Abstract
The area planted with insect-resistant genetically engineered crops expressing Bacillus thuringiensis (Bt) genes has greatly increased in many areas of the world. Given the nearby presence of non-Bt crops (including those planted as refuges) and non-crop habitats, pests targeted by the Bt trait have a choice between Bt and non-Bt crops or weeds, and their host preference may greatly affect insect management and management of pest resistance to Bt proteins. In this study, we examined the oviposition preference of the target pest of Bt rice, Chilo suppressalis, for Bt versus non-Bt rice plants as influenced by previous damage caused by C. suppressalis larvae. The results showed that C. suppressalis females had no oviposition preference for undamaged Bt or non-Bt plants but were repelled by conspecific-damaged plants whether Bt or non-Bt. Consequently, C. suppressalis egg masses were more numerous on Bt plants than on neighbouring non-Bt plants both in greenhouse and in field experiments due to the significantly greater caterpillar damage on non-Bt plants. We also found evidence of poorer performance of C. suppressalis larvae on conspecific-damaged rice plants when compared with undamaged plants. GC-MS analyses showed that larval damage induced the release of volatiles that repelled mated C. suppressalis females in wind tunnel experiments. These findings suggest that Bt rice could act as a dead-end trap crop for C. suppressalis and thereby protect adjacent non-Bt rice plants. The results also indicate that the oviposition behaviour of target pest females should be considered in the development of Bt resistance management strategies.
Keywords: Bt rice, oviposition preference, plant volatile, intraspecific relationship, Bt resistance management
1. Introduction
Over the last 20 years, insect-resistant genetically engineered plants expressing Cry proteins from the bacterium Bacillus thuringiensis (Bt) have been rapidly and widely adopted worldwide. Introduced in 1996, they covered a total area of about 100 million hectares in 2016 [1]. In general, the adoption of Bt crops has provided efficient control of the target pest(s), reduced the application of pesticides and increased yields [2]. These advantages have motivated researchers in China to develop dozens of Bt lines for controlling lepidopteran pests of rice (Oryza sativa L.), including Chilo suppressalis (Crambidae), Scirpophaga incertulas (Crambidae), Sesamia inferens (Noctuidae) and Cnaphalocrocis medinalis (Crambidae) [3,4]. Among these, C. suppressalis is considered the most serious rice pest in China because it attacks rice at all growth stages causing an annual yield loss of 3.1% [3,5].
Although many studies have confirmed that Bt rice lines provide substantial protection against target pests [6], few studies have considered the effects of Bt rice (or Bt maize or cotton) on the oviposition behaviour of target pest females [7–10]. Understanding the effects of Bt crops on oviposition of the target pest is important because Bt crops are generally planted with non-Bt plants. The latter serve as a refuge in that they support pest reproduction and thereby reduce the selection of genotypes with resistance to Bt [11–13]. In the ‘refuge-in-a-bag’ approach, Bt and non-Bt seeds of a crop are mixed and sown together so that the pest females will need to travel only a short distance to select a Bt or non-Bt plant for oviposition [14]. Whether the non-Bt plants are in an adjacent field or are in the same field as the Bt plants, females of mobile target pests will have the opportunity to select between Bt and non-Bt plants for oviposition.
Most models concerning the development of Bt resistance in a target pest assume that oviposition among Bt and non-Bt refuge plants will be random [15,16]. That oviposition may not be random is suggested by two different views on insect behaviour. According to one view, females tend to lay eggs on plants on which their offspring can perform well. Based on this view, researchers have suspected that, given the strong selection pressure caused by Bt plants, female moths (most targeted pests are lepidopterans) may evolve a genetically controlled oviposition preference for alternative non-Bt plants [7]. A different view is based on the observation that female moths often prefer to lay eggs on undamaged rather than on insect-damaged host plants in order to reduce potential competition and improve the performance of their offspring [17]. This phenomenon had been reported for a number of lepidopteran species from different families, including Heliothis virescens (Noctuidae) and Manduca quinquemaculata (Sphingidae) on tobacco [18,19], Ostrinia nubilalis, Ostrinia furnacalis (both Crambidae) and Spodoptera frugiperda (Noctuidae) on corn [20–22], Chilo partellus (Crambidae) on African forage grass [23], Spodoptera littoralis (Noctuidae) on cotton [24] and Manduca sexta (Sphingidae) on tomato [25].
Several studies of lepidopteran oviposition preference have shown that females cannot discriminate between Bt and the corresponding non-Bt cultivars [10,26–29], suggesting that the planting of Bt crops will not alter adult oviposition behaviour. In these cases, however, the experiments only assessed the oviposition preference for undamaged Bt and non-Bt plants. The experiments thus ignored the potential effects of differential larval damage on Bt and non-Bt plants.
In the current study, we conducted laboratory, greenhouse and field experiments to test the hypothesis that females of the striped stem borer C. suppressalis (SSB) prefer to lay eggs on undamaged Bt rice plants over damaged non-Bt rice plants. If this hypothesis is correct, Bt plants would serve as a dead-end trap crop for the adjacent non-Bt plants. We also compared the performance of early-instar SSB on healthy rice plants or plants that had been damaged by conspecifics to determine whether neonate fitness differs on undamaged versus damaged rice plants. Finally, we identified the volatile organic compounds that are released by undamaged and caterpillar-damaged rice plants, which might explain the oviposition response of SBB.
2. Material and methods
(a). Plants and insects
The transgenic Bt rice line T1C-19 and the corresponding non-transformed near isoline Minghui 63 (MH63) were used in all experiments. T1C-19 expresses a synthesized cry1C* gene driven by the maize ubiquitin promoter; the gene encodes the Cry1C protein that targets lepidopteran rice pests [30]. MH63 is an elite indica restorer line for cytoplasmic male sterility in China. All rice seeds were provided by Prof. Yongjun Lin (Huazhong Agricultural University, Wuhan, China).
