Abstract
Social foraging behaviours, which range from cooperative hunting to local enhancement, can result in increased prey capture and access to information, which may significantly reduce time and energy costs of acquiring prey. In colonial species, it has been proposed that the colony itself may act as a site of social information transfer and group formation. However, conclusive evidence from empirical studies is lacking. In particular, most studies in colonial species have generally focussed on behaviours either at the colony or at foraging sites in isolation, and have failed to directly connect social associations at the colony to social foraging. In this study, we simultaneously tracked 85% of a population of Australasian gannets (Morus serrator) over multiple foraging trips, to study social associations at the colony and test whether these associations influence the location of foraging sites. We found that gannets positively associate with conspecifics while departing from the colony and that co-departing gannets have more similar initial foraging patches than individuals that did not associate at the colony. These results provide strong evidence for the theory that the colony may provide a source of information that influences foraging location.
Keywords: social foraging, seabirds, coloniality, social information use, group formation
1. Introduction
Social foraging, when an animal's foraging behaviours, and the resulting costs and benefits, are interdependently linked with the foraging behaviours of others [1], is expected to develop when the benefits of social foraging outweigh the costs, such as increased competition [2]. Social foragers may directly benefit through decreased search time, improved capture rate, access to otherwise unavailable prey, or access to information (reviewed in [1,2]). Additionally, individuals may indirectly benefit from coordinating travel during foraging, through reducing overall energy expenditure by lowering movement costs (i.e. birds flying in formation; [3]) or predator protection effects [2]. Social foraging benefits are predicted to be most prevalent when costs of individual foraging are high [4], resource detectability is low [5], or resources are variable but clumped within the environment [6].
For colonial species, large breeding or roosting aggregations provide significant potential for social foraging opportunities. It has been proposed that colonies can act as a location for information transmission [7] and that the ready availability of social information may be a driving force in the evolution and maintenance of coloniality [8]. However, previous studies examining the potential transmission of information at colonies, through direct between-pair signalling [9] and colony co-departures, have produced mixed results (e.g. [9–12]) and generally focus on either behaviours at the colony or foraging site separately (but see [9,13,14]), and so the link between these behaviours remains unresolved. Notably, without the knowledge of subsequent foraging locations and availability of additional social (i.e. local enhancement [15]) and asocial information sources (i.e. environmental conditions), studies at the colony alone cannot determine whether or how these interactions translate into foraging information.
In this study, we simultaneously tracked 85% of the active breeders from a small colony of Australasian gannets (Morus serrator; hereafter gannets). Gannets, like many seabirds, are colonial, forage in patchy marine environments, and frequently aggregate with both conspecifics and heterospecifics at-sea [16]. Evidence suggests that seabirds use local enhancement by responding to the presence of foraging individuals (e.g. [17]), which can lead to earlier arrival at foraging patches [18]. However, due to the size of most seabird colonies, previous studies have been limited to observing only a very small proportion of the colony, providing an incomplete picture of a colony's behaviours and making it difficult to infer sociality. Here, by concurrently tracking a large proportion of a colony, we test whether gannets preferentially form groups when departing on foraging trips, testing the potential for the use of colony cues. We then evaluate whether this results in collective foraging by determining if initial foraging patches are more similar when birds depart together. Finally, to provide evidence for social foraging opportunities away from the colony site, we investigate the extent to which gannets overlap in their initial foraging patches and determine if co-foraging gannets share more similar departure times, thus examining the link between coordination at the colony and foraging at-sea.
2. Material and methods
We collected behavioural data from adult gannets breeding on a small man-made structure in Port Phillip Bay, southeastern Australia (38°16′42″ S, 144°41′48″ E). One hundred gannets were fitted with GPS data loggers (igotU GT-600; sampling interval = 2 min; see the electronic supplementary material for full details). All complete trips, from 09 January 2015 to 22 January 2015 were analysed, as this period covers the highest proportion of the colony simultaneously tracked.
