous blood that returns to the heart and diffuses into the inner spongy myocardial layer, while the coronary circulation delivers fully oxygenated arterial blood from the gills to the outer compact myocardium [5]. When faced with metabolically demanding situations (e.g. exhaustive exercise and environmental warming), both cardiac output and tissue oxygen extraction typically increase [2,6]. This also leads to an elevated cardiac workload and elevated myocardial oxygen demand, while venous oxygen tension typically declines [2]. In species that lack a coronary supply and solely rely on the luminal oxygen supply, this may lead to a conflict between systemic and myocardial oxygen demands, whereas species with coronaries can increase coronary blood flow to sustain cardiac oxygenation [2,5,6]. Even so, the coronary oxygen delivery capacity has been proposed as a potential limiting factor for cardiac and aerobic performance in species with coronaries [2,4]. For example, coronary ligation in rainbow trout (Oncorhynchus mykiss, Walbaum 1792) impairs ventral aortic blood pressure generation during swimming [7], and reduces maximum swimming speed and acute thermal tolerance [6,8]. Furthermore, coronary perfusion improves both cardiac stroke volume and stroke work in isolated rainbow trout heart preparations [9]. Interestingly, the resting heart rate is elevated in coronary-ligated trout in vivo, presumably to compensate for an impaired stroke volume [6,7,9]. While this resting tachycardia could be expected to reduce the scope for heart rate (fHscope; active-resting heart rates, i.e. fHactive – fHrest) and cardiac output; no study has examined how coronary ligation affects AS, and whether compensatory changes in fHactive could serve to sustain cardiovascular scope in fish with coronary obstruction (e.g. coronary arteriosclerosis).
Here, we tested whether coronary ligation reduces AS and fHscope in trout, or if coronary-ligated fish exhibit compensatory increases in fHactive to maintain AS. Understanding how coronary cardiac oxygenation affects cardiorespiratory performance is necessary to predict how fish with coronaries are affected by environmental and metabolic challenges. Indeed, observations of coronary arteriosclerosis in both wild and farmed salmonid fishes are of growing concern (see [10]), as this may restrict coronary flow and impair cardiorespiratory performance and the capacity to cope with metabolic challenges. This may be particularly important in migratory salmonids that face highly demanding migratory conditions, and where up to 50% of the heart is composed of compact myocardium [2,5,6,11].
2. Material and methods
(a). Experimental animals
Rainbow trout (see table 1 for biometrics) were obtained from a local fish farm (Vänerns Laxodling AB, Sweden). Fish were kept in holding tanks supplied with recirculating aerated freshwater (10°C) under a 12 L : 12 D photoperiod for at least eight weeks prior to experimentation. They were fed three times per week with commercial fish pellets.
Table 1.
Morphological characteristics of the rainbow trout (O. mykiss). BM, body mass; BL, body length (standard); CF, condition factor; RVM, relative ventricle mass. The condition factor of the fish was calculated as (100×body mass)/standard length3. There were no statistical differences between treatment groups for any of the variables.
| treatment group | BM (g) | BL (mm) | CF | RVM (%) |
|---|---|---|---|---|
| control, n = 11 | 1017 ± 58 | 433 ± 5 | 1.25 ± 0.06 | 0.093 ± 0.005 |
| coronary ligated, n = 10 | 1079 ± 49 | 446 ± 8 | 1.22 ± 0.04 | 0.086 ± 0.004 |
(b). Surgery
Fish were anaesthetized in freshwater (10°C) containing MS-222 (tricaine methanesulfonate, 150 mg l−1) buffered with NaHCO3 (300 mg l−1) and then placed on a surgery table with wet foam. Anaesthesia was maintained during surgery by irrigating the gills with 10°C water containing MS-222 (75 mg l−1) and NaHCO3 (150 mg l−1).