Specimens of C. suppressalis used in the experiments were retrieved from a laboratory colony maintained on an artificial diet [31] for over 60 generations with annual introductions of field-collected individuals. The colony was maintained in a climate-controlled chamber (Ningbo Jiangnan, Ningbo, China) at 27 ± 3°C, 75 ± 5% RH and a photoperiod of 16 L : 8 D at the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China.
(b). Initial laboratory and greenhouse experiments
(i). Plant treatment
Pre-germinated seeds of Bt and non-Bt rice were simultaneously sown in the greenhouse at 28 ± 2°C with 65 ± 10% RH and a photoperiod of 16 L : 8 D. After 15 days, the seedlings were individually transplanted into plastic pots (diameter 20 cm, height 18 cm) with holes in the bottom and containing a 3 : 1 mixture of peat and vermiculite (Meihekou Factory, Meihekou, China). Potted plants were placed in a cement pool filled with water to a 2 cm depth. Water was replaced weekly, and nitrogenous fertilizer was applied once each week before tillering and once every two weeks after tillering. Plants were used in the experiments six weeks after transplanting when they were at the tillering stage with 10–12 leaves on the main stem.
For the caterpillar treatments, each Bt or non-Bt rice plant was individually infested with two-third instar C. suppressalis that had been starved for at least 2 h. The rice stems infested with caterpillars were covered with plastic sleeves to prevent insects escaping. Three days later, caterpillars had drilled into the stems and caused visible damage. Caterpillars remained in the plants for the duration of all experiments. A preliminary bioassay confirmed that third instar C. suppressalis could drill into the stems of Bt rice plants and cause damage, although the damage was significantly less on Bt plants than on non-Bt plants [32]. Plants in the treatments without damage (healthy plants) remained uninfested.
(ii). Dual-choice wind tunnel experiments
To test the preference of mated females of C. suppressalis to caterpillar-damaged or undamaged Bt or non-Bt rice plants, a dual-choice wind tunnel experiment was conducted. The wind tunnel was 230 cm long × 80 cm wide × 80 cm high (Bejing Hengfabaishun Commerce Co., Ltd, Beijing, China). The airstream in the tunnel (0.2 m s−1) was produced by a fan (CHW-124-401, BROAD Clean Air Technology Co. Ltd, Changsha, China) and was filtered by passage through active charcoal.
Six choice tests were conducted: (a) undamaged Bt rice versus undamaged non-Bt rice; (b) damaged non-Bt rice versus undamaged non-Bt rice; (c) damaged Bt rice versus undamaged Bt rice; (d) damaged non-Bt rice versus undamaged Bt rice; (e) damaged Bt rice versus undamaged non-Bt rice; and (f) damaged non-Bt rice versus damaged Bt rice. In each choice test, the two types of rice plants (treatments) were placed at the upwind end of the wind tunnel, 0.5 m apart. A plastic plate serving as an insect release platform was placed at the downwind end of the wind tunnel, 1.5 m away from the plants. For insect release, a cubic metal cage (226 cm3) containing one C. suppressalis female that had been mated 2 days earlier was placed on the insect release platform with an opening towards the rice plants. Each released moth was observed for 30 min, and its landing on either of the plants for at least 10 s was recorded. Moths were removed once they had made a choice. The moths that failed to make a choice within the observation period were categorized as ‘no choice’. The positions of the two rice plants were exchanged after each insect observation to eliminate a position bias. The wind tunnel was cleaned with detergent (5%, v/v) followed by alcohol (90%, v/v) and was then washed with clean water at the end of each day. Each moth was tested only once, and a total of 70–120 females were individually tested in each choice experiment. The experiments were conducted between 20.00 and 22.30 under red light (0.4 lux) conditions in a climate-controlled room at 28 ± 2°C and with 75 ± 5% RH.
(iii). Cage experiment in the greenhouse
In the greenhouse, four choice tests were conducted with C. suppressalis females: (a) undamaged Bt rice versus undamaged non-Bt rice; (b) damaged non-Bt rice versus undamaged non-Bt rice; (c) damaged Bt rice versus undamaged Bt rice; and (d) damaged non-Bt rice versus undamaged Bt rice. For each test, four potted plants (two plants of each treatment type) were positioned in the four corners of a cage (60 cm length × 60 cm width × 80 cm height) made of 80-mesh nylon nets. Plants belonging to the same treatment were positioned in opposite corners. A plastic plate containing a cotton ball saturated with a 10% honey-water solution was placed in the centre of the cage, and 10 pairs of newly emerged moths (less than 1 day) were released on the plate. After 72 h, the number of egg masses and number of eggs per egg mass on each plant were determined. Each choice test was repeated 15 to 20 times (replicates). The experiment was performed in a greenhouse at 27 ± 3°C and with 75 ± 10% RH and a photoperiod of 16 L: 8 D.
(c). Experiments under field conditions
The oviposition preference of C. suppressalis females for Bt versus non-Bt rice plants was assessed in a field near Langfang City (39.5° N, 116.4° E), China. Seedlings of Bt and non-Bt rice raised as described above were transplanted into experimental plots (2 × 2 m) on 31 May 2016. Each rice line was represented by eight plots, resulting in 16 plots. Plots were arranged in a 4 × 4 grid such that the Bt plots alternated with the non-Bt plots. Plots were separated by a 1.5-m buffer. The entire experiment (16 plots) was covered with a screened cage (14 × 14 × 2.5 m) made of nylon net (3 mm mesh size) to prevent moths from entering or escaping. The plants were cultivated according to local agricultural practices but without pesticide sprays. On 29 June, 23 July and 23 August 2016, pupae and adults of C. suppressalis were released into the cage (300–500 individuals per release). On 30 July and 30 August 2016, the number of egg masses on each rice plant, and the number of plants damaged by caterpillars in each plot were determined. After the last determination, the number of tillers on 10 randomly selected plants in each plot was determined as an indicator of growth; two plots, one with Bt rice and one with non-Bt rice, were excluded for data analysis of egg density because of significant difference in the number of plant tillers compared to other plots (p < 0.05).