We defined colony departure as the first GPS fix in a trip to cross a 500 m buffer around the colony (within 500 m birds may raft, thus we consider co-departures from either colony or raft). To determine how individuals departed from the colony in relation to others in the colony, we used a 3-min sliding time window to identify individuals departing the colony together (see the electronic supplementary materials for sensitivity of co-departure time-windows).
We used Expectation-Maximization binary Clustering (EMbC; R package EMbC v. 2.0.0 [19]) to identify foraging behaviours (behaviours classified: foraging, commuting and resting; see the electronic supplementary materials for full details). A minimum convex polygon was fitted around the initial foraging patch (defined as more than three consecutive GPS fixes classified as foraging, with bouts merged when separated by less than 5 min of non-foraging; see [20]) of each trip (rgeos v. 0.3-26 [21]). We identified all foraging patches that co-occurred in time (60s buffer) and space (sp package v. 12-5 [22]).
To determine whether overlap in colony departures and foraging patches could be generated by chance, we compared the observed pattern to null models produced through randomizations of the timing of departures and foraging patches (for full details, see the electronic supplementary material). To investigate if gannets that shared foraging patches had more similar colony departures, we calculated the difference in departure times (log transformed; to account for non-normality) between pairs of birds in all co-occurring first foraging patches, and used a two-sample t-test to compare pairs of birds in overlapping and non-overlapping foraging patches.
We calculated the straight line distance between centroids of the first foraging patches of each trip, for each individual and (1) birds on trips that co-departed the colony with the focal trip and (2) all trips in which individuals were not observed co-departing with the focal trip. We used a Kolmogorov–Smirnov test to determine if the distribution of distances varied between each group. All statistical analysis was performed in R v. 3.3.1 [23]. Unless otherwise indicated, data are presented as mean ± s.e.
3. Results
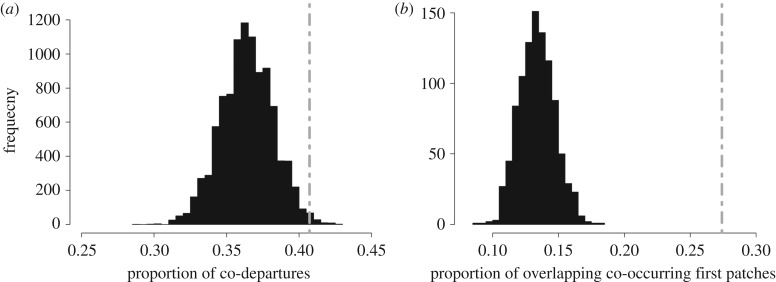
We recorded 938 complete foraging trips (duration: 16.1 ± 0.7 h) from 85 individuals (11.04 ± 0.6 trips per bird), representing 85% of the breeding birds at the time of the study. Gannets preferentially departed the colony with conspecifics (40.7% of trips, 10 000 permutations; p = 0.0068, figure 1a) and co-occurring foraging bouts tended to overlap in foraging area more often than expected by chance (27.4% of co-occurring first foraging bouts overlapped in space; 1000 permutations, p = 0.001, figure 1b).
Figure 1.
Distributions of temporal overlap for (a) the proportion of trips in which at least one pair of individuals co-departed within a 3-min time window and (b) proportion of temporally co-occurring initial foraging patches that overlap in space, compared with the observed values (indicated by dashed line). Null model distributions for co-departures and patch overlap obtained from 10 000 and 1000 data permutations, respectively.
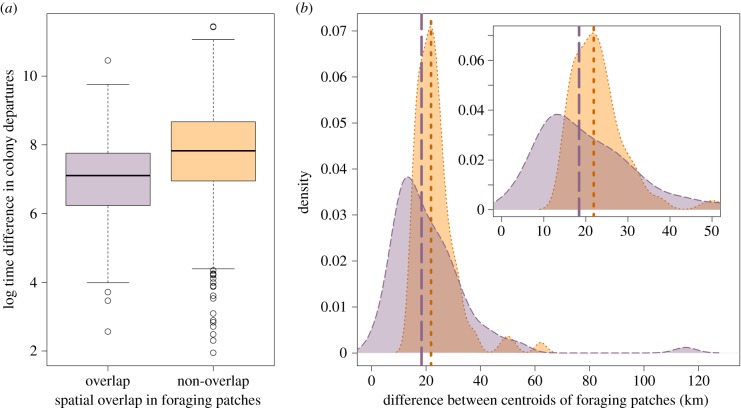
Individuals with overlapping foraging patches had departure times that were 2.6 times closer than those with non-overlapping foraging patches (minutes apart: overlapping = 39 ± 4.9; non-overlapping = 100 ± 4.8; t242 = −7.5, p < 0.0005, figure 2a). Co-departing individuals tended to have first foraging patches closer together than the first patches of birds that did not co-depart (K–S test; D = 0.40, p < 0.0005).