An incision was made in the isthmus to expose the coronary artery [6]. In one experimental group, the common coronary artery was permanently ligated using a 6–0 silk suture (coronary ligated). A second sham-treated group was treated identically except that the coronary artery was not ligated (control). A DST milli-HRT heart rate data storage tag (STAR-ODDI, Gardabaer, Iceland) was then inserted via an approximately 30 mm mid-ventral incision into the abdominal cavity and positioned in an anterior direction towards the pericardial cavity. The DST tag was anchored with a suture to the muscle at the anterior opening of the incision. A PIT tag was also placed in the abdominal cavity for individual identification. The wound was closed using sterile 3–0 monofilament non-absorbable Prolene sutures (Ethicon Ltd, UK). The DSTs, PIT tags and surgical instruments were disinfected using 70% ethanol and thoroughly rinsed with sterile saline.
Following surgery, the fish were placed in opaque holding tanks (130 l) supplied with recirculating aerated freshwater at 10°C, and left to recover for 7 days.
(c). Experimental protocol
For each experimental run, four fish (two from each treatment group) were individually placed in cylindrical Perspex respirometers (10 l) submerged in a holding tank (220 l) supplied with aerated 10°C freshwater and left overnight (approx. 22 h) to measure SMR and fHrest. The following morning, all fish were placed in a separate tank (1500 l) with 10°C freshwater and subjected to a chase stress protocol for 5 min or until fatigue [1,3]. Immediately following the chase, the fish were returned to the respirometers for recordings of MMR and fHactive for 2 h. The chase protocol has been found to provide a reliable measurement of MMR in trout, which is typically higher than the maximum metabolic response obtained during maximum swimming (i.e. at Ucrit) in this species [12]. After the experiments, the fish were euthanized by a cranial blow.
(d). Data acquisition and analysis
SMR and MMR were determined by recordings of whole animal oxygen consumption rate (MO2) via intermittent flow-through respirometry. The partial pressure of oxygen in the water within the respirometer was continuously recorded with oxygen optodes connected to a FireSting system (PyroScience, Aachen, Germany) every 15 min between automated respirometer flush cycles. MO2 was calculated from the decline in the partial pressure of O2 in the respirometers between flush cycles and expressed as mg O2 h−1 kg−1 [1]. Values for SMR were calculated as the 20% quantile of the MO2 values sampled between 00.00 and 04.00 h where the lowest activity was typically observed [13]. The highest MO2 measurement after the chase protocol was designated MMR. AS was calculated as MMR–SMR.. The DSTs were programmed to collect heart rate on day 7 after surgery between 00.00 and 10.00 h at 12 samples h−1, and at 60 samples h−1 between 10.00 and 12.00 h (i.e. during the chase protocol). Values for fHrest were taken as the mean of all values between 00.00 and 04.00 h, and fHactive was calculated as the mean of all values obtained 0–30 min post-chase. fHscope was calculated as fHactive – fHrest. Only readings marked by the software as excellent (quality index 0) were used.
(e). Statistics
All data are presented as means ± s.e.m. Statistical differences (p ≤ 0.05) between treatments were assessed using independent samples t-tests.
3. Results
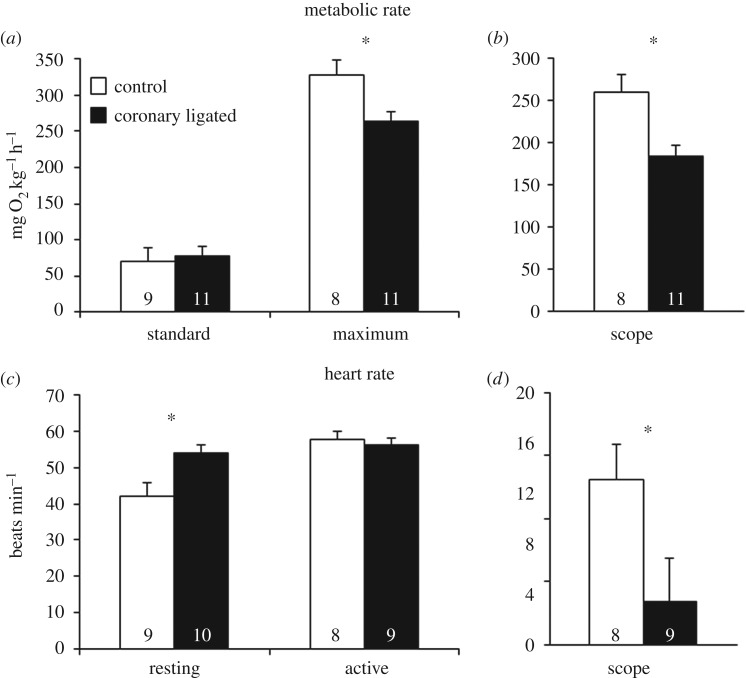
There were no significant differences in morphological characteristics between treatment groups (table 1). Coronary ligation did not alter SMR (figure 1a,b), but significantly reduced MMR (265 versus 329 mg O2 h−1 kg−1; T17 = 2.818; p = 0.012; figure 1a) which lowered AS by 29% compared to control fish (T17 = −3.306; p = 0.004; figure 1b). Coronary-ligated trout had a significantly higher fHrest compared to controls (53.9 ± 2.7 versus 41.9 ± 3.7 beats min−1; T17 = −2.720; p = 0.015; figure 1c). Yet, both groups reached a similar fHactive of around 57 beats min−1, resulting in 74% lower fHscope in coronary-ligated fish compared to controls (3 versus 13 beats min−1; T15 = 2.147; p = 0.049; figure 1c,d).