(d). Caterpillar performance on damaged rice plants in a climate-controlled chamber
A bioassay was conducted to determine the fitness of C. suppressalis larvae that fed on non-Bt rice plants that were damaged or undamaged by conspecifics. Two-day-old larvae of C. suppressalis were weighed on an electronic balance (CPA2250, Sartorius AG, Göttingen, Germany, readability = 0.01 mg) and subsequently placed individually on plants that were undamaged or previously damaged by third instar C. suppressalis; as noted earlier, the instars that had previously damaged the plants were still in place. The plants were prepared as described above. After feeding for 7 days in a climate-controlled chamber (Ningbo Jiangnan, Ningbo, China) at 27 ± 3°C and 75 ± 5% RH, the surviving insects were counted and weighed. The performance of 40 insects was assessed for each of the two treatments.
(e). Response of female C. suppressalis to caterpillar-induced nocturnal plant volatiles
To assess the effects of rice volatiles induced by caterpillar damage on the behaviour of C. suppressalis females, the nocturnal volatiles released by undamaged and caterpillar-damaged plants were collected and identified, and the responses of the females to the key induced volatiles were determined.
(i). Collection and analysis of rice plant volatiles
Non-Bt rice plants at the tillering stage and with 10–12 leaves on the main stem were used in the experiment. The plants remained undamaged or were artificially infested with third instar larvae of C. suppressalis (two larvae per plant) for 72 h before being used for nocturnal volatile collection (20:00–4:00). For volatile collection, the roots of the plants were washed in running water to remove soil. Two caterpillar-damaged or undamaged rice plants were then transplanted into a water-filled conical flask (250 ml) such that their roots were immersed in the water. The flask was then wrapped with aluminium foil (leaving the plant stems and leaves outside) and was placed in a glass bottle (3142 ml). The flasks were placed in a climate-controlled chamber (Ningbo Jiangnan, Ningbo, China) at 27 ± 3°C and 75 ± 5% RH. Before entering the glass bottle, air was filtered through activated charcoal, molecular sieves (5 Å, beads, 8–12 mesh, Sigma-Aldrich, St Louis, Missouri, USA) and silica gel Rubin (cobalt-free drying agent, Sigma-Aldrich, St Louis, Missouri, USA). Plant volatiles were collected in 30-mg Super Q adsorbent traps (80/100 mesh, Alltech Associates, Deerfield, IL, USA) in a glass tube (5 mm diameter, 8 cm height). Traps were rinsed with 250 ml of methylene chloride. A 500-ng quantity of nonyl acetate was added to the samples as an internal standard.
Volatiles were analysed by gas chromatography coupled with a mass spectrometry system (Shimadzu GCMS-QP 2010SE using an RTX-5 MS fused silica capillary column). Samples were injected in a 1-μl volume with a splitless injector held at 230°C. The GC-MS was operated in the scan mode with a mass range of 33–300 amu and was in an electron-impact ionization (EI) mode at 70 eV. The oven temperature was maintained at 40°C for 2 min, and was then increased 6°C min−1 to 250°C, where it was held for 2 min. Volatile compounds were identified by mass spectral matches to library spectra as well as by retention matches to available authentic standards. Quantifications of compounds were based on their integrated areas related to the internal standard. If standards were unavailable, tentative identifications were made based on referenced mass-spectra available from NIST (Scientific Instrument Services, Inc., Ringoes, NJ, USA) or previous study.
(ii). Wind tunnel experiments for identification of key repellent volatiles
No-choice olfactory tests were carried out in a wind tunnel to identify the response of mated females to 10 selected volatile compounds (electronic supplementary material, figure S1). Compounds were selected that were either significantly increased (d-limonene) or newly produced (linalool, 2-heptanol, 2-heptanone, methyl salicylate, α-pinene, α-cedrene, β-myrcene, caryophyllene and 2-nonanone) in response to damage by C. suppressalis larvae. In addition, one mixture containing all 10 compounds and one mixture containing a subset of five compounds (2-heptanol, α-cedrene, β-myrcene, caryophyllene and 2-nonanone) were tested based on the results for the individual compounds. The mixtures were prepared in ratios that corresponded to the ratios of compounds detected in the collection of volatiles from caterpillar-damaged rice plants. The compound 2-nonanone was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China) with 99% purity. The remaining compounds were purchased from Sigma-Aldrich (St Louis, MO, USA) with 97–99% purity.
A rubber septum (Pherobio Technology Co., Ltd, Beijing, China) placed between two pots of undamaged rice plants at the upwind end of the flight tunnel served as the odour source. The septa were loaded with 100 µl of hexane (solvent) containing either 100 µg of an individual compound, mixtures, or no additive (control). After an adaptation period of greater than 30 min in the environment, one mated female was placed on the flight plate at the downwind end of the tunnel, and its flight behaviour was observed for 10 min. For each moth, the landing time on the plants was recorded. The moths that did not land on a plant within the observation period were categorized as ‘no response’.
(f). Data analysis
Dual-choice wind tunnel assays were analysed with χ2 tests, with an expected response of 50% for two treatments. A paired-sample t-test was used to analyse the oviposition data from the greenhouse cage experiment. For the field experiment, oviposition, damage and rice tiller number were analysed by repeated measures ANOVA.