Figure 2.
Similarity between the first foraging patches and departure times of individuals. (a) Log difference in colony departure times for gannets that share a first foraging patch (purple) and those that do not (orange); (b) average difference in distance (km) between central points of the first foraging patches. Co-departing individuals are shown in dashed purple, and non-co-departing pairs are shown in dotted orange. Insert shows 95% of data, for clarity. Lines indicate median values. (Online version in colour.)
4. Discussion
In this study, we used GPS to simultaneously track the foraging movements of 85% of the breeding adults in a colony of Australasian gannets and demonstrate that gannets coordinate foraging movements as part of their overall foraging strategy. Our results, which even with our comprehensive dataset provide a conservative estimate of this coordination (electronic supplementary material, figure S2), show individuals significantly overlap with conspecifics, during both colony departures and subsequently while foraging at-sea. Thus, our study presents robust colony-level support for the existence of social foraging behaviours in colonial seabirds.
As the colony is a central location to which breeding individuals must return, group formation at the colony may be beneficial to avoid locating foraging groups at-sea and/or foraging alone. Indeed, we found that initial foraging patches were more similar for individuals that co-departed the colony, indicating that the colony may provide a site of group formation. Gannets travelling in groups may benefit through multiple mechanisms, including enhanced search ability, access to foraging information [2] and reduced flight costs [3]. These are factors that are difficult to disentangle, likely act in combination and may all be considered aspects of social foraging. However, our data are spatio-temporal co-occurrences, and we did not directly observe inter-individual interactions or determine the effect of the external environment on individual decisions. Thus, our conclusions rely on the assumption that concurrent foraging events represent interdependence in foraging outcomes (social foraging) [1]. Although it is impossible to completely disentangle this pattern from shared environmental drivers using remote tracking data, given the short time-scale over which we measure coordination and the significant overlap observed beyond our null models, we propose that the observed degree of co-occurrence is unlikely to be solely driven by shared external factors. Previous studies of social foraging in colonial seabirds rely on the same assumptions (e.g. [9–11,14]), which are supported by the direct observations of social foraging behaviours [16] that have been found to benefit individuals through reduced foraging time [18] and increased prey capture [24].
As plunge diving is a highly energetically expensive foraging mode [25], gannets may attempt to minimize search time and unsuccessful dives by using conspecific and heterospecific cues. Previous work has highlighted how seabirds respond to aggregations at-sea by joining experimental [26] and natural foraging groups [16–18]. Social foraging can increase prey detection and capture in several species of seabirds through cooperative hunting. For instance, penguin species can cooperatively corral fish shoals [27] and perform synchronised dives (e.g. [28]), which may increase prey detection and/or capture as well as provide group protection through synchronization. In Cape gannets (M. capensis), dive success increases twofold when occurring within seconds of a previous conspecific attack [28], and Australasian gannets exhibit high capture rate (72% success) in mixed-species aggregations [29]. Our results demonstrate that individuals did share foraging areas as predicted, providing further evidence that conspecifics may provide social foraging benefits both at and away from the colony.
Our data simultaneously follow a large proportion of a colony, providing evidence for social foraging behaviours of seabirds and allowing us to capture colony-level social interactions more completely. Although this evidence suggests that social overlap is significant and important across all stages of foraging trips (departure, prey location and foraging), further work modelling individual movements in conjunction with environmental data is necessary to disentangle the effects of social and shared abiotic factors, which can both drive movement decisions. Similarly, future work quantifying the costs and benefits underlying social foraging, such as the energetic gains or losses during group and solitary foraging events, is required to fully understand the consequences of social associations, and would further explain the role social foraging plays within the overall foraging strategy.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Victorian Marine Science Consortium and Parks Victoria for the logistical support. The assistance of the many fieldwork volunteers who made this work possible is gratefully acknowledged.