Figure 1.
Effects of coronary ligation on cardiac and metabolic performance in rainbow trout (O. mykiss). Variables are (a) standard and maximum metabolic rate, (b) AS, (c) resting and active heart rate and (d) scope for heart rate in control (open bars) and coronary-ligated (closed bars) trout. *Significant differences between treatments (p ≤ 0.05). Numbers within bars denote sample sizes.
4. Discussion
We demonstrate that coronary-ligated trout exhibit a 29% reduction in AS due to a 19% reduction in MMR, which likely reflects an impaired cardiac pumping capacity constraining the ability to increase cardiac stroke volume and cardiac output during stress [7,9]. Indeed, salmonids normally increase cardiac output and tissue perfusion by increasing both stroke volume and heart rate [2,5]. However, the coronary-ligated trout also exhibited a reduced fHscope, which was solely due to an increased fHrest, as no compensatory increase in fHactive was observed. Even so, the reduction in AS seems surprisingly small considering that fHscope was reduced by 74% and that a relatively large proportion of the ventricle was likely oxygen-deprived (approx. 35% compact myocardium in this trout strain; A Ekström & E Sandblom 2017, unpublished data). This possibly reflects a compensatory increase in tissue oxygen extraction in the coronary-ligated fish during stress, but additional direct measurements of how maximum cardiac output and the difference between arterial and venous oxygen contents (i.e. tissue oxygen extraction) are affected by coronary ligation are required to substantiate this [2,5].
The elevated fHrest in coronary-ligated trout agrees with previous studies [6,7], and presumably serves to maintain or elevate resting cardiac output when cardiac contractility and stroke volume is impaired from reduced ventricular oxygenation [6,7,9]. In fact, the ventral aortic blood pressure (i.e. cardiac afterload) is reduced in trout after coronary ligation [7], and so the tachycardia likely reflects a reflex response to regulate blood pressure. Preliminary findings from our laboratory suggest that the tachycardia following coronary ligation is mediated by reduced cholinergic tone on the heart (unpublished observation), which is consistent with a barostatic reflex [14].
Considering that AS has been suggested to represent a fundamental fitness trait that governs a range of performance traits in fish [1,2,15], the current finding that coronary flow restrictions negatively affects AS in trout may have broader ecological consequences. For example, both AS and the proportion of compact myocardium are positively correlated with migratory distance and difficulty in various populations of migratory sockeye salmon (Oncorhynchus nerka) [15]. Thus, the occurrence of coronary arteriosclerosis in migratory salmonids should severely constrain aerobic swimming performance during physically demanding spawning migrations, which may ultimately impact negatively on spawning success and overall fitness. Moreover, given the suggested relationships between thermal and aerobic metabolic performance of fishes [1], the ability to tolerate environmental warming and added metabolic demands of climate change may have direct linkages to coronary pathology and perfusion capacity in fish species that possess a coronary circulation.