For analyses of volatiles collected from undamaged and damaged non-Bt plants, one-way ANOVA was used. Differences of specific compounds in two treatments were separated by Tukey honestly significant difference tests. The behavioural response of mated females to single or mixed volatiles was analysed by using the generalized linear model, with plant contact by the moth as the dependent variable and different odours as independent variables, and with a binomial distribution with the logit link function and maximum-likelihood estimation. All analyses were conducted in SPSS 22.0 (IBM SPSS, Somers, NY, USA)
3. Results
(a). Preference of females for undamaged or caterpillar-damaged Bt or non-Bt rice (dual-choice wind tunnel experiment)
When given a choice between undamaged Bt and undamaged non-Bt rice plants in the wind tunnel, C. suppressalis females showed no preference (χ2 = 0.16, d.f. = 1, p = 0.689) (figure 1). However, the females exhibited a strong preference for undamaged plants over plants damaged by C. suppressalis larvae whether the plants were Bt or non-Bt (damaged non-Bt versus undamaged non-Bt: χ2 = 6.37, d.f. = 1, p = 0.012; damaged Bt versus undamaged Bt: χ2 = 4.27, d.f. = 1, p = 0.039; damaged non-Bt versus undamaged Bt: χ2 = 4.13, d.f. = 1, p = 0.042; and damaged Bt versus undamaged non-Bt: χ2 = 9.99, d.f. = 1, p = 0.002) (figure 1). When both Bt and non-Bt plants were damaged by caterpillars, no preference was evident (χ2 = 1.46, d.f. = 1, p = 0.228) (figure 1). Overall, females of C. suppressalis showed no preference for Bt versus non-Bt rice plants but preferred undamaged over caterpillar-damaged rice plants.
Figure 1.
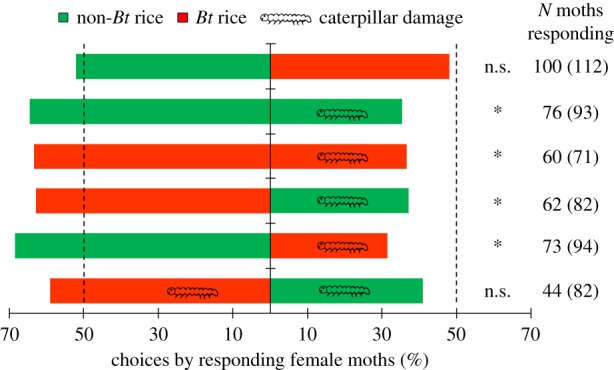
Preference of mated female Chilo suppressalis for undamaged or caterpillar-damaged Bt or non-Bt rice plants (dual-choice wind tunnel experiment). Caterpillar symbols indicate damage by two-third instars of C. suppressalis. Asterisks indicate a significant difference within a choice experiment: *p < 0.05; n.s. indicates a non-significant difference (p > 0.05) (χ2 test). The bars indicate the percentages of females that selected either plant type.
(b). Oviposition preference of females for undamaged or caterpillar-damaged Bt or non-Bt rice (greenhouse cage experiment)
When given a choice between undamaged Bt and undamaged non-Bt rice in the cage experiment, C. suppressalis females laid a similar number of egg masses on the two types of rice plants (t = 1.06, d.f. = 19, p = 0.304) (figure 2). However, females in general laid more egg masses on undamaged over caterpillar-damaged rice plants; the difference was significant for non-Bt rice plants (t = 2.42, d.f. = 18, p = 0.026) but not for Bt rice plants (t = 2.03, d.f. = 14, p = 0.061) (figure 2). When given a choice between undamaged Bt rice and damaged non-Bt rice, females laid significantly more egg masses on the Bt rice plants (t = 3.23, d.f. = 19, p = 0.004). Results for the total number of eggs laid were similar to results for egg masses, i.e. the difference between damaged and undamaged Bt rice was again significant (t = 2.19, d.f. = 14, p = 0.046) (electronic supplementary material, figure S2). Overall, C. suppressalis females preferred to lay eggs on undamaged rather than on caterpillar-damaged rice plants whether the plants were Bt or non-Bt.
Figure 2.

Number of egg masses laid by Chilo suppressalis females on undamaged or caterpillar-damaged Bt or non-Bt rice plants (greenhouse cage experiment). Caterpillar symbols indicate damage by two-third instar C. suppressalis. Each choice test was performed with 15 to 20 replicates, each consisting of a group of 10 pairs of C. suppressalis adults. The number of egg masses represents the total number laid on two rice plants. Asterisks indicate significant differences: *p < 0.05; **p < 0.01; n.s. indicates no significance (p > 0.05) (paired-sample t-test).
(c). Egg densities of C. suppressalis on Bt and non-Bt rice under field conditions
The caterpillar damage rates (% of plants damaged) were 18.7 ± 6.5% on 30 July 2016 and 30.3 ± 5.4% on 30 August 2016 for non-Bt rice plants. The damage rates were significantly lower for Bt rice plants than for non-Bt rice plants on both sampling dates (p < 0.001), i.e. they were less than 3% on Bt rice plants (figure 3). Densities of egg masses were significantly higher on Bt rice plants than on non-Bt rice plants (F1,16 = 5.29, p = 0.042) (figure 3).
Figure 3.

Damage rate (% of plants damaged, bars) by Chilo suppressalis larvae and numbers of egg masses (lines) laid by C. suppressalis females on Bt and non-Bt rice plants under field conditions. Bt and non-Bt rice were planted in 2 × 2 m plots in the field, and the entire plot area was covered with a screened cage (14 × 14 × 2.5 m). Pupae and adults of C. suppressalis were released into the cage on 29 June, 23 July and 23 August 2016. Egg masses on Bt and non-Bt rice plants were counted on 30 July and 30 August. Values are means ± s.e. The caterpillar damage rate was significantly higher (p < 0.001) and egg mass number was significantly lower (p = 0.042) on non-Bt plants than on Bt plants according to a repeated-measures ANOVA.