Ethics
All animal handling followed protocols approved by Deakin University AEC (Approval B20-2013) and Department of Environment and Primary Industry (Victoria, Australia) Wildlife Research Permit 10006878.
Data accessibility
Data are available as the electronic supplementary material.
Authors' contributions
T.B.J., S.C.P., J.P.Y.A. and J.A.G conceived and analysed the study. M.A.R.-M., M.R.W. and J.P.Y.A collected the data. T.B.J. wrote the manuscript and S.C.P., J.A.G. and J.P.Y.A. edited it. All authors contributed to the revising of the final version of this manuscript, approved of and agreed to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Holsworth Wildlife Research Endowment and Deakin University. T.B.J. was supported by a Natural Sciences and Engineering Research Council of Canada postgraduate scholarship.
References
- 1.Giraldeau L-A, Caraco T. 2000. Social foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Beauchamp G. 2014. Social predation: how group living benefits predators and prey. London, UK: Academic Press. [Google Scholar]
- 3.Weimerskirch H, Martin J, Clerquin Y, Alexandre P, Jiraskova S. 2001. Energy saving in flight formation. Nature 413, 697 ( 10.1038/35099670) [DOI] [PubMed] [Google Scholar]
- 4.Galef BG. 2009. Chapter 4. Strategies for social learning: testing predictions from formal theory. In Advances in the study of behavior (eds Brockmann JH, Roper TJ, Naguib M, Wynne-Edwards KE, Mitani JC, Simmons LW), pp. 117–151. New York, NY: Academic Press; ( 10.1016/S0065-3454(09)39004-X) [DOI] [Google Scholar]
- 5.Barrette M, Giraldeau L-A. 2006. Prey crypticity reduces the proportion of group members searching for food. Anim. Behav. 71, 1183–1189. ( 10.1016/j.anbehav.2005.10.008) [DOI] [Google Scholar]
- 6.Barta Z, Szép T. 1992. The role of information transfer under different food patterns: a simulation study. Behav. Ecol. 3, 318–324. ( 10.1093/beheco/3.4.318) [DOI] [Google Scholar]
- 7.Ward P, Zahavi A. 1973. The importance of certain assemblages of birds as ‘information-centres’ for food-finding. Ibis 115, 517–534. ( 10.1111/j.1474-919X.1973.tb01990.x) [DOI] [Google Scholar]
- 8.Evans JC, Votier SC, Dall SRX. 2016. Information use in colonial living. Biol. Rev. 91, 658–672. ( 10.1111/brv.12188) [DOI] [PubMed] [Google Scholar]
- 9.Machovsky-Capuska GE, Hauber ME, Libby E, Amiot C, Raubenheimer D. 2013. The contribution of private and public information in foraging by Australasian gannets. Anim. Cogn. 17, 849–858. ( 10.1007/s10071-013-0716-x) [DOI] [PubMed] [Google Scholar]
- 10.Weimerskirch H, Bertrand S, Silva J, Marques JC, Goya E. 2010. Use of social information in seabirds: compass rafts indicate the heading of food patches. PLoS ONE 5, e9928 ( 10.1371/journal.pone.0009928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racine F, Giraldeau L-A, Patenaude-Monette M, Giroux J-F. 2012. Evidence of social information on food location in a ring-billed gull colony, but the birds do not use it. Anim. Behav. 84, 175–182. ( 10.1016/j.anbehav.2012.04.028) [DOI] [Google Scholar]
- 12.Carter MID, et al. 2016. GPS tracking reveals rafting behaviour of Northern Gannets (Morus bassanus): implications for foraging ecology and conservation. Bird Study 63, 83–95. ( 10.1080/00063657.2015.1134441) [DOI] [Google Scholar]