Supplementary Material
Acknowledgements
We thank students from Hulebäcksgymnasiet and University of Gothenburg for experimental assistance.
Ethics
All experiments complied with ethical permit 165-2015 issued from the regional animal ethics committee.
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
A.E., E.S., J.B. and A.G. designed and conducted the experiments. A.E. analysed the data. All authors contributed to interpreting the data and drafting the manuscript, for which all authors gave final approval and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Swedish Research Council (VR) (E.S. and M.A.) and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) (E.S. and A.G.).
References
- 1.Clark TD, Sandblom E, Jutfelt F. 2013. Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J. Exp. Biol. 216, 2771–2782. ( 10.1242/jeb.084251) [DOI] [PubMed] [Google Scholar]
- 2.Farrell AP, Eliason EJ, Sandblom E, Clark TD. 2009. Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 87, 835–851. ( 10.1139/Z09-092) [DOI] [Google Scholar]
- 3.Sandblom E, et al. 2016. Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat. Commun. 7, 11447 ( 10.1038/ncomms11447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekström A, Brijs J, Clark TD, Gräns A, Jutfelt F, Sandblom E. 2016. Cardiac oxygen limitation during an acute thermal challenge in the European perch: effects of chronic environmental warming and experimental hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R440–R449. ( 10.1152/ajpregu.00530.2015) [DOI] [PubMed] [Google Scholar]
- 5.Farrell AP, Smith FM. 2017. Cardiac form, function and physiology. In Fish physiology, vol 36 (eds Gamperl AK, Gillis TE, Farrell AP, Brauner CJ), pp. 155–264. New York, NY: Academic Press. [Google Scholar]
- 6.Ekström A, Axelsson M, Gräns A, Brijs J, Sandblom E. 2017. Influence of the coronary circulation on thermal tolerance and cardiac performance during warming in rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R549–RR58. ( 10.1152/ajpregu.00536.2016) [DOI] [PubMed] [Google Scholar]
- 7.Steffensen JF, Farrell AP. 1998. Swimming performance, venous oxygen tension and cardiac performance of coronary-ligated rainbow trout, Oncorhynchus mykiss, exposed to progressive hypoxia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 119, 585–592. ( 10.1016/S1095-6433(97)00470-4) [DOI] [PubMed] [Google Scholar]
- 8.Farrell AP. 1987. Coronary flow in a perfused rainbow trout heart. J. Exp. Biol. 129, 107–123. [DOI] [PubMed] [Google Scholar]
- 9.Agnisola C, Petersen L, Mustafa T. 2003. Effect of coronary perfusion on the basal performance, volume loading and oxygen consumption in the isolated resistance-headed heart of the trout Oncorhynchus mykiss. J. Exp. Biol. 206, 4003–4010. ( 10.1242/jeb.00623) [DOI] [PubMed] [Google Scholar]
- 10.Farrell AP. 2002. Coronary arteriosclerosis in salmon: growing old or growing fast? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 723–735. ( 10.1016/S1095-6433(02)00126-5) [DOI] [PubMed] [Google Scholar]
- 11.Brijs J, Sandblom E, Dekens E, Näslund J, Ekström A, Axelsson M. 2016. Cardiac remodeling and increased central venous pressure underlie elevated stroke volume and cardiac output of seawater-acclimated rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R31–R39. [DOI] [PubMed] [Google Scholar]
- 12.Killen SS, Norin T, Halsey LG. 2017. Do method and species lifestyle affect measures of maximum metabolic rate in fishes? J. Fish Biol. 90, 1037–1046. ( 10.1111/jfb.13195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabot D, Steffensen JF, Farrell AP. 2016. The determination of standard metabolic rate in fishes. J. Fish Biol. 88, 81–121. ( 10.1111/jfb.12845) [DOI] [PubMed] [Google Scholar]
- 14.Sandblom E, Axelsson M. 2005. Baroreflex mediated control of heart rate and vascular capacitance in trout. J. Exp. Biol. 208, 821–829. ( 10.1242/jeb.01470) [DOI] [PubMed] [Google Scholar]
- 15.Eliason EJ, et al. 2011. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112. ( 10.1126/science.1199158) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.