(d). Performance of caterpillars on damaged rice plants in a climate-controlled chamber
When 2-day-old C. suppressalis larvae were allowed to feed for 7 days on non-Bt rice plants that were either undamaged or damaged by third instars, the survival rate was significantly lower on damaged plants (75%) than on undamaged plants (92%) (χ2 = 4.50, d.f. = 1, p = 0.034) (electronic supplementary material, figure S3). Similarly, the increase in body weight was significantly lower on damaged plants (3.20 ± 0.22 mg) than on undamaged plants (9.29 ± 0.59 mg) (t = 9.19, d.f. = 65, p < 0.001) (electronic supplementary material, figure S3). Overall, C. suppressalis larvae performed much better on undamaged rice plants than on rice plants previously damaged by conspecifics.
(e). Response of female C. suppressalis to caterpillar-induced nocturnal plant volatiles
A total of 28 compounds were detected in the headspace of non-Bt rice plants damaged by C. suppressalis but only four compounds (α-pinene, d-limonene, methyl salicylate and tetradecane) were detected in the headspace of undamaged non-Bt rice plants (electronic supplementary material, table S1). The contents of d-limonene and tetradecane were significantly higher for damaged than for undamaged plants, and the contents of α-pinene and methl salicylate did not significantly differ between damaged and undamaged plants (electronic supplementary material, table S1).
Based on the GC-MS results (electronic supplementary material, table S1), we conducted a wind tunnel experiment to assess the response of mated C. suppressalis females to each of the following 10 volatile compounds: linalool, 2-heptanol, d-limonene, 2-heptanone, methyl salicylate, α-pinene, α-cedrene, β-myrcene, caryophyllene and 2-nonanone. In the control treatment (rice plants together with a rubber septum loaded with 100 µl of hexane), 51% of females exhibited upwind flight and contacted the rice plants (figure 4). A similar percentage of females responded to linalool, d-limonene, 2-heptanone, methyl salicylate or α-pinene (p > 0.05). Compared to the control treatment, significantly fewer C. suppressalis females showed an upwind flight response to 2-heptanol (χ2 = 3.56, d.f. = 1, p = 0.038), α-cedrene (χ2 = 5.74, d.f. = 1, p = 0.017), β-myrcene (χ2 = 11.751, d.f. = 1, p = 0.0003), caryophyllene (χ2 = 11.372, d.f. = 1, p = 0.001) or 2-nonanone (χ2 = 11.751, d.f. = 1, p = 0.001) (figure 4). Next, we tested the response of mated females to synthetic blends of multiple volatile compounds. The percentage of contact was only 14.3% with the blend containing all 10 compounds and was only 19.3% with the blend containing the five compounds; in both cases, the percentages were significantly lower than that of the control (both p < 0.001).
Figure 4.
Upwind flight responses (percentage of moths that landed on plants) of Chilo suppressalis females to rice plants and rubber septa treated with hexane (control) or with the indicated synthetic volatile compounds dissolved in hexane (wind tunnel experiment). * and ** indicate percentages that were significantly lower (p < 0.05 and 0.01, respectively), i.e. indicated repellence.
4. Discussion
The rapid increase in the planting of Bt-transgenic crop varieties has greatly changed crop production and protection in large areas of the world. With the combined presence of non-Bt crops (including those planted as refuges) and non-crop habitats, pests targeted by the Bt trait have a choice between Bt and non-Bt crops or weeds. The host preference of these pests has important consequences for both insect pest management and insect resistance management.
Our assays showed that C. suppressalis females have no oviposition preference for healthy Bt versus non-Bt rice plants. These results are consistent with previous studies with cotton, maize, rice and oilseed rape, suggesting that the transformation and the expression of the cry genes does not affect the oviposition behaviour of the target pests [10,26–29]. Our previous study also confirmed that undamaged Bt and non-Bt rice plants (the same rice lines used as in the current study) emitted the same number of volatiles and there were no significant differences in the quantity of each volatile compound between the treatments [33]. We did document, however, a significant oviposition preference of C. suppressalis females for undamaged rice plants versus plants damaged by conspecific larvae, whether the plants were Bt or non-Bt. To our knowledge, this is the first report indicating that C. suppressalis females avoid laying eggs on host plants damaged by conspecifics. As noted in the introduction, this phenomenon has been previously observed for a number of other lepidopteran species. However, this behaviour is not exhibited by all insects. Leaf beetles (Coleoptera: Chrysomelidae), for example, preferred damaged plants to undamaged plants [34–37]. Even some lepidopterans prefer damaged plants. S. littoralis, for example, preferentially oviposited on cotton plants damaged by third to fourth instar larvae rather than on undamaged plants [38]. We have also found that the mated females of another lepidopteran rice pest, C. medinalis, showed no oviposition preference between undamaged and conspecific-damaged rice plants (YQ Wang & YH Li 2018, unpublished data).
The question remains why C. suppressalis females prefer to lay eggs on healthy rice plants. According to the ‘mother knows best’ principle, one reason could be that the offspring perform better on undamaged rice plants than on damaged rice plants [17]. Our results support this hypothesis in that the survival and body weight of C. suppressalis larvae were lower on caterpillar-damaged rice plants than on healthy rice plants. A possible explanation is the induced production of trypsin proteinase inhibitors in rice plants in response to C. suppressalis infestation [39]. As has been reported for many moth species, females appear to recognize damaged plants by the volatile compounds released [18,25,40,41]. Our GC-MS analyses combined with the wind tunnel experiments indicated that five volatile compounds, namely 2-heptanol, α-cedrene, β-myrcene, caryophyllene and 2-nonanone, were mainly responsible for the repellence of damaged plants.