- 13.Cook TR, Gubiani R, Ryan PG, Muzaffar SB. 2017. Group foraging in Socotra cormorants: a biologging approach to the study of a complex behavior. Ecol. Evol. 7, 2025–2038. ( 10.1002/ece3.2750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton GJ, Hoskins AJ, Berlincourt M, Arnould JPY. 2017. Departure time influences foraging associations in little penguins. PLoS ONE 12, e0182734 ( 10.1371/journal.pone.0182734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pöysä H. 1992. Group foraging in patchy environments: the importance of coarse-level local enhancement. Ornis Scandinavica (Scandinavian J. Ornithol.) 23, 159–166. ( 10.2307/3676444) [DOI] [Google Scholar]
- 16.Vaughn R, Würsig B, Packard J. 2010. Dolphin prey herding: prey ball mobility relative to dolphin group and prey ball sizes, multispecies associates, and feeding duration. Mar. Mamm. Sci. 26, 213–225. ( 10.1111/j.1748-7692.2009.00317.x) [DOI] [Google Scholar]
- 17.Tremblay Y, Thiebault A, Mullers R, Pistorius P. 2014. Bird-borne video-cameras show that seabird movement patterns relate to previously unrevealed proximate environment, not prey. PLoS ONE 9, e88424 ( 10.1371/journal.pone.0088424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiebault A, Mullers R, Pistorius P, Meza-Torres MA, Dubroca L, Green D, Tremblay Y. 2014. From colony to first patch: processes of prey searching and social information in Cape Gannets. Auk 131, 595–609. ( 10.1642/AUK-13-209.1) [DOI] [Google Scholar]
- 19.Garriga J, Palmer JRB, Oltra A, Bartumeus F. 2016. Expectation-maximization binary clustering for behavioural annotation. PLoS ONE 11, e0151984 ( 10.1371/journal.pone.0151984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez L, Borsa P, Cruz S, de Grissac S, Hennicke J, Lallemand J, Prudor A, Weimerskirch H. 2017. Geographical variation in the foraging behaviour of the pantropical red-footed booby. Mar. Ecol. Prog. Ser. 568, 217–230. ( 10.3354/meps12052) [DOI] [Google Scholar]
- 21.Bivand R, Rundel C.2017. Interface to geometry engine—open source (GEOS). See https://CRAN.R-project.org/package=rgeos .
- 22.Pebesma E, Bivand R.2017. Classes and methods for spatial data. See https://cran.r-project.org/package=sp .
- 23.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 24.Thiebault A, Semeria M, Lett C, Tremblay Y. 2016. How to capture fish in a school? Effect of successive predator attacks on seabird feeding success. J. Anim. Ecol. 85, 157–167. ( 10.1111/1365-2656.12455) [DOI] [PubMed] [Google Scholar]
- 25.Green JA, White CR, Bunce A, Frappell PB, Butler PJ. 2009. Energetic consequences of plunge diving in gannets. Endangered Species Res. 10, 269–279. ( 10.3354/esr00223) [DOI] [Google Scholar]
- 26.Bairos-Novak KR, Crook KA, Davoren GK. 2015. Relative importance of local enhancement as a search strategy for breeding seabirds: an experimental approach. Anim. Behav. 106, 71–78. ( 10.1016/j.anbehav.2015.05.002) [DOI] [Google Scholar]
- 27.Ryan PG, Edwards L, Pichegru L. 2012. African penguins Spheniscus demersus, bait balls and the Allee effect. Ardea 100, 89–94. ( 10.5253/078.100.0113) [DOI] [Google Scholar]
- 28.Berlincourt M, Arnould JPY. 2014. At-sea associations in foraging Little Peguins. PLoS ONE 9, e105065 ( 10.1371/journal.pone.0105065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machovsky Capuska G, Vaughn R, Würsig B, Katzir G, Raubenheimer D. 2011. Dive strategies and foraging effort in the Australasian gannet Morus serrator revealed by underwater videography. Mar. Ecol. Prog. Ser. 442, 255–261. ( 10.3354/meps09458) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as the electronic supplementary material.