Based on the current finding that C. suppressalis females avoid laying eggs on plants previously damaged by conspecific larvae and based on the previous finding that neonate C. suppressalis cannot survive or cause noticeable damage on Bt rice plants in the field [42], we expect that C. suppressalis females will migrate from damaged non-Bt rice fields to protected Bt rice fields for egg laying when the Bt and non-Bt plants are in close proximity. As a consequence, the offspring produced on Bt rice plants will be killed by the Bt protein. This is inconsistent with the ‘mother knows best’ theory. As suggested by Jongsma et al. [7], female moths appear to be unable to detect the presence of the Bt proteins and may therefore fail to evolve an avoidance behaviour. Our results thus indicate that Bt rice could serve as a dead-end trap crop for C. suppressalis and would protect adjacent non-Bt rice as suggested by Shelton et al. [43]. Given the small-scale rice farming systems prevalent in China [44], the effects on C. suppressalis populations might be significant. This, however, needs to be confirmed under field conditions once Bt rice is cultivated on larger areas. Interestingly, planthoppers such as Nilaparvata lugens (Hemiptera: Delphacidae) show a different behaviour. When given the choice, they prefer to oviposit on caterpillar-damaged (non-Bt) rice plants compared to undamaged (Bt) rice plants [32]. Non-Bt rice refuges planted adjacent to Bt rice might thus act as a trap crop and protect Bt rice from this non-target pest.
Another important implication of our results relates to the evolution of Bt resistance in the target pests, which is a major threat for the sustainable use of Bt crops [12,13,45]. Currently, the most commonly practised strategy for delaying the evolution of Bt resistance is the establishment of refuges with non-Bt plants [11,12]. Because C. suppressalis is highly specialized for rice, structured non-Bt rice refuges rather than natural refuges adjacent to Bt rice will be necessary [16]. However, the value of the refuges in delaying the development of Bt resistance is based on the assumption of a random oviposition and mating by the target pest across the Bt plants and the non-Bt plants in the refuges [15,46]. Our results suggest that this assumption is invalid for C. suppressalis because females will avoid ovipositing on the non-Bt refuge plants once such plants are damaged. Data modelling for a different Lepidoptera species (Spodoptera frugiperda; Noctuidae) suggest that oviposition preference for Bt over non-Bt plants will significantly affect the speed of resistance evolution to the Bt trait [8]. This should be taken into account when designing the resistance management plan for Bt rice in China, especially because C. suppressalis can have up to five generations per year [47].
The current findings indicate that C. suppressalis females will show a strong oviposition preference for Bt over non-Bt rice plants once the plants have been exposed to the pest. This occurs because pest damage will be much higher on the non-Bt rice and because the females prefer to oviposit on undamaged plants. The results suggest that by acting as a dead-end trap crop, Bt rice could help protect adjacent non-Bt rice plants against C. suppressalis.
Acknowledgements
We thank Prof. Yongjun Lin (Huazhong Agricultural University) for providing rice seeds. We acknowledge Bruce Jaffee (http://jaffeerevises.com) for critical comments and language editing on an earlier version of the manuscript.
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.nn514kg [48].
Authors' contributions
Y.L. and Y.J. designed the study. Y.J. and X.H. performed all of the experiments. Y.L., Y.J., X.H., K.W. and J.R. analysed data and wrote the manuscript. Y.P. provided experimental materials and participated in the discussion of this project. All the authors have read and approved the manuscript for publication.
Competing interests
The authors declare that they have no conflict of interests.
Funding
The study was supported by the National GMO New Variety Breeding Program of PRC (2016ZX08011-001).
References
- 1.ISAAA. 2016. Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52 Ithaca, NY: ISAAA; See http://isaaa.org/resources/publications/briefs/52/executivesummary/default.asp (accessed 27 June 2018). [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine (NASEM). 2006. Genetically engineered crops: experiences and prospects. Washington, DC: National Academies Press; See https://www.nap.edu/catalog/23395/genetically-engineered-crops-experiences-and-prospects (accessed 27 June 2018). [PubMed] [Google Scholar]
- 3.Chen M, Shelton A, Ye GY. 2011. Insect-resistant genetically modified rice in China: from research to commercialization. Annu. Rev. Entomol. 56, 81–101. ( 10.1146/annurev-ento-120709-144810) [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Hallerman EM, Liu Q, Wu K, Peng Y. 2016. The development and status of Bt rice in China. Plant Biotechnol. J. 14, 839–848. ( 10.1111/pbi.12464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng CF, Wang HT, Gao LD, Xuan JW. 2003. The occurrence status, damage cost estimate and control strategies of stem borers in China. Plant Protect. 29, 37–39. ( 10.3969/j.issn.0529-1542.2003.01.012) [DOI] [Google Scholar]
- 6.Liu Q, Hallerman E, Peng Y, Li Y. 2016. Development of Bt rice and Bt maize in China and their efficacy in target pest control. Int. J. Mol. Sci. 17, 1561 ( 10.3390/ijms17101561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jongsma MA, Gould F, Legros M, Yang L, van Loon JJA, Dicke M. 2010. Insect oviposition behavior affects the evolution of adaptation to Bt crops: consequences for refuge policies. Evol. Ecol. 24, 1017–1030. ( 10.1007/s10682-010-9368-3) [DOI] [Google Scholar]
- 8.Téllez-Rodríguez P, Raymond B, Morán-Bertot I, Rodríguez-Cabrera L, Wright DJ, Borroto CG, Ayra-Pardo C. 2014. Strong oviposition preference for Bt over non-Bt maize in Spodoptera frugiperda and its implications for the evolution of resistance. BMC Biol. 12, 3790–3795. ( 10.1186/1741-7007-12-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han P, Velasco-Hernández MC, Ramirez-Romero R, Desneux N. 2016. Behavioral effects of insect-resistant genetically modified crops on phytophagous and beneficial arthropods: a review. J. Pest Sci. 89, 859–883. ( 10.1007/s10340-016-0791-2) [DOI] [Google Scholar]
- 10.Sun X, Zhou W, Liu H, Zhang A, Ai CR, Zhou SS, Zhou CX, Wang MQ. 2013. Transgenic Bt rice does not challenge host preference of the target pest of rice leaffolder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). PLoS ONE 8, e79032 ( 10.1371/journal.pone.0079032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould F. 2000. Testing Bt refuge strategies in the field. Nat. Biotechnol. 18, 266–267. ( 10.1038/73693) [DOI] [PubMed] [Google Scholar]
- 12.Tabashnik BE, Brévault T, Carrière Y. 2013. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521. ( 10.1038/nbt.2597) [DOI] [PubMed] [Google Scholar]
- 13.Tabashnik BE, Carrière Y. 2017. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 35, 926–935. ( 10.1038/nbt.3974) [DOI] [PubMed] [Google Scholar]
- 14.Burkness EC, Cira TM, Moser SE, Hutchison WD. 2015. Bt maize seed mixtures for Helicoverpa zea (Lepidoptera: Noctuidae): larval movement, development, and survival on non-transgenic maize. J. Econ. Entomol. 108, 2761–2769. ( 10.1093/jee/tov253) [DOI] [PubMed] [Google Scholar]
- 15.Caprio MA. 2001. Source–sink dynamics between transgenic and non-transgenic habitats and their role in the evolution of resistance. J. Econ. Entomol. 94, 698–705. ( 10.1603/0022-0493-94.3.698) [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Gao Y, Wu K. 2017. Function and effectiveness of natural refuge in IRM strategies for Bt crops . Curr. Opin. Insect Sci. 21, 1–6. ( 10.1016/j.cois.2017.04.007) [DOI] [PubMed] [Google Scholar]
- 17.Gripenberg S, Mayhew PJ, Parnell M, Roslin T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393. ( 10.1111/j.1461-0248.2009.01433.x) [DOI] [PubMed] [Google Scholar]
- 18.De Moraes CM, Mescher MC, Tumlinson JH. 2001. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 410, 577–580. ( 10.1038/35069058) [DOI] [PubMed] [Google Scholar]
- 19.Kessler A, Baldwin IT. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 291, 2141–2144. ( 10.1126/science.291.5511.2141) [DOI] [PubMed] [Google Scholar]
- 20.Harmon JP, White JA, Andow DA. 2003. Oviposition behavior of Ostrinia nubilalis (Lepidoptera: Crambidae) in response to potential intra- and interspecific interactions. Environ. Entomol. 32, 334–339. ( 10.1603/0046-225X-32.2.334) [DOI] [Google Scholar]
- 21.Huang CH, Yan FM, Byers JA, Wang RJ, Xu CR. 2009. Volatiles induced by the larvae of the Asian corn borer (Ostrinia furnacalis) in maize plants affect behavior of conspecific larvae and female adults. Insect Sci. 16, 311–320. ( 10.1111/j.1744-7917.2009.01257.x) [DOI] [Google Scholar]
- 22.Signoretti A, Peñaflor M, Bento J. 2012. Fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), female moths respond to herbivore-induced corn volatiles. Neotrop. Entomol. 41, 22–26. ( 10.1007/s13744-011-0003-y) [DOI] [PubMed] [Google Scholar]
- 23.Bruce TJA, Midega CAO, Birkett MA, Pickett JA, Khan ZR. 2010. Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol. Lett. 6, 314–317. ( 10.1098/rsbl.2009.0953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zakir A, Bengtsson M, Sadek MM, Hansson BS, Witzgall P, Anderson P. 2013. Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J. Exp. Biol. 216, 3257–3263. ( 10.1242/jeb.083188) [DOI] [PubMed] [Google Scholar]
- 25.Reisenman CE, Riffell JA, Duffy K, Pesque A, Mikles D, Goodwin B. 2013. Species-specific effects of herbivory on the oviposition behavior of the moth Manduca sexta. J. Chem. Ecol. 39, 76–89. ( 10.1007/s10886-012-0228-1) [DOI] [PubMed] [Google Scholar]
- 26.Hellmich RL, Higgins LS, Witkowski JF, Campbell JE, Lewis LC. 1999. Oviposition by European corn borer (Lepidoptera: Crambidae) in response to various transgenic corn events. J. Econ. Entomol. 92, 1014–1020. ( 10.1093/jee/92.5.1014) [DOI] [Google Scholar]
- 27.Kjær C, Damgaard C, Lauritzen AJ. 2010. Assessment of effects of Bt-oilseed rape on large white butterfly (Pieris brassicae) in natural habitats. Entomol. Exp. Appl. 134, 304–311. ( 10.1111/j.1570-7458.2009.00958.x) [DOI] [Google Scholar]
- 28.Lei Z, Liu TX, Greenberg SM. 2009. Feeding, oviposition and survival of Liriomyza trifolii (Diptera: Agromyzidae) on Bt and non-Bt cottons. Bull. Entomol. Res. 99, 253–261. ( 10.1017/S0007485308006317) [DOI] [PubMed] [Google Scholar]
- 29.Dos Santos VB, Silva LB, Carneiro E, Silva AF, Lopes GN, Pavan BE, Rodrigues TF. 2017. Comparative study of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) preference for Bt and non-Bt soybean cultivars. Am. J. Plant Sci. 8, 2483–2500. ( 10.4236/ajps.2017.810169) [DOI] [Google Scholar]
- 30.Tang W, Chen H, Xu C, Li X, Lin Y, Zhang Q. 2006. Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Mol. Breed. 18, 1–10. ( 10.1007/s11032-006-9002-9) [DOI] [Google Scholar]
- 31.Han L, Li S, Liu P, Peng Y, Hou M. 2012. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 105, 253–258. ( 10.1603/AN10170) [DOI] [Google Scholar]
- 32.Wang X, Liu Q, Meissle M, Peng Y, Wu K, Romeis J, Li Y. 2018. Bt rice could provide ecological resistance to non-target planthoppers. Plant Biotechnol. J. ( 10.1111/pbi.12911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Romeis J, Yu H, Zhang Y, Li Y, Peng Y. 2015. Bt rice does not disrupt the host-searching behavior of the parasitoid Cotesia chilonis. Sci. Rep. 5, 15295 (doi.org:10.1038/srep15295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng C, Weiss MJ. 1992. Evidence of an aggregation pheromone in the flea beetle, Phyllotreta Cruciferae (Goeze) (Coleoptera: Chrysomelidae). J. Chem. Ecol. 18, 875–884. ( 10.1007/BF00988328) [DOI] [PubMed] [Google Scholar]
- 35.Bolter CJ, Dicke M, Loon JJAV, Visser JH, Posthumus MA. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol. 23, 1003–1023. ( 10.1023/B:JOEC.0000006385.70652.5e) [DOI] [Google Scholar]
- 36.Kalberer NM, Turlings TCJ, Rahier M. 2001. Attraction of a leaf beetle (Oreina cacaliae) to damaged host plants. J. Chem. Ecol. 27, 647–661. ( 10.1023/A:1010389500009) [DOI] [PubMed] [Google Scholar]
- 37.Piesik D, Lemńczyk G, Skoczek A, Lamparski R, Bocianowski J, Kotwica K, Delaney KJ. 2011. Fusarium infection in maize: volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus. J. Plant Physiol. 168, 1534–1542. ( 10.1016/j.jplph.2011.01.032) [DOI] [PubMed] [Google Scholar]
- 38.Anderson P, Alborn H. 1999. Effects on oviposition behaviour and larval development of Spodoptera littoralis by herbivore-induced changes in cotton plants. Entomol. Exp. Appl. 92, 45–51. ( 10.1046/j.1570-7458.1999.00523.x) [DOI] [Google Scholar]
- 39.Zhou G, Wang X, Yan F, Wang X, Li R, Cheng J, Lou Y. 2011. Genome-wide transcriptional changes and defence-related chemical profiling of rice in response to infestation by the rice striped stem borer Chilo suppressalis. Physiol. Plant. 143, 21–40. ( 10.1111/j.1399-3054.2011.01483.x) [DOI] [PubMed] [Google Scholar]
- 40.Almohamad R, Verheggen FJ, Francis F, Lognay G, Haubruge E. 2010. Assessment of oviposition site quality by aphidophagous hoverflies: reaction to conspecific larvae. Anim. Behav. 79, 589–594. ( 10.1016/j.anbehav.2009.11.032) [DOI] [Google Scholar]
- 41.Martins CB, Zarbin PH. 2013. Volatile organic compounds of conspecific-damaged Eucalyptus benthamii influence responses of mated females of Thaumastocoris peregrinus. J. Chem. Ecol. 39, 602–611. ( 10.1007/s10886-013-0287-y) [DOI] [PubMed] [Google Scholar]
- 42.Wang YN, Ke KQ, Li YH, Han LZ, Liu YM, Hua HX, Peng YF. 2016. Comparison of three transgenic Bt rice lines for insecticidal protein expression and resistance against a target pest, Chilo suppressalis (Lepidoptera: Crambidae). Insect Sci. 23, 78–87. ( 10.1111/1744-7917.12178) [DOI] [PubMed] [Google Scholar]
- 43.Shelton AM, Badenes-Perez FR. 2006. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 51, 285–308. ( 10.1146/annurev.ento.51.110104.150959) [DOI] [PubMed] [Google Scholar]
- 44.Fan S, Chan-Kang C. 2005. Is small beautiful? Farm size, productivity, and poverty in Asian agriculture. Agricult. Econ. 32, 135–146. ( 10.1111/j.0169-5150.2004.00019.x) [DOI] [Google Scholar]
- 45.Wan P, et al. 2017. Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm. Proc. Natl Acad. Sci. USA . 114, 5413–5418. ( 10.1073/pnas.1700396114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gould F, 1998. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43, 701–726. ( 10.1146/annurev.ento.43.1.701) [DOI] [PubMed] [Google Scholar]
- 47.Heinrichs EA, Nwilene FE, Stout M, Hadi BAR, Freitas T. 2017. Rice insect pests and their management. Cambridge, UK: Burleigh Dodds Science Publishing; See https://iapps2010.me/2017/06/13/new-book-rice-insect-pests-and-their-management/ (accessed 27 June 2018). [Google Scholar]
- 48.Jiao Y, Hu X, Peng Y, Wu K, Romeis J, Li Y.2018. Data from: Bt rice plants may protect neighbouring non-Bt rice plants against the striped stem borer, Chilo suppressalis. Dryad Digital Repository . ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jiao Y, Hu X, Peng Y, Wu K, Romeis J, Li Y.2018. Data from: Bt rice plants may protect neighbouring non-Bt rice plants against the striped stem borer, Chilo suppressalis. Dryad Digital Repository . ( ) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.nn514kg [48].



